Abstract
TAFRO syndrome is a systemic inflammatory disorder that is characterized by thrombocytopenia, anasarca, myelofibrosis, renal dysfunction, and organomegaly. Although thrombocytopenia is one of the major features of TAFRO syndrome, complications of disseminated intravascular coagulation (DIC) are not common. The therapeutic strategy for TAFRO syndrome complicated by DIC has not been established. We herein describe a case of TAFRO syndrome with DIC that was successfully treated with tocilizumab (an anti-IL-6 receptor antibody) and recombinant thrombomodulin (rTM). This case suggests a possible therapeutic benefit of rTM in patients with TAFRO syndrome complicated by DIC.
Keywords: TAFRO syndrome, disseminated intravascular coagulation, tocilizumab, recombinant thrombomodulin
Introduction
TAFRO syndrome (thrombocytopenia, anasarca, myelofibrosis, renal dysfunction, and organomegaly) is a systemic inflammatory disorder with Castleman-like pathological features that has recently been identified in Japan (1). In 2015, the diagnostic criteria, disease severity classification, and treatment strategy for this syndrome were reported (2); however, the pathophysiology of TAFRO syndrome is not fully understood. This rare disease is difficult to accurately diagnose. TAFRO syndrome may have a fatal outcome if the patient does not receive timely and appropriate treatment. In particular, patients who develop disseminated intravascular coagulation (DIC) have a poor prognosis (3-5). A therapeutic strategy for TAFRO syndrome, particularly in patients with DIC, has yet to be established. We herein describe a case of TAFRO syndrome with DIC that was successfully treated with tocilizumab (TCZ; an anti-IL-6 receptor antibody) and recombinant thrombomodulin (rTM).
Case Report
A 38-year-old man was referred to our hospital for unilateral massive pleural effusion. His symptoms included fever, cough, dyspnea, and epigastric pain. He was a current smoker with an 18 pack-year history and had a history of duodenal ulcer. He had developed fever and abdominal discomfort 4 weeks before his admission, and had attended several outpatient clinics where he had been diagnosed with an upper respiratory tract infection and a duodenal ulcer. However, antibiotics and a proton pump inhibitor did not improve his symptoms. He was thus admitted for further evaluation and treatment.
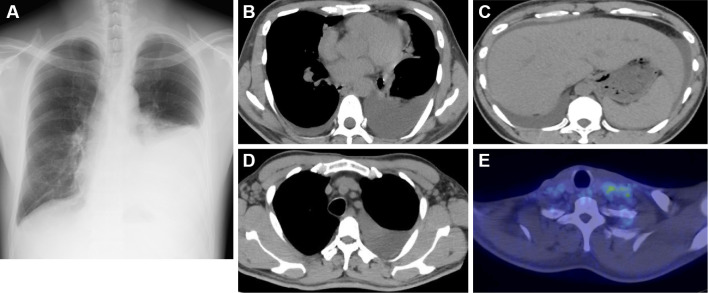
On admission, a physical examination revealed hepatomegaly, cervical lymph node swelling, and reduced breath sounds in the left lung fields. The laboratory findings included thrombocytopenia, renal dysfunction, elevated C-reactive protein (CRP), and elevated alkaline phosphatase (ALP) (Table 1). A chest radiograph showed a left pleural effusion (Fig. 1A), and chest-abdominal computed tomography (CT) showed a massive left pleural effusion, a small right pleural effusion, ascites, and hepatomegaly (Fig. 1B and C). The CT scan also revealed the enlargement of the supraclavicular, axillary (Fig. 1D), para-aortic, and inguinal lymph nodes. Furthermore, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) imaging demonstrated a mild FDG uptake in the left supraclavicular lymph nodes (Fig. 1E). The pleural effusion was exudative with no evidence of malignancy or infection. Taken together, these findings suggested an underlying lymphoproliferative disease with inflammatory features. His symptoms worsened on day 2, prompting treatment with methylprednisolone pulse therapy (1,000 mg/day for 3 days), which had to be started before obtaining a definitive diagnosis.
Table 1.
Laboratory Data on Admission.
| Complete blood cell count | Blood chemistry | Immunologic test | ||||||||
| White blood cell | 10,400 | /μL | Total protein | 5.8 | g/dL | RF | 4 | IU/mL | ||
| Neutrophil | 76.2 | % | Albumin | 2.1 | g/dL | IgG | 1,329 | mg/dL | ||
| Lymphocyte | 14.6 | % | Total bilirubin | 1 | mg/dL | IgG4 | 22.5 | mg/dL | ||
| Eosinophil | 0.6 | % | AST | 12 | U/L | IgA | 213 | mg/dL | ||
| Basophil | 0.5 | % | ALT | 5 | U/L | IgM | 93 | mg/dL | ||
| Monocyte | 8.1 | % | LDH | 334 | U/L | ANA | <×40 | |||
| Red blood cell | 478×104 | /μL | ALP | 880 | U/L | anti-ds-DNA IgG | - | |||
| Hemoglobin | 12.9 | g/dL | γ-GTP | 149 | U/L | PR3-ANCA | 0.8 | IU/mL | ||
| Hematocrit | 37.8 | % | BUN | 38 | mg/dL | MPO-ANCA | <0.5 | IU/mL | ||
| Platelet | 7.8×104 | /μL | Creatinine | 2.8 | mg/dL | Direct Coombs test | - | |||
| CRP | 15.23 | mg/dL | PA-IgG | 16.2 | ng/107 cells | |||||
| Glucose | 124 | mg/dL | ||||||||
| Coagulation system | ||||||||||
| PT | 14.6 | sec | Cytokines | Pleural effusion | ||||||
| PT-INR | 1.22 | sIL-2R | 800 | U/mL | Total protein | 3 | g/dL | |||
| APTT | 29.9 | sec | IL-6 | 26.6 | pg/mL | LDH | 101 | U/L | ||
| Fibrinogen | 539 | mg/dL | VEGF | 107 | pg/mL | Cell count | 106 | /μL | ||
| FDP | 60.7 | μg/mL | Lymphocyte | 72.6 | % | |||||
| D-dimer | 17 | μg/mL | Urine test | Monocyte | 24.4 | % | ||||
| Antithrombin III | 74.6 | % | Protein | 2+ | Mesothelial cell | 2.6 | % | |||
| TAT | 5.4 | ng/mL | Glucose | - | Eosinophil | 0.4 | % | |||
| soluble fibrin monomer | 4.9 | μg/mL | Occult blood | 2+ | ||||||
| PIC | 5.6 | μg/mL | Granular cast | - | ||||||
PT: prothrombin time, PT-INR: prothrombin time-international normalized ratio, APTT: activated partial thromboplastin time, FDP: fibrinogen degradation products, TAT: thrombin- antithrombin complex, PIC: plasmin-α2 plasmin inhibitor complex, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, BUN: blood urea nitrogen, CRP: C-reactive protein, sIL-2R: soluble interleukin-2 receptor, IL-6: interleukin-6, VEGF: vascular endothelial growth factor, RF: rheumatoid factor, ANA: antinuclear antibody, anti-dsDNA IgG: anti-double-stranded DNA IgG antibody, PR3-ANCA: proteinase 3-anti neutrophil cytoplasmic antibody, MPO-ANCA: myeloperoxidase-anti neutrophil cytoplasmic antibody, PA-IgG: platelet-associated IgG
Figure 1.
Chest radiography, CT, and FDG-PET images on admission. (A) The chest radiograph showed a left pleural effusion. (B, D) Chest CT revealed bilateral pleural effusion and the enlargement of the axillary lymph nodes. (C) Abdominal CT revealed ascites and hepatomegaly. (E) The FDG-PET images showed a mild FDG uptake in the left supraclavicular lymph nodes (maximum standardized uptake value 3.1).
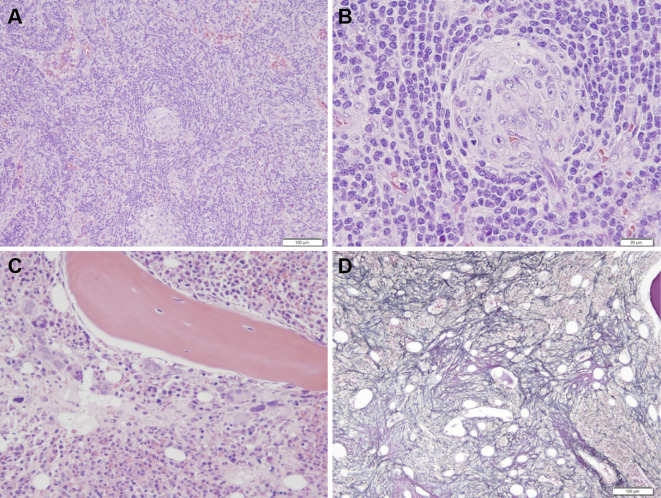
To make a definitive diagnosis, a left supraclavicular lymph node was biopsied. A histopathological examination revealed interfollicular expansion and atrophic germinal centers penetrated by blood vessels (Fig. 2A and B). The pathological findings resembled those of the hyaline-vascular (HV) type of CD. The patient's serum was negative for antibodies against human immunodeficiency virus (HIV) and human herpes virus type 8 (HHV-8), which have been associated with CD in Western countries. Moreover, the level of serum interleukin-6 (IL-6), which plays a central role in the pathogenesis of CD, was elevated to 26.6 pg/mL, suggesting a possible diagnosis of idiopathic multicentric CD (MCD) (6). However, his other clinical features, including thrombocytopenia, anasarca, elevated ALP, and a lack of hypergammaglobulinemia, were inconsistent with MCD (Table 1). Moreover, bone marrow aspiration resulted in a dry tap, and a bone marrow biopsy revealed an increased number of megakaryocytes and reticulin fibrosis (Fig. 2C and D). Based on the clinical symptoms, laboratory data, and pathological findings, he was diagnosed with TAFRO syndrome on day 10.
Figure 2.
The histopathological findings. (A) and (B) The histological appearance of the left cervical lymph node. (A) Hematoxylin and Eosin (H&E) staining, 40×. (B) H&E staining, 200×. The lymph node showed interfollicular expansion and atrophic germinal centers penetrated by blood vessels. (C) and (D) The histological appearance of the bone marrow. (C) H&E staining, 200×. The examination of the bone marrow revealed hyperplastic marrow with increased megakaryocytes. (D) Silver staining, 100×. Reticulin fibrosis was observed.
The patient's urine volume decreased to less than 50 mL/day, despite the administration of methylprednisolone pulse therapy (1,000 mg/day, for 3 days), followed by prednisolone (PSL) (60 mg, daily). Hemodialysis was thus initiated on day 4. Immunosuppressive therapy with cyclosporin A (CyA) was also initiated, with the CyA dose adjusted to achieve a target trough level of 150-250 ng/mL.
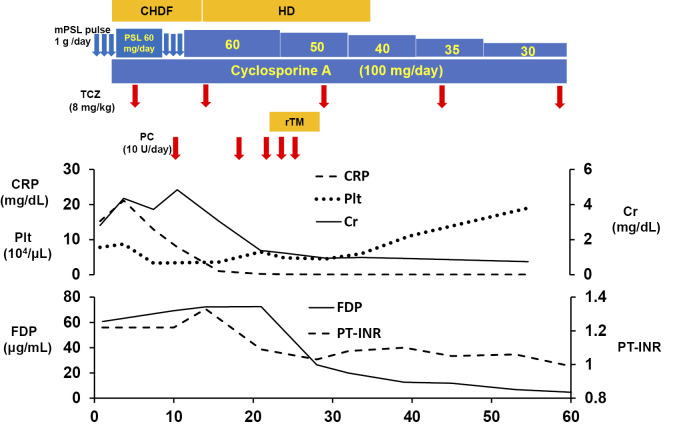
Despite combined treatment with corticosteroids and CyA, he had persistent thrombocytopenia, renal dysfunction, and massive ascites. On day 7, intravenous TCZ (8 mg/kg) was initiated, based on previous studies that have shown TCZ can be an effective adjunct to corticosteroids (7-11). Two weeks after the initiation of TCZ, his general condition improved, as evidenced by reductions in fever, systemic inflammation, renal dysfunction, and anasarca. Furthermore, the serum IL-6 level decreased in parallel with his clinical improvement. However, the thrombocytopenia did not improve, and he was refractory to platelet transfusion (Fig. 3). On day 14, the patient's platelet count decreased to 3.4×104/μL. In addition, a coagulation analysis revealed a prolonged prothrombin time-international normalization ratio (1.33), and increased levels of fibrin/fibrinogen degradation products (FDP; 72.3 μg/mL), D-dimer (21.6 μg/mL), and thrombin-antithrombin complex (TAT; 5.4 ng/mL). He was thus diagnosed with DIC based on both the Japanese Ministry of Health and Welfare's old diagnostic criteria for DIC (JMHW criteria) and the International Society of Thrombosis and Hemostasis (ISTH) criteria. To treat the coagulation abnormalities, rTM (380 U/kg) was administered intravenously from day 23 to day 28. Following rTM treatment, the platelet count gradually increased and the coagulation disorder was effectively controlled. Thereafter, the PSL dose was tapered, and TCZ was administered every two weeks. On day 35, hemodialysis was discontinued, and he was discharged on day 60 (Fig. 3). Currently, at one year after the onset of disease, he continues to receive combined treatment with daily oral prednisolone (5 mg) and CyA, and intravenous TCZ every three weeks. The patient's serum IL-6 level returned to normal and there has been no sign of relapse.
Figure 3.
The clinical course and treatment. mPSL: methylprednisolone, PSL: prednisolone, TCZ: tocilizumab, rTM: recombinant thrombomodulin, PC: platelet concentrate transfusion, CHDF: continuous hemodiafiltration, HD: hemodialysis, CRP: C-reactive protein, Plt: platelet, Cr: creatinine, FDP: fibrin/fibrinogen degradation products, PT-INR: prothrombin time-international normalized ratio
Discussion
TAFRO syndrome is a systemic inflammatory disorder that is difficult to accurately diagnose, in part, because it is a rare disease that has only recently been described (1). Unfortunately, the outcome of TAFRO syndrome may be fatal unless appropriate treatment is initiated in a timely manner.
In 2015, the diagnostic criteria, disease severity, and treatment strategies for TAFRO syndrome were reported (2). The diagnosis requires the presence of all of the major features of the diagnostic criteria and at least two of the four minor features. In the present case, the patient displayed all of the major and minor features of the diagnostic criteria for TAFRO syndrome. Patients with TAFRO syndrome are classified into five groups based on disease severity, which is defined by anasarca, thrombocytopenia, inflammation, and renal insufficiency. The treatment strategy includes glucocorticoid treatment alone or in combination with CyA, TCZ, and/or rituximab (2).
In most reported cases, patients with TAFRO syndrome have been diagnosed by hematologists. However, in the present case, the patient was referred to the Pulmonology department due to massive pleural effusion. The effusion was a unique feature in this case and made an accurate diagnosis difficult. Another notable feature of this case was that the patient had a very aggressive clinical course; the disease severity was classified as grade 5 (very severe). In addition, the patient developed DIC in spite of combined treatment with glucocorticoids, CyA, and TCZ. The addition of rTM effectively treated the DIC, which developed in association with TAFRO syndrome.
Thrombocytopenia is one of the major features of TAFRO syndrome. Although the mechanisms underlying the development of thrombocytopenia in TAFRO syndrome are still unclear, an autoimmune etiology has been suggested (7, 12, 13). There have only been four reported cases of TAFRO syndrome that met the diagnostic criteria for DIC of both the JMHW and ISTH, which suggests that DIC is an uncommon complication of TAFRO syndrome (Table 2) (3-5). These 4 patients were treated with corticosteroids, CyA, or tocilizumab, either alone or in combination; anticoagulant therapy was not initiated. Unfortunately, 3 of the 4 patients (75%) died. The general mortality rate for patients with TAFRO syndrome has been reported to be approximately 12% (2, 14). These data suggest that patients with TAFRO syndrome complicated by DIC have a significantly worse prognosis.
Table 2.
Characteristics of Reported Cases of TAFRO Syndrome Complicated with DIC.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Our case | |
|---|---|---|---|---|---|
| Age/Sex | 57/F | 49/M | 56/F | 78/F | 38/M |
| Platelet (103/μL) | 13 | 10 | 44 | <50 | 34 |
| PT-INR | 1.44 | 1.36 | 0.94 | 1.17 | 1.33 |
| Fibrinogen (mg/dL) | 461 | 777 | 532 | 543 | 654 |
| FDP (μg/mL) | 32.1 | 22.3 | 42.2 | 51.3 | 72.3 |
| Treatment | GC+CHOEP | GC+IVIG | GC+CyA | GC+TCZ | GC+CyA+TCZ+rTM |
| Outcome | died | died | remission | died | remission |
| References | 3 | 4 | 4 | 5 |
PT-INR: prothrombin time-international normalized ratio, FDP: fibrin/fibrinogen degradation products, GC:glucocorticoid, CHOEP: cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone, IVIG: intravenous immunoglobulin, CyA: cyclosporin A, TCZ: tocilizumab, rTM: recombinant thrombomodulin
In the present case, rTM was effective in the treatment of DIC associated with TAFRO syndrome. To our knowledge, this is the first report to describe the successful treatment of DIC due to TAFRO syndrome using rTM. rTM is a new anticoagulant that was recently developed in Japan. It exerts anticoagulant effects by inactivating thrombin (15). The pathophysiological mechanisms of DIC in TAFRO syndrome remain uncertain. In the present case, plasmin-α2 plasmin inhibitor complex (PIC), a marker of fibrinolysis activation, was only mildly elevated. This type of DIC, called DIC with suppressed fibrinolysis, is typically seen in sepsis, in which inflammatory cytokines play a central role (16). In addition, hyper-cytokine storms, including IL-6 and vascular endothelial growth factor (VEGF), may be involved in the onset of TAFRO syndrome (3). Taken together, these data suggest that the inflammatory cytokine-initiated activation of tissue factor and the decreased expression of thrombomodulin on endothelial cells, leading to thrombin generation and clot formation is one of the pathophysiological mechanisms of DIC in TAFRO syndrome. Thus, rTM may be effective in the treatment of DIC in TAFRO syndrome through its inhibition of thrombin generation. In addition, several studies have shown that rTM therapy did not increase the bleeding risk in comparison to heparin therapy (15). Thus, rTM appears to be a safe treatment for patients with TAFRO syndrome and severe thrombocytopenia.
The exact cause of DIC in TAFRO syndrome is unknown, but it may occur due to a cytokine storm, including IL-6 and VEGF. Although IL-6 and VEGF are not always elevated in patients with TAFRO syndrome, these inflammatory cytokines have been reported to be elevated in patients with TAFRO syndrome complicated by DIC (3-5). In addition, these patients demonstrated rapid and aggressive clinical decline. Notably, there was no association between the severity of thrombocytopenia and the presence of DIC. It is possible that autoimmune mechanisms (7, 12, 13) and platelet consumption lead to thrombocytopenia in patients with TAFRO syndrome complicated by DIC, and that cytokine storms lead to an aggressive clinical decline.
In the present case, multi-drug therapy, including corticosteroids, CyA, and TCZ, was administered and it is difficult to say which medication was the most effective. However, the decrease in the serum IL-6 level was correlated with the patient's clinical improvement, and this occurred after several doses of TCZ. Thus, we suspect that TCZ was the most effective medication in the present case. The 2015 version of the treatment strategy for TAFRO syndrome did not recommend a defined treatment period (2). According to previous case reports, combination therapy may be discontinued one to two years after the disease onset (7, 17). In the present case, we plan to carefully taper down combination therapy now that one year has passed since the disease onset.
In CD patients who achieved complete resolution, the serum IL-6 levels gradually decreased to the basal levels after treatment with TCZ, probably due to inhibition of the autocrine IL-6 signaling loop (18). In the present case, the serum IL-6 levels similarly decreased after treatment with TCZ. However, the patient's DIC did not improve, even after the decrease in serum IL-6 concentration. It has been reported that the serum levels of IL-6 and other cytokines, such as VEGF, are elevated in patients with TAFRO syndrome (8). Thus, we hypothesize that other inflammatory cytokines may be associated with DIC, regardless of the IL-6 concentration.
Although the etiology of the patient's pleural effusion remains unclear, the overexpression of cytokines such as IL-6 and VEGF may be involved (13, 19). In the present case, the serum IL-6 level and the plasma VEGF level were high, suggesting that the bilateral pleural effusions might have been associated with overexpression of these cytokines.
In conclusion, TAFRO syndrome is difficult to accurately diagnose, and TAFRO syndrome may have a fatal outcome-especially in patients with DIC-unless it is treated rapidly and appropriately. In patients with concomitant DIC, immunosuppressive and anti-inflammatory therapies are unlikely to improve the coagulation disorder. We reported the first case of TAFRO syndrome with DIC in a patient who was successfully treated with rTM and tocilizumab. If DIC is identified at the time of the diagnosis of TAFRO syndrome, the administration of an anticoagulant agent such as rTM in addition to immunosuppressive and anti-inflammatory therapy, may be a reasonable therapeutic approach, and may improve the prognosis. Clinical trials are needed to determine the efficacy of rTM in these patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Kawabata H, Takai K, Kojima M, et al. . Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J Clin Exp Hematop 53: 57-61, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Masaki Y, Kawabata H, Takai K, et al. . Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol 103: 686-692, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Masaki Y, Nakajima A, Iwao H, et al. . Japanese variant of multicentric Castleman's disease associated with serositis and thrombocytopenia - a report with two cases: is TAFRO syndrome (Castleman-Kojima disease) a distinct clinicopathological entity? J Clin Exp Hematop 53: 79-85, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Takai K, Nikkuni K, Momoi A, Nagai K, Igarashi N, Saeki T. Thrombocytopenia with reticulin fibrosis accompanied by fever, anasarca and hepatosplenomegaly: a clinical report of five cases. J Clin Exp Hematop 53: 63-68, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Awano N, Inomata M, Sonoda Y, et al. . A case of multicentric castleman's disease of mixed-type, which showed constellation of symptoms, i.e., thrombocytopenia, anasarca, anemia, fever, myelofibrosis, and lymphadenopathy. J Clin Exp Hematop 53: 101-105, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood 123: 2924-2933, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Kawabata H, Kotani S, Matsumura Y, et al. . Successful treatment of a patient with multicentric Castleman's disease who presented with thrombocytopenia, ascites, renal failure and myelofibrosis using tocilizumab, an anti-interleukin-6 receptor antibody. Intern Med 52: 1503-1507, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Kubokawa I, Yachie A, Hayakawa A, et al. . The first report of adolescent TAFRO syndrome, a unique clinicopathologic variant of multicentric Castleman's disease. BMC Pediatr 14: 139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sakashita K, Murata K, Inagaki Y, Oota S, Takamori M. An anterior mediastinal lesion in TAFRO syndrome showing complete remission after glucocorticoid and tocilizumab therapy. Respirol Case Rep 4: e00173, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujiwara S, Mochinaga H, Nakata H, et al. . Successful treatment of TAFRO syndrome, a variant type of multicentric Castleman disease with thrombotic microangiopathy, with anti-IL-6 receptor antibody and steroids. Int J Hematol 103: 718-723, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Sakai K, Maeda T, Kuriyama A, Shimada N, Notohara K, Ueda Y. TAFRO syndrome successfully treated with tocilizumab: a case report and systematic review. Mod Rheumatol: 1-6, 2016(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 12. Inoue M, Ankou M, Hua J, Iwaki Y, Hagihara M, Ota Y. Complete resolution of TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly) after immunosuppressive therapies using corticosteroids and cyclosporin A: a case report. J Clin Exp Hematop 53: 95-99, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Iwaki N, Sato Y, Takata K, et al. . Atypical hyaline vascular-type castleman's disease with thrombocytopenia, anasarca, fever, and systemic lymphadenopathy. J Clin Exp Hematop 53: 87-93, 2013. [DOI] [PubMed] [Google Scholar]
- 14. Iwaki N, Fajgenbaum DC, Nabel CS, et al. . Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol 91: 220-226, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Saito H, Maruyama I, Shimazaki S, et al. . Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost 5: 31-41, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care 2: 20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sakashita K, Murata K, Inagaki Y, Oota S, Takamori M. An anterior mediastinal lesion in TAFRO syndrome showing complete remission after glucocorticoid and tocilizumab therapy. Respirol Case Rep 4: e00173, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawabata H, Kadowaki N, Nishikori M, et al. . Clinical features and treatment of multicentric castleman's disease: a retrospective study of 21 Japanese patients at a single institute. J Clin Exp Hematop 53: 69-77, 2013. [DOI] [PubMed] [Google Scholar]
- 19. Tatekawa S, Umemura K, Fukuyama R, et al. . Thalidomide for tocilizumab-resistant ascites with TAFRO syndrome. Clin Case Rep 3: 472-478, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]