Abstract
Background and objective:
Cholangiocarcinoma remains a serious public health concern in Thailand. While many of the risk factors for cholangiocarcinoma in western countries are well-recognized, it remains unclear whether they are the same in Thailand. We set out to investigate the risk factors for cholangiocarcinoma in Thailand.
Methods:
Starting March 4, 2016, we reviewed studies found using pre-specified keywords on SCOPUS, Pro Quest Science Direct, PubMed, and online public access catalog of Khon Kaen University. Two review authors independently screened studies for inclusion criteria, extracted data, and assessed the studied Risk of Bias. The Newcastle-Ottawa Scale and the Joanna Briggs Institute Critical Appraisal Tools were used to assess the quality of included studies. The risk effects of factors were estimated as a pooled adjusted odds ratio with a 95% confidence interval. The heterogeneity of results was considered using the I-square, Tau-square and Chi-square statistics.
Results:
A strong association was found between cholangiocarcinoma and age, Opisthorchis viverrini infection, eating raw cyprinoid fish, family history of cancer, liquor consumption, and taking praziquantel. There was only a mild association found between eating nitrite-containing foods, fresh vegetables, education, smoking behavior, and sex. No association was found between cholangiocarcinoma and eating fermented fish (Pla-ra), northeastern Thai or Chinese sausage, sticky rice, meat, chewing betel nut, or eating fruit. There were two protective factors including fresh vegetables consumption and education attainment.
Conclusion:
There are unique risk factors of cholangiocarcinoma in Thailand, including age, Opisthorchis viverrini infection, eating raw cyprinoid fish, family history of cancer, liquor consumption, and taking praziquantel.
Keywords: Cholangiocarcinoma, risk factors, systematic review, Thailand
Introduction
Cholangiocarcinoma (CCA) is a bile duct cancer, which originates in biliary epithelial cells, and occurs in the intrahepatic and extrahepatic regions of the bile duct, but it does not include malignancies in the gallbladder or the ampulla of Vater (Green et al., 1991; Bhudhisawasdi et al., 2012). The epidemiology of CCA varies by region. The incidence is trending upward in China, Korea, and Thailand, and particularly in the northeastern region of Thailand which had the highest worldwide incidence (85 per 100,000 per year) (Banales et al., 2016).
In Thailand, the age-standardized rate (ASR) of liver cancer and bile duct cancer between 1988 and 2012 was between 40.5 and 33.9 per 100,000 in males and 16.3 and 12.9 per 100,000 in females. The most common histological type was CCA, accounting for between 82.0% and 89.0% of all detected primary liver cancers. (Vatanasapt et al., 1993; Deerasamee et al., 1999; Sriplung et al., 2003; Khuhaprema et al., 2007; Khuhaprema et al., 2010; Khuhaprema et al., 2012; Khuhaprema et al., 2013; Imsamran et al., 2015) The rate has been slight decreasing and a high incidence of CCA persists.
Systematic reviews indicate that the factors associated with CCA are liver fluke infection, hepatitis B and C, liquor consumption, and diabetes mellitus. (Xia et al., 2015; Palmer and Patel, 2012) Some studies, however, suggest that factors like praziquantel use (PZQ) and liquor consumption. (Kamsa-ard et al., 2013; Li et al., 2011; Ye et al., 2013) are not significantly associated with CCA.
In Thailand, intense debate surrounds the subject on which factors are associated with CCA. For instance, family history of cancer and sticky rice consumption and there are no systematic reviews have been conducted to confirm or refute the hypothesis of association. (Poomphakwaen et al., 2009; Chernrungroj, 2000; Honjo et al., 2005; Parkin et al., 1991).
Research on such potential factors such as sex, age, highest educational attainment, betel nut chewing, and type of diet (viz., nitrite-containing food like northeast Thai sausage, fermented fish (Pla-ra), raw fish (Lap-Pla), partially cooked fish (Koi-Pla) or other meat, or certain vegetables) have been conducted but a definitive causal relationship with CCA among Thai people has not been defined.
In sum, there have not been any reported systematic, analytical series investigating the likely potential risk factors. There is also disagreement in the literature; consequently, the main purpose of the present study was to investigate the risk factors for CCA in Thailand.
Materials and methods
1. Study selection
1.1. Types of studies
Studies were included if they used any analytical design (i.e., case-control, matched case-control, nested case-control, cohort and cross-sectional designs) to investigate the association between potential risk factors variables and CCA in Thailand.
1.2. Selection of studies
Screens showing the title, abstract, and finally the full text of the publications were independently evaluated by two researchers (Siriporn Kamsa-ard; SK1 and Supot Kamsa-ard; SK2) and disagreement was resolved through discussion with our CCA and cancer epidemiology expert co-authors Vor Luvira; VL and Krittika Suwanrungruang; KS, respectively. Selecting studies to include in the review was performed in Covidence (Covidence, 2016).
1.3. Risk factors
The risk factors of interest were: 1) demographic data such as age, sex, education, and family history of cancer; 2) health behavior (i.e., liquor consumption, smoking, and betel nut chewing); 3) OV infestation and PZQ treatment; and, 4) diet (viz., consumption of raw fish, Pla-ra, nitrite-containing foods, sausage, meat, sticky rice, vegetables, and fruits).
1.4. Outcome
The outcome was CCA.
2. Literature research
In addition to hand-searching journals through indices, we searched SCOPUS, ProQuest, ScienceDirect, PubMed, ProQuest (including: Dissertation and Theses Global), Online Public Access Catalog of Khon Kaen University (KKU Web OPAC), and Google Scholar up to and including March 4, 2016 without any language restriction.
Search terms covering the names and synonyms of “cholangiocarcinoma”, “cancer”, “risk factor” and “Thailand” were used singly and in combination. The more detailed terms used for “cholangiocarcinoma” included intrahepatic bile ducts, intrahepatic bile duct carcinoma, intrahepatic bile duct cancers, bile duct adenocarcinoma, bile duct cystadenocarcinoma, bile duct cancer, Klatskin’s tumor, perihilar bile duct cancers, hilar bile duct cancers, and distal bile duct cancers. The more detailed terms used for “cancer” were tumor(s), malignancy, and carcinoma. The more detailed terms used for “risk factor” were risk, association, relationship, relation, correlation, and connection.
3. Study inclusion/exclusion criteria
The inclusion criteria for the selection of primary studies were as follows: (1) human research; (2) the article investigated the factors affecting and risk of CCA; and (3) the outcome was “CCA”; (4) the research setting was in Thailand; and, (5) published in Thai or English.
The exclusion criteria were: (1) non-human research (including: genetic determinants of risk for CCA); and (2) systematic review
Figure 1 is a flow diagram showing the article selection process. Mendeley was used to delete the duplicate records (Mendeley, 2016).
Figure 1.
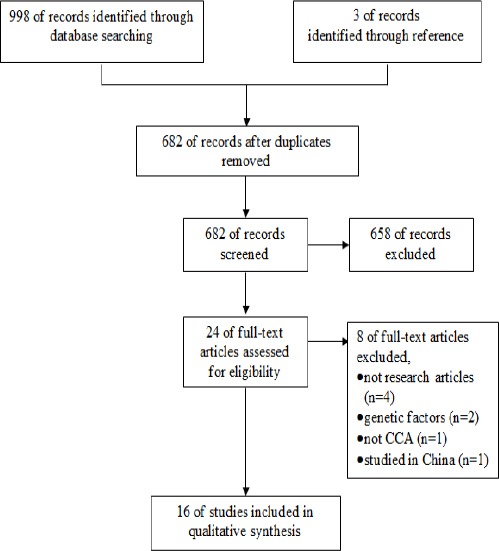
Study Flow Diagram
4. Data extraction and management
Research design found, including case-control, matched case-control, and nested case-control design. This was an 8-item scale covering the assessment of case selection and control selection, study comparability, and exposure. For the purpose of the present review, the scale was adapted so that each primary study was rated in terms of a ‘low’, ‘high’ or ‘unclear’ risk of bias (Table 1). There are five items for cross-sectional study (Table 1a) and 8-item scale for case-control study (Table 1b). Independent ratings were made by SK1 and SK2, and any disagreements were resolved through discussion with co-researchers (VL and KS). Review Manager (RevMan) 5.0 was used for data extraction and management.
Table 1.
Risk of Bias (ROB) Items. (a) Adapted from the Joanna Briggs Institute (JBI)s for Cross-Sectional Study
| Risk of bias items |
|---|
| SELECTION |
| 1. Selection of Sample |
| Were the criteria for inclusion in the sample clearly defined? |
| a) yes, low risk of bias (clear inclusion and exclusion criteria e.g., risk, stage of disease progression) |
| b) yes, high risk of bias (e.g., not stated in part (a)) |
| c) no description, unclear risk of bias |
| EXPOSURE |
| 1. Ascertainment of exposure |
| Was the exposure measured in a valid and reliable way? |
| a) yes, low risk of bias (clearly describe the method of measurement of exposure, assessing validity requires a ’gold standard’, reliability refers to intra and inter-observed reliability) |
| b) yes, high risk of bias (e.g., not stated in part (a)) |
| c) no description of source, unclear risk of bias |
| 2. Ascertainment of outcome |
| Were the outcome measured in the valid and reliable way? |
| a) yes, low risk of bias (e.g., measurement tools used were validated instruments; e.g., histological proved) |
| b) yes, high risk of bias (e.g., not stated in part (a)) |
| c) no description of source, unclear risk of bias |
| 3. Confounding factors |
| Were confounding factors indentified? |
| a) yes, low risk of bias (strategies to deal with effects of confounding factors e.g., study design or in data analysis (multiple analysis), matching or stratifying sampling of participants |
| b) yes, high risk of bias (e.g., not stated in part (a)) |
| c) no description of source, unclear risk of bias |
| 4. Non-Response rate |
| a) yes, low risk of bias (e.g., same rate for both groups) |
| b) yes, high risk of bias (e.g., non-respondents described; rate different and no designation) |
| c) no description of source, unclear risk of bias |
Table 1.
Risk of Bias (ROB) Items. (b) Adapted from Newcastle-Ottawa Scale (NOS)s for Case-Control Study
| Risk of bias items |
|---|
| SELECTION |
| 1. Is the Case Definition Adequate? |
| (a) yes, low risk of bias (e.g., eligibility criteria/ operational definition) |
| (b) yes, high risk of bias (e.g., not stated in part (a)) |
| (c) no description, unclear risk of bias |
| 2. Representativeness of the Cases |
| (a) yes, low risk of bias (e.g., consecutive representative series of cases) |
| (b) yes, high risk of bias (e.g., not satisfying requirements in part (a), or not stated) |
| (c) no description, unclear risk of bias |
| 3. Selection of Controls |
| a) yes, low risk of bias (e.g., community controls) |
| b) yes, high risk of bias (e.g., hospital controls) |
| c) no description, unclear risk of bias |
| 4. Definition of Controls |
| a) yes, low risk of bias (e.g., no history of CCA) |
| b) yes, high risk of bias (e.g., no mention of history of CCA) |
| c) no description of source, unclear risk of bias |
| COMPARABILITY |
| 1. Comparability of cases and controls on the basis of the design or analysis |
| a) yes, low risk of bias (e.g., matching consideration by age, multiple analysis with age adjusted) |
| b) yes, high risk of bias (e.g., not stated in part (a)) |
| c) no description of source, unclear risk of bias |
| EXPOSURE |
| 1. Ascertainment of exposure |
| a) yes, low risk of bias (secure record e.g., surgical records; structured interview where blind to case/control status) |
| b) yes, high risk of bias (e.g., interviewer not blinded to case/control status; written self report or medical record only) |
| c) no description of source, unclear risk of bias |
| 2. Same method of ascertainment for cases and controls |
| a) yes, low risk of bias (e.g., use of a structured questionnaire) |
| b) yes, high risk of bias (e.g., not stated in part (a)) |
| c) no description of source, unclear risk of bias |
| 3. Non-Response rate |
| a) yes, low risk of bias (e.g., same rate for both groups) |
| b) yes, high risk of bias (e.g., non-respondents described; rate different and no designation) |
| c) no description of source, unclear risk of bias |
5. Assessment of risk of bias of primary studies
The particular instrument chosen for evaluating the studies according to the risk of bias (RoB) tool was the Newcastle-Ottawa Scale (NOS) for assessing the quality of case-control studies (Wells, 2013). To evaluate cross-sectional studies, we used the Joanna Briggs Institute (JBI) Critical Appraisal Tools (Joanna Briggs Institute, 2016).
6. Statistical method
6.1. Measures of association
The magnitude of the associations between the factors affecting CCA was presented as the adjusted odds ratios (adjusted ORs) with their 95% confidence intervals (95% CI).
6.2. Assessment of heterogeneity
Heterogeneity of the results across studies was assessed using the Cochran’s Q test, and I2. An unacceptable degree of inconsistency across the study outcomes was considered to have occurred when the p-value of the Cochran’s Q test statistic was <0.10 and the I2 was >75%. Publication bias was assessed visually using a funnel plot (Higgins et al., 2003).
6.3. Pooling association
A random effects model was used to pool the association effects across the studies when there was evidence of an unacceptable degree of heterogeneity that could not be explained. All analyses were conducted using RevMan 5.0.
7. Ethical considerations
The study met specified criteria exempting it from review by the Khon Kaen University Ethics Committee for Human Research.
Results
1. Characteristics of included studies
A total of 16 studies were included.
1.1. Study design
Three cross-sectional studies (Elkins et al., 1990; Haswell-Elkins et al., 1994; Mairiang et al., 1992) and thirteen case-control studies (Kamsa-ard S et al., 2013; Poomphakwaen et al., 2009; Chernrungroj, 2000; Honjo et al., 2005; Parkin et al., 1991; Itoh et al., 1994; Kaewpitoon et al., 2014; Kamsa-ard et al., 2009; Kurathong et al., 1985; Manwong et al., 2013; Songserm et al., 2012; Songserm et al., 2014; Srivatanakul et al., 2010) were included.
1.2. Risk factors for CCA
The possible risk factors for CCA evaluated in the meta-analyses were 1) age, 2) sex, 3) education, 4) a family history of cancer, 5) smoking, 6) alcohol consumption, 7) betel nut chewing, 8) OV infection, 9) PZQ treatment, and eating 10) raw fish, 11) Pla-ra, 12) nitrite-containing foods, 13) local sausage, 14) meats, 15) sticky rice, 16) vegetables, and 17) fruits.
2. RoB
The review authors assessed the quality of the 16 studies using the ROB scale. One study was judged as having a “low risk of bias” (low risk for all items). Seven studies were assessed as having a “high risk of bias” (at least one item high risk) and 8 as having an “unclear risk of bias” (at least one item unclear and no item for high risk) (Figure 2).
Figure 2.
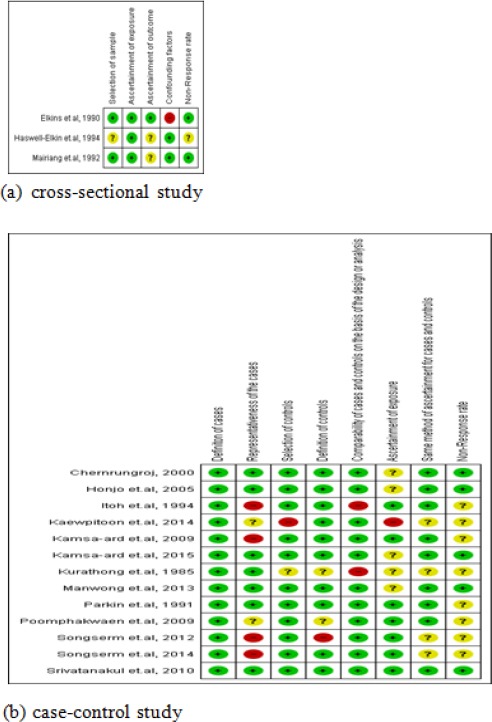
Risk of Bias Summary: Review Authors’ Judgments about Each Risk of Bias Item for Each of the Included studies.
3. Risk factors for CCA in Thailand
3.1) Strong association (lower limits for 95% CI of ORadj ≥ 1.50)
3.1.1. Age
There were two age-focused studies. One was a cross-sectional study involving 1,807 subjects. Older subjects were 6.58 times more likely to have CCA than younger subjects (pooled OR=6.58; 95% CI: 1.42, 30.47). The other study was a case-control study including 227 subjects and older subjects were 34.77 times more likely to have CCA than younger subjects (pooled OR=34.77; 95% CI: 4.64, 260.57) (Figure 3).
Figure 3.
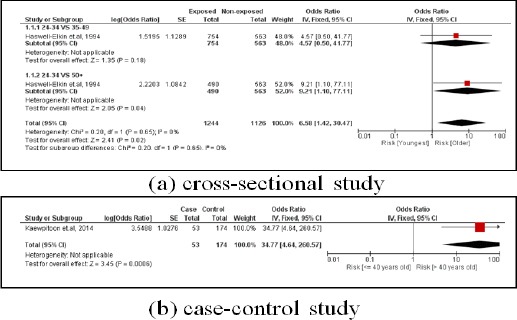
Meta-Analysis Forest Plots of the Relationship between Age and CCA
3.1.2. OV infection
There were 11 studies. Three were cross-sectional, involving 1,894 subjects. The subjects with an OV infection were 5.54 times more likely to develop CCA than subjects who did not (pooled OR=5.54; 95% CI: 1.92, 15.98). Eight studies were case-controlled studies involving 2,092 subjects. Those subjects with an OV infection were 6.35 times more likely to develop CCA than those who did not (pooled OR=6.35; 95% CI: 2.87, 14.05) (Figure 4).
Figure 4.
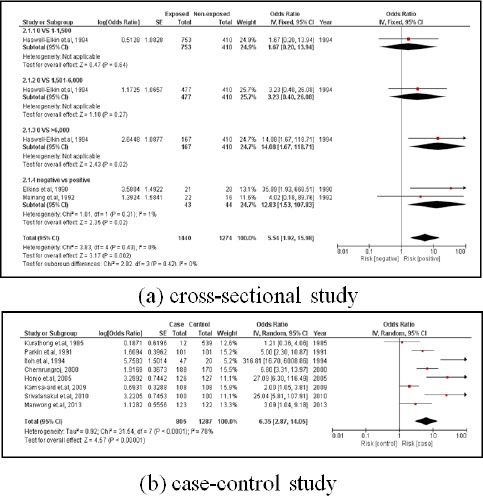
Meta-Analysis Forest Plots of the Relationship between OV Infection and CCA
3.1.3. Eating raw fish
There were 3 case-controlled studies, involving 1,920 subjects. Those who consumed raw fish were 2.54 times more likely to develop CCA than those who did not (pooled OR=2.54; 95% CI: 1.94, 3.35) (Figure 5).
Figure 5.
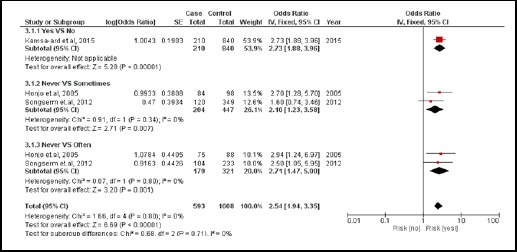
Meta-Analysis Forest Plots of the Relationship between Eating Raw Fish and CCA
3.1.4. Family history of cancer
There were 4 were case-controlled studies, involving 1,912 subjects. Those with a family history of cancer were 2.48 times more likely to develop CCA than those who did not (pooled OR=2.48; 95% CI: 1.91, 3.21) (Figure 6).
Figure 6.
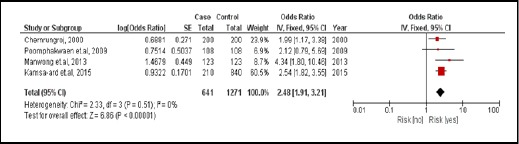
Meta-Analysis Forest Plots of the Relationship between Family History of Cancer and CCA
3.1.5. Liquor consumption
There were 6 case-controlled studies, involving 2,431 subjects. Subjects who consumed liquor were 3.01 times more likely to develop CCA than those who did not (pooled OR=3.01; 95% CI: 2.00, 4.54) (Figure 7).
Figure 7.
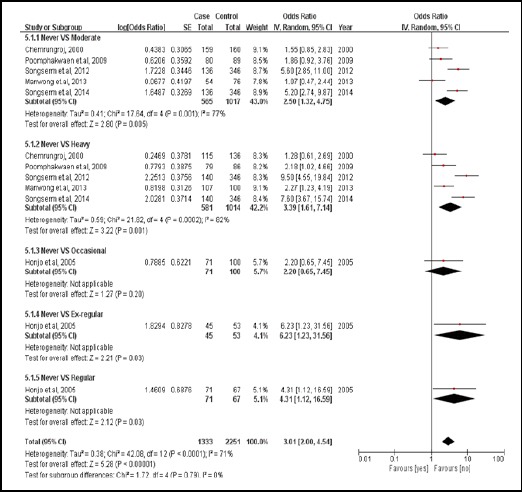
Meta-Analysis Forest Plots of the Relationship between Liquor Consumption and CCA
3.1.6. PZQ use
There were 4 case-controlled studies, involving 1,921 subjects. Those who used PZQ were 2.02 times more likely to develop CCA than those who had not (pooled OR=2.02; 95% CI: 1.59, 2.57) (Figure 8).
Figure 8.
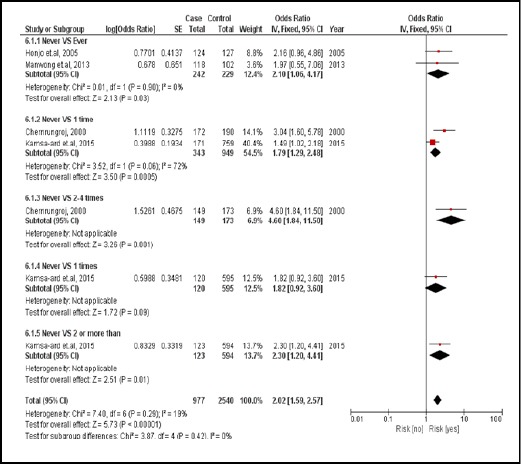
Meta-Analysis Forest Plots of the Relationship between PZQ Treatment and CCA
3.2. Mild association (lower limits for 95% CI of ORadj included 1.01-1.49 or upper limits included 0.67–0.99)
3.2.1. Nitrite-containing foods
There were 2 case-controlled studies, involving 616 subjects. Subjects who consumed nitrite-containing foods were 2.08 times more likely to develop CCA than those who did not (pooled OR=2.08; 95% CI: 1.47, 2.96) (Figure 9).
Figure 9.
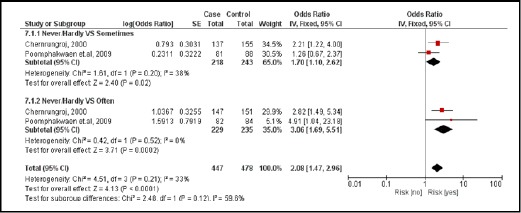
Meta-Analysis Forest Plots of the Relationship between Nitrite-Containing Food and CCA
3.2.2. Smoking behavior
There were 5 case-controlled studies, involving 2,278 subjects. Smoker had a 1.46 times greater likelihood of developing CCA than those who did not (pooled OR=1.46; 95% CI: 1.10, 1.94) (Figure 10).
Figure 10.
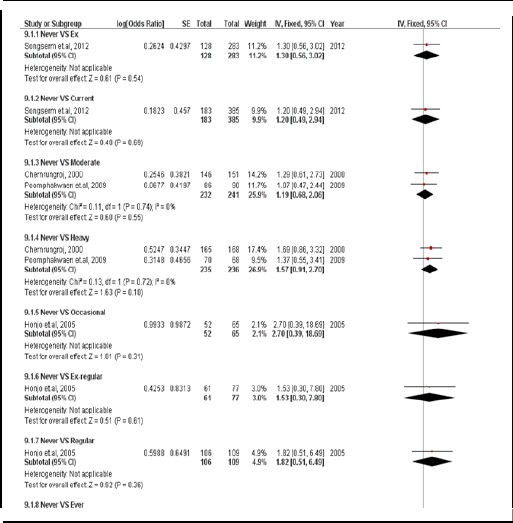
Meta-Analysis Forest Plots of the Relationship between Smoking Behavior and CCA
3.2.3. Sex
There were 2 studies. The first was a cross-sectional study involving 1,807 subjects, and males were 3.00 times more likely to develop CCA than females (pooled OR=3.00; 95% CI: 0.80, 11.25). The second was a case-controlled study, involving 227 subjects. Males had a 2.33 times greater likelihood of developing CCA than females (pooled OR=2.33; 95% CI: 1.03, 5.27) (Figure 11).
Figure 11.
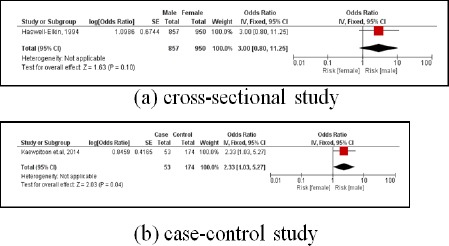
Meta-Analysis Forest Plots of the Relationship between Sex and CCA
3.2.4. Vegetables consumption
There were 5 case-controlled studies, involving 1,747 subjects. Those who regularly consumed vegetables were 0.45 times for developing CCA compared with those who did not (pooled OR=0.45; 95% CI: 0.27, 0.75). It was the protective factor (Figure 12).
Figure 12.
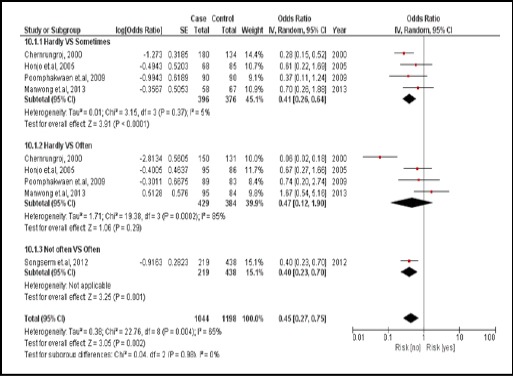
Meta-Analysis Forest Plots of the Relationship between Vegetables Consumption and CCA
3.2.5. Education attainment
There were 4 case-controlled studies, involving 2,372 subjects. Those with a higher educational attainment were 0.68 times for developing CCA compared with those with a primary school education (a pool OR=0.68; 95% CI: 0.51, 0.93) It was the protective factor (Figure 13).
Figure 13.
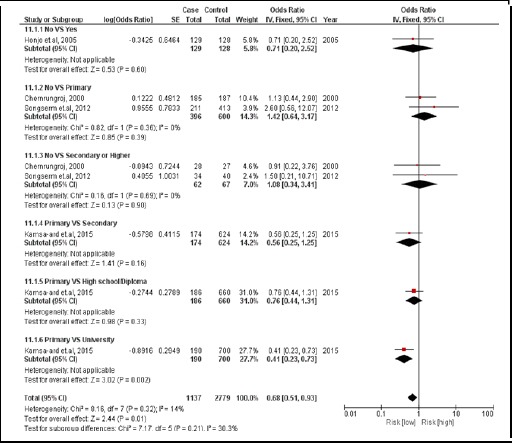
Meta-Analysis Forest Plots of the Relationship between Education Attainment and CCA
3.3. No association (95% CI of ORadj included 1.00)
3.3.1. Pla-ra
There were 2 case-controlled studies, involving 435 subjects. Those who consumed Pla-ra were 1.61 times more likely to develop CCA than those who did not (pooled OR=1.61; 95% CI: 0.76, 3.41) (Figure 14).
Figure 14.
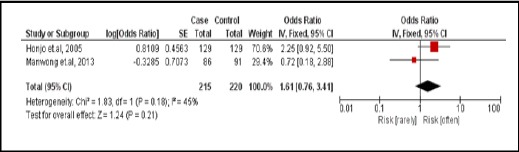
Meta-Analysis Forest Plots of the Relationship between Eating Pla-ra and CCA
3.3.2. Local northeastern Thai / Chinese sausage
There were 2 case-controlled studies, involving 738 subjects. Those who consumed the sausage were 1.13 times more likely to develop CCA than those who did not (pooled OR=1.13; 95% CI: 0.71, 1.79) (Figure 15).
Figure 15.

Meta-Analysis Forest Plots of the Relationship between Eating Sausage and CCA
3.3.3. Eating glutinous (sticky) rice
There were 3 case-controlled studies, involving 842 subjects. Those who regularly consumed sticky rice were 1.30 times more likely to develop CCA than those who did not (pooled OR=1.30; 95% CI: 0.85, 2.01) (Figure 16).
Figure 16.
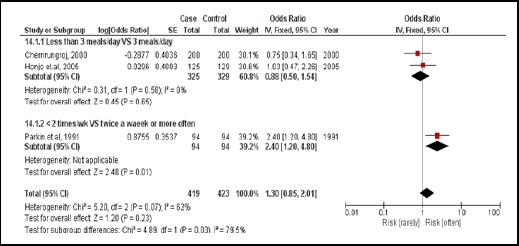
Meta-Analysis Forest Plots of the Relationship between Eating Sticky Rice and CCA
3.3.4. Meat consumption
There were 2 case-controlled studies, involving 616 subjects. Those who regularly consumed meat were 1.03 times more likely to develop CCA than those who did not (pooled OR=1.03; 95% CI: 0.57, 1.85) (Figure 17).
Figure 17.
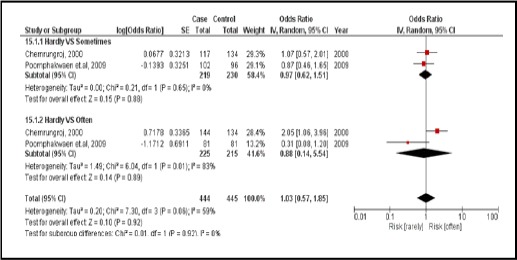
Meta-Analysis Forest Plots of the Relationship between Meats Consumption and CCA
3.3.5. Betel chewing nut
There were 3 case-controlled studies, involving 709 subjects. Those who regularly chewed betel nut were 1.45 times more likely to develop CCA than those who did not (pooled OR=1.45; 95% CI: 0.69, 3.02) (Figure 18).
Figure 18.
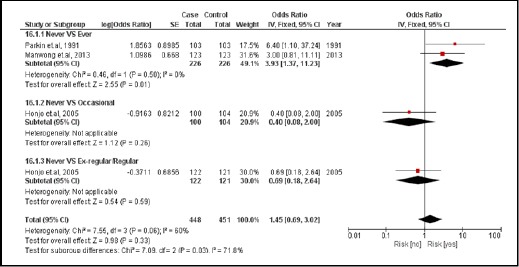
Meta-Analysis Forest Plots of the Relationship between Betel Nut Chewing and CCA
3.3.6. Fruits consumption
There were 4 case-controlled studies, involving 1,404 subjects. Those who regularly consumed fruit had a 0.66 times for developing CCA compared with those who did not (pooled OR=0.66; 95% CI: 0.43, 1.02) (Figure 19).
Figure 19.
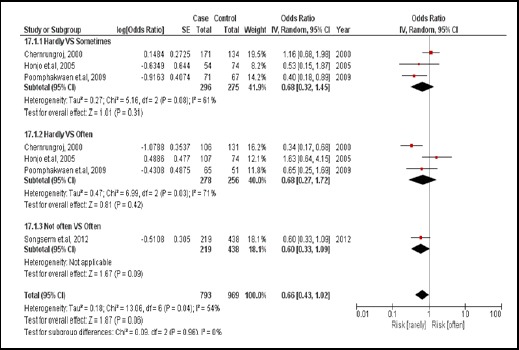
Meta-Analysis Forest Plots of the Relationship between Fruit Consumption and CCA
Discussion
The aim of this systematic review was to investigate the risk factors for CCA in Thailand. Sixteen epidemiological studies were met the eligibility requirements for inclusion in the review. The main finding illustrated that there is evidence that some factors are strongly associated with CCA such as age, OV infection, eating raw fish, family history of cancer, liquor consuming behavior, and taking PZQ. Eating nitrite-containing foods, fresh vegetables, education, smoking, and sex had only a mild association with CCA. Eating fresh vegetables and education were the protective factors. No association was found between CCA and eating Pla-ra, northeastern Thai / Chinese sausage, sticky rice, meat, chewing betel nut, and eating fruit.
A ROB graph illustrates the proportion of studies with a ‘yes’, ‘no’, or ‘unclear’ judgment. There was a “low” risk of bias on one study, an “unclear risk” for 8 and a “high” risk for 7.
Generalizations of this present study
The results of the current study can be generalized to the local Thai population, as the 16 articles reviewed were conducted in Thailand. The potential local risk factors included demographic variables (sex, age, education, and family history of cancer), determinants of health (alcohol consumption, smoking behavior, and chewing betel nut), and behaviors potentially related to CCA (viz., OV infection, PZQ treatment, eating raw fish, Pla-ra and local sausage/other sausage, local nitrite-containing foods, meats consumption, eating sticky rice, vegetables consumption and fruits consumption).
Strategies to minimize potential biases during data extraction
Two independent ratings (SK1 and SK2) were extracted from the studies, and any disagreements were resolved through discussion with the third researcher (VL – an expert in CCA) and the fourth researcher (KS – an epidemiologist in cancer research). The evidence from this present study should therefore be reliable.
Assessment of study quality
The quality of the studies was assessed using the NOS and JBI Critical Appraisal Tools. The scale comprises 8 items that cover 3 dimensions (Tables 1a and 1b). There was only 1 study which rated ‘low’ vis-à-vis bias, while 7 rated ‘high’, and 8 ‘unclear’. The 16 studies, however, carried out multiple analyses and accounted for various potential risk factors with respect to CCA. Controlling different confounders from each study and different classifications of independent variable demonstrated differences between studies, so that the 5 primary factors are: 1) OV infection, 2) liquor-consuming behavior, 3) vegetable consumption, 4) meat consumption, and 5) fruit consumption.
1) OV infection, even through the result have illustrated to diversity main finding risk factors to CCA. However, the magnitude of effect (adjusted OR) were in the same direction of magnitude. That is, almost studies were included in the meta-analysis which reported OV infection associated to risk of CCA, except a study by Kurathong et al., (1985) which suggested there were no significantly associated risk factors for CCA. The latter conclusion was likely based on an inadequate sample size.
2) Liquor consumption. Comparing moderate drinkers and non-drinkers, all of the reviewed articles showed no statistically significant association with CCA. The exception was reported by Songserm et al., (2012) who defined moderate drinking as 0.3-3.5 g/d (1 unit=8 g). (Choices NHS, 2017) Meanwhile Chernrungroj (2000) reported that moderate drinking was 2-14 g/d. (National Institute on Alcohol Abuse and Alcoholism, 2017) The level of consumption of a “moderate drinker” varied markedly between Songserm et al., (2012) and Chernrungroj (2000) and Poomphakwaen et al. (2009), so the results of the various studies are difficult to compare.
3) Vegetable consumption. The results generally illustrated the same trend of protection against CCA the greater the amount of vegetables consumed. Manwong et al., (2013) by contrast reported that more frequent vegetable consumption led to a greater risk of CCA. The difference in results may be types of vegetables. Manwong et al., (2013) classified two types of vegetables including vegetables grown in water and northeastern Thailand.
4) Meat consumption. According to Chernrungroj et al., (2000) meat included beef, pork, and products such as sausage and fermented products. Poomphakwaen et al., (2009) included beef and pork and by contrast did not confirm any association from meat consumption.
5) Fruit consumption. Honjo et al., (2005) categorized the frequency of consumption in terms of units of time, which differs from other studies. (Poomphakwaen et al., 2009; Chernrungroj, 2000; Songserm et al., 2012) Honjo et al., (2005) studied vegetable consumption categorized as < 1 time per week, 1 times per week, and ≥ 2 times per week while the other studies categorized as < 1 time per day, 1.0-2.08 times per day, and > 2.08 times per day, (Poomphakwaen et al., 2009; Chernrungroj, 2000) < 1 times per day and > 1 times per day. (Songserm et al., 2012) The result from Honjo et al. is not consistent with other studies.
Consistency of the main finding when comparing with other systematic reviews
1) PZQ treatment. We conducted a systematic review and found only one study that investigated the association between PZQ treatment and CCA in Thailand (Kamsa-ard et al., 2013). It found that was not significantly associated with the risk of CCA (OR=1.84, 95% CI: 0.81, 4.16). By comparison, the present study found a strong association between PZQ and risk of CCA (OR=2.02, 95% CI: 1.59, 2.57). The inconsistency may be because the previous study included two articles while the present study included four studied in a meta analysis.
2) OV infection. When considering OV infection, Xia et al. (2015) and Shin et al. (2010) demonstrated that liver fluke infection is strongly associated with risk of CCA (OR=4.17; 95% CI: 2.81, 6.19 and 4.84; 95%CI: 2.79, 8.41, respectively), which agrees with the present study (OR=6.05; 95% CI: 3.20, 11.42). The previous study (Xia et al., 2015; Shin et al., 2010), however, did not classify type of liver flukes, and there are two common species (viz., Clonorchis sinensis and O. viverrini). In Thailand, OV infection is the most common. (Sripa et al., 2011)
3) Liquor consumption. Previous research has shown that drinking is associated with an increased risk of CCA in Thailand (OR=2.81; 95% CI: 1.52, 5.21) (Palmer and Patel, 2012). This finding is consistent with the present study (OR=3.01; 95% CI: 2.00, 4.54). There were two other studies that reported no associated to risk with CCA. The odds of developing CCA were 1.14 and 1.09, respectively (OR=1.14; 95% CI: 0.75, 1.75 and 1.09; 95% CI: 0.87, 1.37). (Li et al., 2011; Ye et al., 2013)
In addition, the main finding from Li et al., (2011) was limited as they recruited only Chinese with extrahepatic CCA while in another study Ye et al., (2013) recruited all nationalities with extrahepatic CCA.
Palmer and Patel (2012) demonstrated the risk of intrahepatic CCA associated with liquor consumption. This finding is consistent with the present study which provided strong evidence of intrahepatic over against extrahepatic CCA throughout Thailand. This present study did not, however, categorize the type of CCA.
Recent evidence supports the hypothesis that the perihilar large duct type and the peripheral small bile duct type CCA have different carcinogenesis and risk factors. Chronic biliary inflammation (e.g., parasitic infection, nitrosamine and hepatolithiasis) induces neoplastic changes in the large bile ducts, whereas chronic hepatitis or cirrhosis induces the peripheral small duct type. (Aishima and Oda, 2015) Chronic liquor consumption is associated with intrahepatic rather than extrahepatic CCA.
4) Smoking. The present study demonstrated that smoking is weakly associated with an increased risk of CCA in Thailand. The odds of developing CCA were 1.46 (95% CI: 1.10, 1.94), compared with the 1.31 (95% CI: 0.95, 1.82) (Palmer and Patel, 2012) and 1.23 (95% CI: 1.01, 1.50) Ye et al. (2013) of previous studies.
Strengths of the study
The present study is the first correctly designed, systematic review of currently available evidence for investigating potential risk factors for an increased risk of CCA among people in Thailand. The literature reviewed was done using SCOPUS, ProQuest, ScienceDirect, PubMed, KKU Web OPAC, and ProQuest Dissertation and theses Global, which increased the reliability of the conclusions.
Limitations of the study
This systematic review was limited by the criteria used to select and/or reject the eligibility of reviewed studies. For example, studies about genetic factors and non-human research were excluded. The present study did, nevertheless, demonstrate possible epidemiological risk factors for CCA among Thai people. Controlling different confounders from each study affect to differences between studies.
The main finding is that some factors, the main finding is that some factors are strongly associated with CCA including: age, OV infection, eating raw fish, family history of cancer, alcohol consumption and taking repeated PZQ treatment.
Implications
The results of the current study indicate that the factors strongly associated with CCA are age, OV infection, eating raw fish, family history of cancer, liquor consumption, and repeated PZQ use. The public should be made aware of these risks to health, particularly OV infection/re-infection.
Implication for further research
The policy of the Ministry of Public Health recommends “Do not eat unboiled Pla-ra” because it is a putative risk for CCA. This present study, however, revealed that consuming “Pla-ra” is not associated with the risk of CCA. This contradiction may be due to the magnitude of the effect or that it depends on the amount and type consumed, whether raw, partially raw, or boiled. Thus, further research is needed to clarify the relationship between the amount and type of “Pla-ra” consumed.
Statement conflict of Interest
All authors declare that they have no competing interests regarding the publication of this paper.
Acknowledgements
The authors thank (a) the Faculty of Medicine, Khon Kaen University, Thailand, for financial support (Grant number: SY58201) and (b) Mr. Bryan Roderick Hamman for assistance with the English-language presentation of the manuscript under the aegis of the publication clinic, Khon Kaen University.
References
- 1.Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015;22:94–100. doi: 10.1002/jhbp.154. [DOI] [PubMed] [Google Scholar]
- 2.Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European network for the study of cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–80. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 3.Bhudhisawasdi V, Khuntikeo N, Chur-in S, et al. Cholangiocarcinoma: experience of Srinagarind Hospital. Srinagarind Med J. 2012;2:331–9. [Google Scholar]
- 4.Chernrungroj G. Risk factor for cholangiocarcinoma: a case-control study. Yale University; 2000. [Google Scholar]
- 5.Choices NHS. Alcohol units - Live Well - NHS Choices [Internet] 2017. [[Accessed 11 Jul 2017]]. Available : http://www.nhs.uk/Livewell/alcohol/Pages/alcohol-units.aspx .
- 6.Cochrane Community. RevMan 5 download [Internet] 2016. [[Accessed 23 Jun 2016]]. Available : http://community.cochrane.org/tools/review-production-tools/revman-5/revman-5-download . [Google Scholar]
- 7.Covidence. Accelerate your systematic review [Internet] 2016. [[Accessed 26 Apr 2016]]. Available : https://www.covidence.org/ [Google Scholar]
- 8.Deerasamee S, Martin N, Sontipong S, et al. Cancer in Thailand Vol II 1992-1994. IARC technical report No. 34. chapter I introducton. International agency for research on cancer, Lyon. 1999:24. [Google Scholar]
- 9.Elkins DB, Haswell-Elkins MR, Mairiang E, et al. A high frequency of hepatobiliary disease and suspected cholangiocarcinoma associated with heavy Opisthorchis viverrini infection in a small community in north-east Thailand. Trans R Soc Trop Med Hyg. 1990;84:715–9. doi: 10.1016/0035-9203(90)90159-c. [DOI] [PubMed] [Google Scholar]
- 10.Green A, Uttaravichien T, Bhudhisawasdi V, et al. Cholangiocarcinoma in north east Thailand. A hospital-based study. Trop Geogr Med. 1991;43:193–8. [PubMed] [Google Scholar]
- 11.Haswell-Elkins MR, Mairiang E, Mairiang P, et al. Cross-sectional study of opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int J Cancer. 1994;59:505–9. doi: 10.1002/ijc.2910590412. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honjo S, Srivatanakul P, Sriplung H, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005;117:854–60. doi: 10.1002/ijc.21146. [DOI] [PubMed] [Google Scholar]
- 14.Imsamran W, Chaiwerawattana A, Wiangnon S, et al. Cancer in Thailand Vol VIII 2010-2012. chapter II. Cancer incidence in Thailand. Bangkok: Bangkok medical publisher; 2015. p. 10. [Google Scholar]
- 15.Itoh M, Pairojkul C, Thamawit W, et al. Association of antibodies to opisthorchis viverrini with hepatobiliary disease in Northeastern Thailand. Am J Trop Med Hyg. 1994;51:424–9. [PubMed] [Google Scholar]
- 16.Joanna Briggs Institute. Critical appraisal tools [Internet] 2016. [[Accessed 7 Jul 2016]]. Available: http://joannabriggs.org/research/critical-appraisal-tools.html . [Google Scholar]
- 17.Kaewpitoon S, Kaewpitoon N, Yodklaw E. Risk factor associated with cholangiocarcinoma, Satuek hospital, Buriram province, Thailand. Thammasat Med J. 2014;14:163–9. [in Thai] [Google Scholar]
- 18.Kamsa-ard S, Promthet S, Sithithaworn P, et al. Predictive statistical model for the risk of cholangiocarcinoma in Northeast Thailand. Srinagarind Med J. 2009;24:231–9. [in Thai] [Google Scholar]
- 19.Kamsa-ard S, Laopaiboon M, Luvira V, Bhudhisawasdi V. Association between praziquantel and cholangiocarcinoma in patients infected with Opisthorchis viverrini: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2013;14:7011–6. doi: 10.7314/apjcp.2013.14.11.7011. [DOI] [PubMed] [Google Scholar]
- 20.Khuhaprema T, Srivatanakul P, Sriplung H, et al. Cancer in Thailand Vol. IV 1998-2000. Chapter II: Cancer incidence in Thailand. Bangkok: Bangkok medical publisher; 2007. p. 11. [Google Scholar]
- 21.Khuhaprema T, Srivatanakul P, Attasara P, et al. Cancer in Thailand Vol. V 2001-2003. Chapter II: Cancer incidence in Thailand. Bangkok: Bangkok medical Publisher; 2010. p. 7. [Google Scholar]
- 22.Khuhaprema T, Attasara P, Sriplung H, et al. Cancer in Thailand Vol. VI 2004-2006. Chapter II: Cancer incidence in Thailand. Bangkok: Bangkok medical Publisher; 2012. p. 7. [Google Scholar]
- 23.Khuhaprema T, Attasara P, Sriplung H, Wiangnon S, Sangrajrang S. Cancer in Thailand Vol. VII 2007-2009. Chapter II: Cancer incidence in Thailand. Bangkok: Bangkok medical Publisher; 2013. p. 12. [Google Scholar]
- 24.Kurathong S, Lerdverasirikul P, Wongpaitoon V, et al. Opisthorchis viverrini infection and cholangiocarcinoma. A prospective, case-controlled study. Gastroenterology. 1985;89:151–6. doi: 10.1016/0016-5085(85)90755-3. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Yang H, Cao J. Association between alcohol consumption and cancers in the Chinese population--a systematic review and meta-analysis. PLoS One. 2011;6:e18776. doi: 10.1371/journal.pone.0018776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mairiang E, Elkins DB, Mairiang P, et al. Relationship between intensity of opisthorchis viverrini infection and hepatobiliary disease detected by ultrasonography. J Gastroenterol Hepatol. 1992;7:17–21. doi: 10.1111/j.1440-1746.1992.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 27.Manwong M, Songserm N, Promthet S, Matsuo K. Risk factors for cholangiocarcinoma in the lower part of Northeast Thailand: a hospital-based case-control study. Asian Pac J Cancer Prev. 2013;14:5953–6. doi: 10.7314/apjcp.2013.14.10.5953. [DOI] [PubMed] [Google Scholar]
- 28.Mendeley. Empowering researchers to organize their references [Internet] 2016. [[Accessed 19 Apr 2016]]. Available: https://www.mendeley.com/ [Google Scholar]
- 29.National Institute on Alcohol Abuse and Alcoholism (NIAAA) What is a standard drink? [Internet] 2017. [[Accessed 11 Jul 2017]]. Available: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink . [Google Scholar]
- 30.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkin DM, Srivatanakul P, Khlat M, et al. Liver cancer in Thailand I. A case-control study of cholangiocarcinoma. Int J Cancer. 1991;48:323–8. doi: 10.1002/ijc.2910480302. [DOI] [PubMed] [Google Scholar]
- 32.Poomphakwaen K, Promthet S, Kamsa-Ard S, et al. Risk factors for cholangiocarcinoma in Khon Kaen, Thailand: a nested case-control study. Asian Pac J Cancer Prev. 2009;10:251–8. [PubMed] [Google Scholar]
- 33.Shin HR, Oh JK, Masuyer E, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579–85. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Songserm N, Promthet S, Pientong C, et al. Gene-environment interaction involved in cholangiocarcinoma in the Thai population: polymorphisms of DNA repair genes, smoking and use of alcohol. BMJ Open. 2014;4:e005447. doi: 10.1136/bmjopen-2014-005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Songserm N, Promthet S, Sithithaworn P, et al. Risk factors for cholangiocarcinoma in high-risk area of Thailand: role of lifestyle, diet and methylenetetrahydrofolate reductase polymorphisms. Cancer Epidemiol. 2012;36:89–94. doi: 10.1016/j.canep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Sripa B, Bethony JM, Sithithaworn P, et al. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011;120:158–68. doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sriplung H, Sontipong S, Martin N, et al. Chapter II: Cancer incidence in Thailand. Bangkok: Bangkok medical publisher; 2003. Cancer in Thailand Vol III 1995-1997; p. 13. [Google Scholar]
- 38.Srivatanakul P, Honjo S, Kittiwatanachot P, et al. Hepatitis viruses and risk of cholangiocarcinoma in northeast Thailand. Asian Pac J Cancer Prev. 2010;11:985–8. [PubMed] [Google Scholar]
- 39.Vatanasapt V, Martin N, Sriplung H, et al. Cancer in Thailand 1988-1991 IARC technical report No. 16. Chapter 6 results. International agency for research on cancer, Lyon. 1993:59. [Google Scholar]
- 40.Wells GA, Shea B, Higgins JP, et al. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Res Synth Methods. 2013;4:63–77. doi: 10.1002/jrsm.1077. [DOI] [PubMed] [Google Scholar]
- 41.Xia J, Jiang S, Peng HJ. Association between Liver Fluke Infection and Hepatobiliary Pathological Changes: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0132673. doi: 10.1371/journal.pone.0132673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye XH, Huai JP, Ding J, Chen YP, Sun XC. Smoking, alcohol consumption, and the risk of extrahepatic cholangiocarcinoma: a meta-analysis. World J Gastroenterol. 2013;19:8780–8. doi: 10.3748/wjg.v19.i46.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]


