Abstract
We herein report a case of immune-related colitis induced by the long-term use of nivolumab. A 62-year-old Japanese man was treated with nivolumab at 3 mg/kg every 2 weeks for advanced lung adenocarcinoma. The patient was admitted to our hospital due to non-bloody watery diarrhea after the 70th dose of nivolumab. A biopsy specimen of the colon mucosa revealed evidence of colitis with cryptitis and crypt microabscesses. He was diagnosed with immune-related colitis and started on predonisolone 60 mg/day. Subsequently, his symptoms remarkably resolved. Consideration of immune-related adverse events up to several years after the initiation of nivolumab is important.
Keywords: nivolumab, immune-related adverse event, colitis, non-small cell lung cancer, immune-checkpoint-inhibitor
Introduction
Nivolumab is a fully human IgG4 programmed death 1 (PD-1) immune checkpoint inhibitor (ICI) antibody that binds to the PD-1 receptor and blocks its interaction with programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2) and reactivates T cell-mediated anti-tumor immunity (1). Nivolumab has shown superiority to docetaxel with regard to the overall survival in patients with advanced, previously treated squamous and non-squamous non-small cell lung cancer (NSCLC) (2, 3). Therefore, nivolumab is currently approved for the treatment of NSCLC in Japan. The safety profile of nivolumab is also favorable in comparison with docetaxel (2, 3).
However, nivolumab has been shown to induce immune-related adverse events (irAEs) that differ markedly from those of cytotoxic chemotherapy. The irAEs include various organ disorders, such as gastro-intestinal, skin, liver, lung, endocrine, renal and joint disorders. Furthermore, most irAEs occur within six months of the initiation of anti-PD-1 blockade (4). Few reports have described irAEs due to the long-term use of ICIs (5).
We herein report a case of immune-related colitis induced by the long-term use of nivolumab.
Case Report
A 62-year-old Japanese man was admitted to our hospital due to non-bloody watery diarrhea. He had been diagnosed with Stage IV lung adenocarcinoma with right adrenal metastasis and left iliopsoas metastasis 3 years previously and had been started on cisplatin (CDDP) 75 mg/m2 + pemetrexed (PEM) 500 mg/m2 on Day 1 of a 21-day cycle. After he completed four cycles of chemotherapy, the objective response was stable disease according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST).
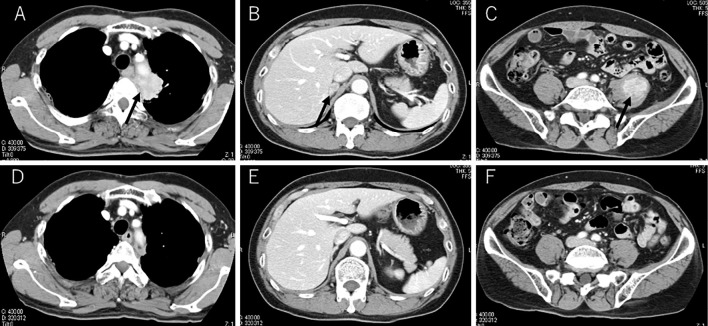
However, follow-up computed tomography (CT) revealed enlargement of primary and metastatic lesions (Fig. 1A-C). These CT findings met the criteria of progressive disease according to the RECIST. Therefore, he was treated with nivolumab 3 mg/kg every 2 weeks as second-line chemotherapy. The primary and metastatic lesions maintained a long-term partial response (Fig. 1D-F). After the 70th dose of nivolumab, he experienced non-bloody watery diarrhea 30 times a day in the 2 days before admission. The patient, an ex-smoker, drank alcoholic beverages occasionally. He had no remarkable medical history and no periodic medication. Furthermore, he had not travelled outside of Japan recently. His Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 1. He presented with tachycardia, and a physical examination revealed hyperactive bowel sounds and left flank tenderness. There was no evidence of peritoneal irritation signs. Furthermore, laboratory tests demonstrated an increased white blood cell count and C-reactive protein levels; however, there was no elevation of carcinoembryonic antigen or cytokeratine 19 fragments. Laboratory tests also showed a normal thyroid level and adrenal function (Table). An analysis of a stool culture was negative for Clostridium difficile toxin and norovirus antigen.
Figure 1.
CT scans showed a primary lesion in the left upper lobe (black arrow), enlarged right adrenal gland (black arrow) and enlarged left iliopsoas (black arrow) with contrast enhancement (A-C). CT images showed a reduction in the primary and metastatic lesions at the 70th dose of nivolumab (D-F).
Table.
Laboratory Findings on Admission.
| <Blood count> | <Biochemistry> | <Endocrine> | ||||||||
| WBC | 12,300 | /μL | TP | 7.4 | g/dL | TSH | 1.03 | μIU/mL | ||
| Neut | 87 | % | Alb | 4.5 | mg/dL | F-T3 | 2.8 | pg/mL | ||
| Lymph | 8 | % | T-Bil | 1.1 | mg/dL | F-T4 | 1.1 | ng/dL | ||
| Mono | 4 | % | AST | 25 | IU/L | ACTH | 32.7 | pg/mL | ||
| Eosino | 1 | % | ALT | 22 | IU/L | Cortisol | 21.4 | μg/dL | ||
| RBC | 528×104 | /μL | LDH | 231 | IU/L | |||||
| Hb | 16.7 | g/dL | CK | 61 | IU/L | <Tumor marker> | ||||
| Ht | 49.2 | % | BUN | 22.6 | mg/dL | CEA | 1.2 | ng/mL | ||
| MCV | 93.1 | fl | Cre | 1.04 | mg/dL | CYFRA | 1.06 | mg/dL | ||
| MCHC | 33.8 | % | Glu | 93 | mg/dL | |||||
| Plt | 22×104 | /μL | Na | 136 | mEq/L | |||||
| K | 4.26 | mEq/L | ||||||||
| Cl | 102.5 | mEq/L | ||||||||
| CRP | 4.8 | mg/dL | ||||||||
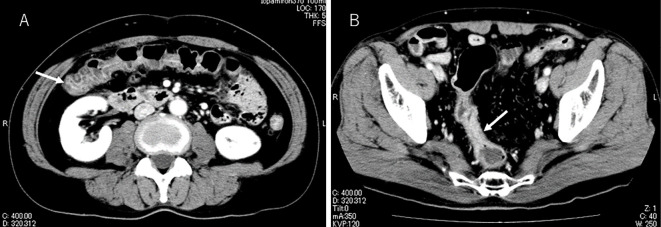
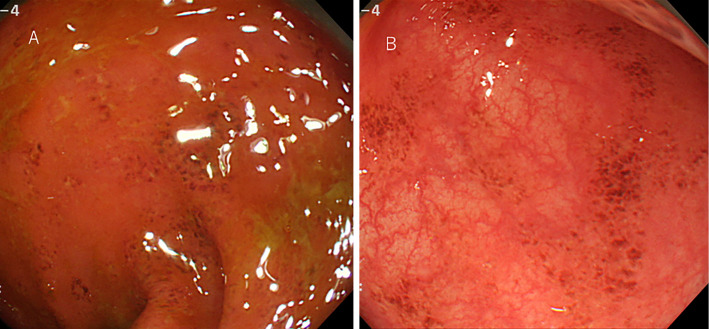
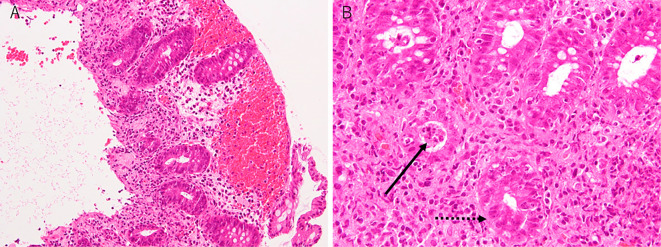
Contrast-enhanced abdominal CT showed colon wall thickening and edematous changes (Fig. 2A and B). Subsequently, he was referred to a gastroenterologist. Colonoscopy revealed mucosal erythema and edema and the loss of the vascular pattern of the sigmoid colon (Fig. 3). A biopsy of the mucosal erythema was performed. Neutrophilic and lymphocytic infiltration of the colon mucosa and mucosal erosion and hemorrhaging were observed. Cryptitis and crypt microabscesses were also observed (Hematoxylin and Eosin staining) (Fig. 4). Therefore, he was diagnosed with grade 3 immune-related colitis according to Common Terminology Criteria for Adverse Events version 4.0. Initial treatment involved abstinence from food with intravenous nutrition and ciprofloxacin 600 mg/day. He was also treated with predonisolone at 60 mg/day on the third day of admission. He started oral intake on the sixth day of admission. His symptoms remarkably improved after the initiation of predonisolone, and the dose of predonisolone was gradually reduced. He was discharged on day 21 of admission with oral predonisolone at 30 mg/day. Subsequently, the dose of predonisolone was tapered and discontinued without relapse of colitis in the outpatient department. There was also no evidence of lung cancer recurrence at 13 months after the withdrawal of nivolumab.
Figure 2.
Contrast-enhanced abdominal CT revealed edematous changes in the transverse colon (A; white arrow) and wall thickening of the sigmoid colon (B; white arrow).
Figure 3.
Colonoscopy showed mucosal edema and the loss of the vascular pattern (A) as well as mucosal erythema of the sigmoid colon (B).
Figure 4.
Hematoxylin and Eosin staining of the biopsy specimen. Neutrophilic and lymphocytic infiltration of the colon mucosa and mucosal erosion or hemorrhaging were observed (A). Under high magnification, cryptitis (dot black arrow) and crypt microabscesses (black arrow) were observed (B).
Discussion
This patient course provides an important clinical suggestion: We should consider irAEs up to several years after the initiation of nivolumab. Furthermore, close collaboration between organ specialists is critical for the management of irAEs.
irAEs have been reported to occur in up to 58-74% of lung cancer patients treated with nivolumab (2, 3, 6). Most irAEs occur within 6 months of the initiation of anti-PD-1 blockade (4). The median time to the occurrence of gastrointestinal disorders was 3.0-4.7 weeks during the use of nivolumab (2, 3). However, in the KEYNOTE-006 study, the number of patients with grade 3, 4 or 5 adverse events increased as the duration of pembrolizumab use became longer (7). To our knowledge, there has only been one report of irAEs due to the long-term use of ICI. Nishino et al. reported a case of pneumonitis induced by nivolumab and ipilimumab sequentially. The onset of the irAE occurred 24.3 months after the initiation of therapy (5). In our case, immune-related colitis occurred 32.5 months after treatment.
It is important to distinguish infectious enterocolitis from immune-related colitis. A stool examination, such as a fecal culture for Clostridium difficile toxin and viral antigen, is necessary for the exclusion of infectious enterocolitis (4, 8). Abdominal CT is useful for understanding the severity and extent of colitis and can exclude intestinal perforation. For the colonoscopic findings, the site, distribution and findings of the lesion may be helpful in distinguishing immune-related colitis from other types of colitis. Colonoscopy with a biopsy is helpful for the confirmation of colitis (4). The differential diagnosis of colitis is broad, and expert knowledge is important for the accurate diagnosis. Therefore, referral to a gastroenterologist is essential for the management of immune-related colitis.
Ipilimumab is an anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibody. Immune-related gastrointestinal disorder is more common during ipilimumab administration than nivolumab (4). In a previous study, Beck et al. reviewed 41 patients diagnosed with grade 3 or 4 enterocolitis related to ipilimumab. The most common symptom of enterocolitis was diarrhea (40 of 41 patients). The histopathological features included neutrophilic and lymphocytic inflammation, cryptitis and crypt abscess, similar to inflammatory bowel disease (9). These findings were consistent with our case. Therefore, it seems that there is some kind of autoimmune mechanism underlying the development of immune-related colitis.
Regarding the treatment of grade 3 or 4 immune-related colitis, ICIs should be withheld, and systemic corticosteroids at 1-2 mg/kg/day predonisolone or equivalent should be administered (8). Steroid tapering should be performed over at least one month in order to prevent recurrence (10). Therefore, we chose predonisolone at 60 mg/day (1 mg/kg/day) and were able to taper and discontinue predonisolone without relapse.
The number of cases of long-term use of ICIs for lung cancer might increase in the future. Accordingly, our understanding of irAEs might improve. Therefore, it is important to highlight irAEs when they are encountered. Check sheets about irAE symptoms and patient education on irAEs are also important for early detection and may aid in the prediction and prevention of irAEs.
In conclusion, we should consider the possibility of irAEs up to several years after the initiation of nivolumab. The early detection and management of irAEs is important.
Author's disclosure of potential Conflicts of Interest (COI).
Miyako Satouchi: Honoraria, Bristol-Myers Squibb and Ono Pharmaceutica.
References
- 1. Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24: 207-212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P, et al. . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123-135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz-Ares L, Horn L, et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373: 1627-1639, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michot JM, Bigenwald C, Champiat S, et al. . Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54: 139-148, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med 373: 288-290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rizvi NA, Mazières J, Planchard D, et al. . Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16: 257-265, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robert C, Schachter J, Long GV, et al. . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372: 2521-2532, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 4: 560-575, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beck KE, Blansfield JA, Tran KQ, et al. . Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 24: 2283-2289, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eigentler TK, Hassel JC, Berking C, et al. . Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 45: 7-18, 2016. [DOI] [PubMed] [Google Scholar]