Abstract
Background:
Cancer is a significant problem in modern medicine, also is the most common cause of death after cardiovascular diseases, and in need of targeted drug release. Although, chemotherapy is an important candidate in cancer treatment, but it has many side effects on healthy tissues of the body. Therefore, Nano technology is used for specific function, by the least side effects and damage to normal cells.
Materials and method:
In this study, the pharmacological properties of PEGylated Nano-niosomal Gingerol was examined. Noisome were prepared using reverse phase evaporation method, which contains specific proportion of cholesterol, span60 and polyethylene glycol. Then, PEGylated the prepared formulation by PEG6600. The amount of release and encapsulation of the drug was investigated. The percentage of remains of cancer cell line T47D treated with PEGylated niosomal Gingerol.
Results:
The average diameter of the nanoparticles, size distribution and zeta potential were reported for PEGylated niosomal sample 35.65 nm, 0.17 and 21 mv, and for PEGylated niosomal drug sample 256.9 nm, 0.23 and 28 mv, respectively. The amount of OD for encapsulated drug was 0.198, also the amount of concentration of the drug which is not encapsulated, was 0.77947 μl of the drug per ml. This value of encapsulated drug was 76.38 percent.
Conclusion:
The results showed that IC50 of the formulation of PEGylated nanoniosomal Gingerol is less than the standard drug. It seems, the cause of this phenomenon is due to the effect of Polyethylene glycol, in more stability and slower drug release, in the formulation of PEGylated niosome. Also, Polyethylene glycol makes increase in the drug dealing and its greater influence with the target cell. In this study, more than 76% of the Gingerol drug in PEGylated nanoniosomal formulation were enclose. Also, we could reduce the amount of drug release, as much as possible.
Keywords: Breast cancer, drug delivery, reverse phase evaporation noisome-T47D
Introduction
Nowadays, breast cancer has become prevalent in human societies and Cancer has become as the second most common cause of death in developing countries (Tabrizi and Bidgoli, 2015), the disease is in common among women. Breast anatomy is different in women and men. This has been interpreted more. Milk glands in the body of women are the secondary organs of the reproductive system, which has grown significantly during maturity, but its full function growth mode is after pregnancy. Breasts are as a form of underdeveloped, in men. Each breast forms a circular prominence, on the frontal and surround chest wall, on the pectoralis major muscle, after maturity. Breast cancer is a cancer that begins in the breast tissue. Symptoms of breast cancer can be a lump in the breast, change in breast shape, skin deep, liquid discharge from the nipple, or scaling part of the skin. In cases where the disease has spread in other organs these symptoms can be bone pain, swollen lymph nodes, difficulty breathing, or have jaundice (Langer, 2000).
Breast cancer risk factors include: Obesity, lack of physical exercise, drinking alcohol, hormone replacement therapy during menopause, ion beams, early menarche and late childbirth or not having children (Tabrizi and Hosseini, 2015). For about 5 to 10% of cases of this disease are genetic (Lanza et al., 2002). America Preventive Services Task Force recommends that women aged 50 to 74 conduct screening every two years (Mathiowitz, 1999). For preventing breast cancer in high risk individuals may be using drugs tamoxifen or raloxifene, removing both breasts through surgery is a useful preventive measure in some high risk women (O’Driscoll et al., 2003). The survival rate in developed countries is high, so that 80% and 90% of people in England and America are alive at least five years. In the developing country the survival rate is lower. Worldwide, breast cancer is the most important cancer in women, and 25% of all cancers are accounted for (Reed and Tomaselli, 2000). Risk factors in breast cancer are: Age, Sex, Race, Genetic risk factors, Family history, the onset of menstrual periods, Menopause, Breast-feeding and pregnancy, Alcohol, Smoking, diet.
Materials and Methods
Niosomal preparation
In this study, two methods were experienced, among the variety of techniques to produce Nano-niosomal. For reasons of safety issues in the production process, and non-toxic materials, reverse phase technique was chosen for niosomal preparation.
Determination characterizations of particles
Measured particles diameter, after making PEGylated niosomal formulations and encapsulation of the drug. To do this, the sample of PEGylated niosomal drug and the PEGylated niosomal sample were diluted, using phosphate buffer one to hundred, initially. Then, the average particles diameter and Zeta potential were measured, by using a Zeta Sizer. Furthermore, 200 μl of the formulations prepared were poured on the lam, to dry. After that, photos were taken from the formulations prepared, by using Scanning Electron Microscope (SEM).
Loading and Encapsulation efficiency
Only one factor is important to succeed nanoparticle systems: high capacity of drug loading. Which reduces the amount of drug carriers in drug delivery management. Drug loading inside the nanoparticles is done in two ways: A. during production of the nanoparticles, B. By performing absorption (after the formation of the nanoparticles and by keeping theme in the drug solution). It is clear, the first method (incorporation of drug during formation of the drug), has more ability to absorb the drug.
In this study, the first method was inevitable, because the drug solvent was organic material of ethanol and cannot be solved in PBS. The adsorption isotherm for drug-release system presents vital information for the best formulation of the drug binding capacity to the surface of the nanoparticles and the amount of drug absorbed. Studies have shown absorption of the drug to the surface of the nanoparticles is compliance Langmuir adsorption isotherm. Absorption capacity is depends on the amount of hydrophobic and the specific surface of the nanoparticles. It is expected to see with increasing concentrations of monomeric, increasing binding of drug in trapping of drugs. But in some cases, this is done as reverse. This show that optimization should be done for monomer concentration. Type of surface-active substances, stabilizers and the amount of environmental acidity are effective factors, in drug loading. Also, the amount of environmental electrically charged and chemical of the solution are effective in drug loading.
To calculate the percentage of encapsulation of niosome, we took 1.5 milliliter of each formulation and pour them in separate vials, they were centrifuges for 45 minutes, at room temperature, at speed of 50,000 revolutions per minute. Then, we took of the supernatant that is containing free drug, read OD in 254 nm. Knowing the total amount of drug used in the formulation and using the following formula, the amount of encapsulation was calculated.

Formula 1
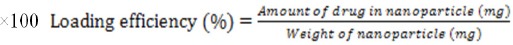
Formula 2
Drug release
The release of the drug from the niosome, determined by the Membrane Penetration techniques. We conducted the following experiment to know, within a specified time, how much encapsulated Gingerol is released. We took from PEGylated niosomal drug, 1 milliliter, initially. And send theme in dialysis bags (Cut-off 8000). On the other hand, moved 20 milliliter of buffer BPS into two graduated cylinder. These bags were placed in the buffer BPS, such those are suspended. Then, threw the magnet into graduated cylinder, fully covered its openings using Para film, to external factors does not effect on it (Figure 1-3).
Figure 1.
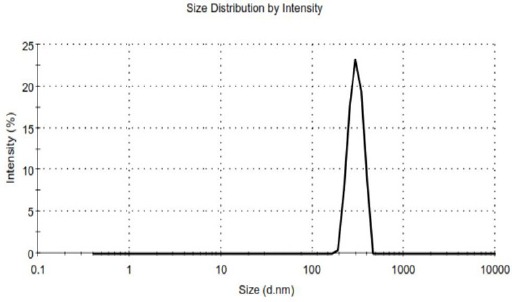
The Curve of Zeta Sizer for the Nano Drug Sample
Figure 2.
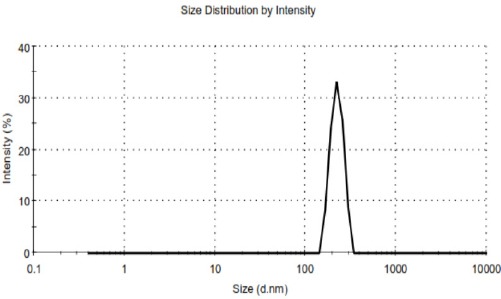
The Curve of Zeta Sizer for Nano Control Sample
Figure 3.
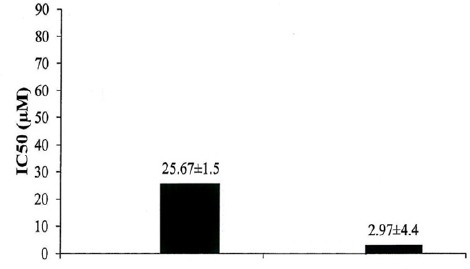
The Comparison of (μM) IC50 in Prepared Formulation on Cell Line T47D
It should be noted that, be sure to use gloves for carrying dialysis bags or filling them with samples. After that, we put on the graduated cylinder on the mixer, by speed of 100 rpm and room temperature. The operation conducted during two days. PBS buffer samples was taken, at various time intervals. Eventually, OD of the solutions measured, by using a spectrophotometer at a wavelength of 254. On the other hand, we solved 1 mg of the drug, in 1 ml of PBS. We investigated the drug release, in times and with the conditions mentioned.
MTT
The yellow MTT revived to purple formazan, in the mitochondria of living cells. Absorption of the colored solution can be measured, in 540 nm wavelength by spectrophotometry. The maximum absorption depends on solvent used. This revival activity done only when mitochondrial reductase enzymes are active. So, this conversion can be linked to the number of living cells, directly. The amount of cell survival will obtain when the amount of the products of the purple formazan produced, which produced by cells exposed to toxic substances, compared with the amount of the products of the formazan produced in untreated cells. As mentioned, by using a spectrophotometer, we did colorimetric. The results were reported as IC50, indicates the amount of the drug which at that dose, 50% of cancer cells, are alive. MTT solution is solvable in balanced salt solutions or cell culture without phenol and red, and it is light yellow. Mitochondrial dehydrogenase of living cells break the Tetrazolium ring, and purple crystals of formazan MTT is produced. Crystals can be solved in acid isopropanol. Purple solution created be measured bysperoscopy.
Evaluation of the drug release
From PBS buffer volume was take and measured the OD value of that, by using spectrophotometer, which can be seen in the table below. However, obtain the percentage of drug released, after 48 hours, by using the standard curve.
Results
OD measured by Spectrophotometer, 0.198 reported.
Characterization of nanoniosome
As mentioned, we measured the average diameter of the nanoparticles, size distribution and zeta potential of pharmaceutical formulations and drug-free, by using a Zeta Sizer. It was reported for PEGylated niosomal sample 35.65 nm, 0.17 and 21 mv, and for PEGylated niosomal drug sample 256.9 nm, 0.23 and 28 mv.
Determine the percentage of loading and encapsulation of the drug
In the previous parts, we calculated OD amount of the prepared formulation. By replacement in OD equation, according to C, we count the encapsulated drug. The amount of OD was 0.198. We calculate the amount of concentration of the drug is not encapsulated, that it is 0.77947 μl of the drug per ml. After that, we reduced this value from the initial value of the drug per milliliter. So, the resulting number is the value of encapsulated drug.
Table 1.
OD Values of PBS Buffers Containing Samples of the Drug and the Standard Drug
| Time (hour) | PEGylated Niosomal | STANDARD DRUG |
|---|---|---|
| 2 | 15.55142962 | 263.3527389 |
| 4 | 17.68190836 | 529.6625824 |
| 6 | 19.41292235 | 795.9724258 |
| 8 | 20.74447156 | 1062.282269 |
| 20 | 23.40757012 | 2660.14133 |
| 24 | 24.47280937 | 3192.761017 |
| 28 | 25.13858398 | 3725.380704 |
| 44 | 26.20382335 | 5855.859451 |
| 48 | 26.73644304 | 6388.479138 |
Table 2.
OD Value of Diluted Samples
| Concentration (μl/ml) | OD |
|---|---|
| 0.0625 | 0.019 |
| 0.125 | 0.048 |
| 0.25 | 0.019 |
| 0.5 | 0.153 |
| 1 | 0.237 |
Table 3.
The Comparison of (μg/μl) IC50 in Prepared Formulation on Cell Line T47D
| Sample | T47D | |
|---|---|---|
| PEGylated niosomal drug | Standard drug | |
| IC50 (μg/μl) | 0.00044 | 0.003804 |
However, reduce the concentration of the encapsulated drug from this value, and multiply in 100, to obtain the percentage of encapsulated drug. This value is 76.38 percent. Besides that, by using the formula, the amount of drug load was obtained.
Discussion
Cancer is a disease of abnormal proliferation of cells in the body. Due to late diagnosis and lack of effective treatments. Suitable blood compatibility in the uploaded Nanoparticles with the drug is very important (Knox et al., 1986; Kemnitzer et al., 2004). Cancer is a lack of coordination between growth and cell death that results is the accumulation of an excessive number of cells. World Health Organization statistics show that one woman out of every 55 women in the world has conflict with the cancer at some point of her life. Usually, this disease effects on women over 50 years. The present study investigates the Breast Cancer cell line T47D. Recently, a lot of works have been conducted to develop a delivery system by changing the process to control the destiny of drugs, particularly drug distribution within the organism (Roudsari et al., 2016). Because of late diagnosis and lack of effective treatments, the disease a high mortality rate among women. Treatment Methods divided into three major categories: Surgery, Chemotherapy and Radiation therapy that all of these have some problems and side effects. Nanoparticles are trying to minimize these negative effects.
In this study, investigated toxic effects of PEGylated niosomal Gingerol drug on cell line T47D. The results of the investigation of loading Gingerol showed that the amount of encapsulation of the PEGylated nanoniosomal drug is acceptable. Due to be determined dose of drug released, at various times, we used dialysis. Gingerol release from the nanoparticles was including initial phase of rapid release and followed by slow release phase. Reducing in drug released in the initial burst that almost for both samples occurred in the first 6 hours, depends on the composition of the nanoparticles formulation. Decay of PEGylated niosomal sample was much faster compared to the standard drug, which seems is due to the presence of Polyethylene glycol. It would causes the drug passes a longer distance, therefore, duration of the distribution in longer for the release. Investigated cytotoxic effect of two formulations of PEGylated niosomal Gingerol and the standard drug by MTT test. In this experiment, the formulation of PEGylated nanoniosomal drug-free has the least toxic on T47D. The results showed that IC50 of the formulation of PEGylated nanoniosomal Gingerol is less than the standard drug. It seems, the cause of this phenomenon is due to the effect of Polyethylene glycol, in more stability and slower drug release, in the formulation of PEGylated niosome. Also, Polyethylene glycol makes increase in the drug dealing and its greater influence with the target cell. In this study, we succeeded to enclose more than 76% of the Gingerol drug in PEGylated nanoniosomal formulation. Also, we could reduce the amount of drug release, as much as possible.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgements
I appreciate the guidance of Professor Azim Akbarzadeh and Doctor Marzieh Motiee poor.
References
- 1.Izadi M, Shahemabadi HE, Kanaani L, et al. Investigation the characteristics of carboplatin loaded onto pegylated liposomal nanoparticles on the rat Glioma cell line C6. Adv Biores. 2016;7:113–18. [Google Scholar]
- 2.Kemnitzer W, Drewe J, Jiang S, et al. Discovery of 4-Aryl-4 H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based high-throughput screening assay structure- activity relationships of the 4-Aryl group. J Med Chem. 2004;47:6299–310. doi: 10.1021/jm049640t. [DOI] [PubMed] [Google Scholar]
- 3.Kemnitzer W, Kasibhatla S, Jiang S, et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based high-throughput screening assay. Structure–activity relationships of the 7-and 5-, 6-, 8-positions. Bioorg Med Chem Lett. 2005;15:4745–51. doi: 10.1016/j.bmcl.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 4.Kerr JF, Winterford CM, Harmon BV. Apoptosis Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–26. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Knox RJ, Friedlos F, Lydall DA, Roberts JJ. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum (II) and cis-diammine-(1, 1 cyclobutanedicarboxylato) platinum (II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986;46:1972–79. [PubMed] [Google Scholar]
- 6.Langer R. Biomaterials in drug delivery and tissue engineering: one laboratory's experience. Acc Chem Res. 2000;33:94–101. doi: 10.1021/ar9800993. [DOI] [PubMed] [Google Scholar]
- 7.Lanza R, Langer R, Vacanti JP. Principles of tissue engineering (second ed.) Academic press New York. 2002:995. [Google Scholar]
- 8.Mathiowitz E. Encyclopedia of controlled drug delivery. New York: Wiley; 1999. p. 45. [Google Scholar]
- 9.O'Driscoll L, Linehan R, Clynes M. Survivin: role in normal cells and in pathological conditions. Curr Cancer Drug Targets. 2003;3:131–52. doi: 10.2174/1568009033482038. [DOI] [PubMed] [Google Scholar]
- 10.Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941–53. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 11.Reed JC, Tomaselli KJ. Drug discovery opportunities from apoptosis research. Curr Opin Biotechnol. 2000;11:586–92. doi: 10.1016/s0958-1669(00)00148-8. [DOI] [PubMed] [Google Scholar]
- 12.Robertson GS, Crocker SJ, Nicholson DW, Schulz JB. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000;10:283–92. doi: 10.1111/j.1750-3639.2000.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roudsari MH, Saeidi N, Kabiri N, et al. Investigation of characteristics and behavior of loaded carboplatin on the, liposomes nanoparticles, on the lung and ovarian cancer: an in-vitro evaluation. Asian Pac J Cancer Biol. 2016;1:9–12. [Google Scholar]
- 14.Tabrizi MM, Bidgoli SA. Increased risk of childhood Acute Lymphoblastic Leukemia (ALL) by prenatal and postnatal exposures to high voltage power lines: A case control study in Isfahan-Iran. Asian Pac J Cancer Prev. 2015;16:2347–50. doi: 10.7314/apjcp.2015.16.6.2347. [DOI] [PubMed] [Google Scholar]
- 15.Tabrizi MM, Hosseini SA. Role of electromagnetic field exposure in childhood acute lymphoblastic leukemia and no impact of urinary alpha amylase - a case control study in Tehran, Iran. Asian Pac J Cancer Prev. 2015;16:7613–18. doi: 10.7314/apjcp.2015.16.17.7613. [DOI] [PubMed] [Google Scholar]


