Abstract
Background:
The aberrant expression of surface receptors on immunocytes may represent potential markers of tumor escape for nasopharyngeal carcinoma (NPC). The aim of this study was to investigate the expression of representative receptors on natural killer (NK) cells and NK group 2, member D (NKG2D) on immunocytes in the peripheral blood of patients with NPC.
Methods:
Patients (n = 64) with NPC prior to initiation of treatment were defined as the study group. Healthy volunteers (n = 31) served as the control group. The expression of NK cells and NKT cells; the triggering receptors NKp30, NKp44, and NKp46 on NK cells; the activating receptor NKG2D on NK cells, CD4+ T cells, and CD8+ T cells; and the inhibitory receptors CD158b and CD159a on NK cells were analyzed by flow cytometry in the two groups.
Results:
Here, our study showed that no differences were observed in terms of the numbers of NK cells or NKT cells, or the expression of CD158b and CD159a on the surface of NK cells between the two groups. Nevertheless, the expression levels of NKp30 and NKp46 on NK cells in the NPC patients were significantly lower than in the healthy individuals (P < 0.05). No differences existed in the expression of NKG2D on NK cells, but NKG2D on CD8+ T cells showed a markedly lower expression in the study group (P < 0.001).
Conclusions:
Our findings may reflect a possible mechanism of immune evasion for NPC. The enhancement of immunotherapy concerning NKp30, NKp46, and NKG2D may be an innovative treatment strategy for patients with NPC.
Keywords: Nasopharyngeal carcinoma, surface receptors, NK cells, NKG2D, Flow cytometry
Introduction
Nasopharyngeal carcinoma (NPC) is regarded as the most common epithelial tumor of the head and neck in Southeast Asia, especially in South China (Wei and Sham, 2005). With intensity-modulated radiation therapy combined with chemotherapy, the 5-year estimated local recurrence-free rate for patients with NPC exceeds 90% (Lee et al., 2014; Lin et al., 2014; Sun et al., 2014). However, the 5-year overall survival rate is only around 80%, and distant metastases have become the primary reason for treatment failure (Lee et al., 2014; Lin et al., 2014; Sun et al., 2014; Wang et al., 2014; Zong et al., 2015). Therefore, there is an urgent need to find additional effective therapeutic modalities. Determining ways to further decrease the risk of distant metastases is a hot topic in the current treatment of NPC, and immunotherapy might be a novel treatment for cancer.
Human natural killer (NK) cells, a type of lymphoid cells, play an integral role in innate immunity to resist against infections and tumors by their cytotoxic activity (Pardoll, 2001). The cytotoxicity of NK cells is usually involved by the regulation of both the activating and inhibitory surface reporters. Natural cytotoxicity receptors (NCRs), such as NKp30, NKp44, and NKp46, as well as NK group 2, member D (NKG2D) are representative activating receptors that could be combined with their corresponding ligands to trigger the activity of NK cells (Pardoll, 2001; Koch et al., 2013). However, inhibitory receptors such as CD158b and CD159a can confer inhibitory signals to NK cells by immunoreceptor tyrosine-based inhibition motifs to reduce the cytotoxicity of NK cells (Konjevic et al., 2009). When major histocompatibility complex-I expression on tumor cells is low or absent, tumor cells will be identified by NK cells, and their activating receptors will play major roles to enhance the cytotoxicity (Zheng et al., 2006). In addition, NKG2D is expressed on the membranes of NKT cells, CD8+ T cells, γδ T cells, and particular subsets of CD4+ T cells and binds to distinct ligands of tumors to play an increasingly important role in both innate and adaptive immunity (Raulet et al., 2013). Hence, NK cell receptors and NKG2D-mediated immunotherapy may be a powerful avenue for the treatment of cancer.
At present, increasing research on both NK cell surface receptors and NKG2D in cervical cancer, acute myelocytic leukemia, and viral infections have demonstrated the positive correlation between the downregulated killing effects of NK cells and the low expression of NCRs or NKG2D (De Maria et al., 2003; Garcia-Iglesias et al., 2009). In addition, the negative correlation between inhibitory receptors of NK cells and tumor progression has been revealed (Konjevic et al., 2009). Furthermore, the alteration of NKG2D on certain T lymphocytes has been proven to correlate closely with immunosuppression in the host (Clayton et al., 2008). However, the expression of representative receptors on NK cells and NKG2D on immunocytes in patients with NPC has not been well investigated. Thus, there is a pressing need to investigate the possibly aberrant expression of these receptors to provide a new pathway for tumor immune evasion and to explore a novel immunotherapeutic intervention for patients with NPC.
Therefore, the aim of this study was to investigate the expression of receptors on NK cells and NKG2D on immunocytes by flow cytometry in the peripheral blood of patients with NPC as compared with that of healthy controls in order to provide a reference for the clinical management of NPC.
Materials and methods
Patients
The study group consisted of 64 newly diagnosed and previously untreated patients with NPC. The clinical data of the patients referred to Fujian Provincial Cancer Hospital between June 2015 and November 2015 were reviewed. Of the 64 patients, 38 were male individuals and 26 were female individuals, with a male/female ratio of 1.46:1. The median age of the patients with NPC was 40 years old (range: 25–49 years old). All patients with NPC were staged by the 7th edition of the American Joint Committee on Cancer staging system (Pan et al., 2015). Twelve patients (18.75%) were defined as having stage I–II cancer, and the remaining 52 patients (81.25%) were defined as having stage III–IVB cancer. The control group was composed of 31 healthy volunteers. Subjects with a history of diabetes mellitus, hypertension, coronary artery disease, or infectious disease were excluded. Of the 31 healthy individuals, 13 were males and 18 were females, with a male/female ratio of 1:1.38. The median age of the controls was 40 years old (range: 26–57 years old).
Flow cytometry
Peripheral blood samples were obtained and analyzed while still fresh (within 30 min of collection). Flow cytometry was employed to analyze the proportion of immune cells in the peripheral blood of NPC patients and healthy controls. Blood samples (50 μL each) were aliquoted into 11 tubes. Then, 10 μL of fluorescein isothiocyanate-, allophycocyanin-, or phycoerythrin-conjugated mouse monoclonal antibodies (MAbs) to CD3 (Clone UCHT1), CD4 (Clone RPA-T4), CD8 (Clone RPA-T8), CD56 (Clone NCAM 16.2), CD158b (Clone CH-L), NKp30 (Clone p30-15), NKp44 (Clone p44-8), NKp46 (Clone 9E2, also known as 9-E2), and NKG2D (Clone 1D11) from BD Biosciences Company (San Jose, CA, USA) and phycoerythrin-conjugated anti-human CD159a (NKG2A) from Beckman Coulter Inc. (Brea, CA, USA) were considered as markers of surface receptor for analyses. After incubation for 30 min at 4 °C with the appropriate fluorochrome-conjugated MAb, the cells were washed by three times with phosphate-buffered saline solution. Triple- or quadruple-color flow cytometry was conducted on a FACSCalibur instrument (Becton Dickinson, San Jose, CA, USA). Statistical data were obtained and counted with Cell Quest software.
Statistical analysis
The analyses were all conducted by using SPSS for Windows, version 17.0 (SPSS, Inc., Chicago, IL, USA). The Student’s t-test or the nonparametric Mann-Whitney U test was employed to have a comparison among the continuous quantitative parameters. All data were presented as the mean ± standard deviation or median (interquartile range), on the basis of the distribution of different variables. A two-tailed P value of less than 0.05 was deemed to have a statistically significant difference.
Results
The proportion of immune cells detected in the peripheral blood between the two groups. When the percentages of immune cells between the NPC patients and the healthy controls were compared by flow cytometry, no statistically significant differences were found in terms of the proportion of NK cells, NKT cells, CD3+ T cells, CD4+ T cells, or CD8+ T cells (all P > 0.05), as displayed in Table 1.
Table 1.
NK Cells, NKT Cells and T Lymphocytes
| Patients group | Healthy controls | Pa | |
|---|---|---|---|
| NK lymphocytes(%) | 18.67±11.07 | 15.06±8.77 | 0.116 |
| NKT lyphocytes (%) | 4.51±2.84 | 5.53±3.24 | 0.119 |
| CD3+ T lymphocytes (%) | 69.93±11.64 | 71.30±8.97 | 0.563 |
| CD4+ T lymphocytes (%) | 36.35±9.94 | 36.67±6.45 | 0.851 |
| CD8+ T lymphocytes (%) | 27.50±10.37 | 27.90±7.27 | 0.847 |
Student’s t-test, two sided P.value<0.05
The expression of certain surface receptors on NK cells. To further analyze the activities of NK cells, some receptors on NK cells were also tested by flow cytometry. From Table 2, With regard to the inhibitory receptors on NK cells, no significant differences existed in terms of the percentages of CD158b and CD159a between the patients and the controls. As for the activating receptors on NK cells, the percentage of NKp44 was also not found to be statistically significant. However, the expression levels of NKp30 and NKp46 on NK cells in the NPC patients were shown to be markedly lower than in the healthy controls, with P values of 0.018 and <0.001, respectively (Figure 1, 2).
Table 2.
The Percentage of Receptors Expressed on NK Cells
| Patients group | Healthy controls | P | |
|---|---|---|---|
| CD158b | 45.68±21.24 | 42.08±18.91 | 0.424a |
| CD159a | 28.27±19.10 | 31.54±14.07 | 0.399a |
| NKp30* | 49.81±20.78 | 60.31±17.77 | 0.018a |
| NKp44 | 1.0 (0.1) | 0.3 (0.1) | 0.081b |
| NKp46* | 44.21±21.53 | 60.57±17.71 | <0.001a |
Student’s t-test;
Mann-Whitney U test;
According to Student’s t-test; the value of P <0.05 indicates statistical significant.
Figure 1.
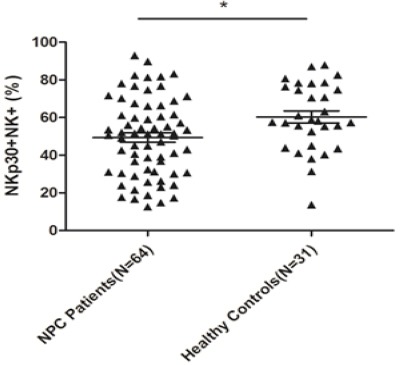
A Comparison of the Expression Level of NKp30 on NK Cells Between NPC Patients and Healthy Controls by Flow Cytometry. The expression level of NKp30 was significantly lower in 64 NPC peripheral blood samples as compared to that in 31 healthy peripheral blood samples (* P <0.05).
Figure 2.
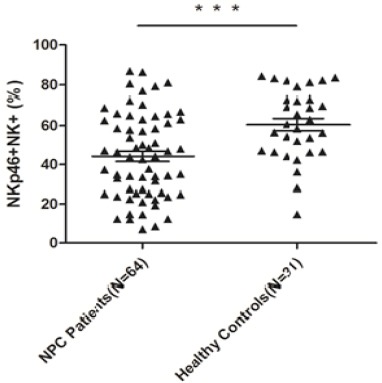
A Comparison of the Expression Level of NKp46 on NK Cells Between NPC Patients and Healthy Controls by Flow Cytometry. The expression level of NKp30 was markedly decreased in 64 NPC peripheral blood samples as compared to that in 31 healthy peripheral blood samples (*** P <0.001).
The expression of NKG2D on immunocytes. NKG2D, a key activating receptor that plays an important role in both innate and adaptive immunity, was expressed not only on NK cells but also on other T lymphocytes. As shown in Table 3, no significant differences existed in terms of the expression of NKG2D on NK cells and CD4+ T cells; however, the expression level of NKG2D on CD8+ T lymphocytes in NPC patients was found to be significantly decreased than that of healthy individuals (P < 0.001) (Figure 3).
Table 3.
NKG2D Expressed on NK Cells and T Lymphocytes
| Patients group | Healthy controls | P | |
|---|---|---|---|
| NKG2D on NK cells (%) | 95.45 (92.83) | 96.10 (92.00) | 0.877b |
| NKG2D on CD4+ T lymphocytes (%) | 1.05 (0.50) | 0.6 (0.4) | 0.061b |
| NKG2D on CD8+ T lymphocytes (%)* | 94.14 ± 2.90 | 96.05 ± 1.96 | <0.001a |
Student’s t-test;
Mann-Whitney U test;
P <0.05 indicates statistical significance between the two groups.
Figure 3.
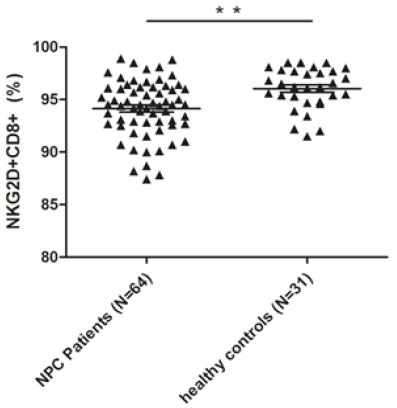
A Comparison of the Expression Level of NKG2D on CD8+ T Lymphocytes Between NPC Patients and Healthy Controls by Flow Cytometry. The expression level of NKG2D on CD8+ T lymphocytes was much lower in 64 NPC peripheral blood samples as compared to that in 31 healthy peripheral blood samples (** P <0.005).
Discussion
Over the past several decades, several studies on T lymphocyte dysregulation in the peripheral blood of patients with NPC have shown an association between tumor progression and immunosuppression (Li et al., 2007; Fogg et al., 2013; Chia et al., 2014); however, no research on NK cells and their surface receptors or the expression of NKG2D on immunocytes has been reported to date. Our study represents the first study to investigate the aberrant expression of surface receptors on NK cells and NKG2D on immunocytes in NPC patients, revealing the possible mechanism of tumor escape for NPC. In the present study, our results showed that NPC patients had a lower expression of NKp30, NKp46, and NKG2D on CD8+ T cells than healthy individuals. Therefore, NKp30, NKp46, and NKG2D may be potential biomarkers of immune escape for NPC.
NK cells are known as the first line of defense against infection and tumor cells in the innate immune system; thus, the number of NK cells may increase as a result of activation of innate immunity by the malignant process in NPC (Hu et al., 2012). In our study, however, no statistically notable differences were discovered in the proportion of NK cells between NPC patients and healthy controls, or between NKT cells and certain subsets of T lymphocytes. This finding was in line with the study by Zheng et al., who demonstrated that the frequencies of NK cells were similar between the two groups (Zheng et al., 2006). Thus, we need to investigate further the representative surface receptors on NK cells to evaluate NK cell cytotoxicity.
On further analysis of surface receptors on NK lymphocytes, significantly decreased proportions of NKp30 and NKp46 were observed in NPC patients as compared with the healthy individuals. This finding may suggest that the cytotoxic effect of NK cells was breached due to the decreased expression of NKp30 and NKp46 in NPC patients. NKp30 and NKp46 are both members of the NCR family on NK cells to be correlated with the ability to recognize and lyse transformed and tumor cells (Garcia-Iglesias et al., 2009). NKp30 has been shown to bind to a tumor-specific B7 family homolog (B7-H6) (Brandt et al., 2009) and the nuclear factor HLA-B-associated transcript 3 (BAT3) (Pogge von Strandmann et al., 2007) both can directly trigger NKp30-mediated NK cell cytolytic effects. In addition, heparin sulfate/heparin sequences on cancer cells have been hypothesized to be a type of NKp46-binding motif (Hecht et al., 2009). Hence, the decreased proportions of NKp30 and NKp46 in our study may reveal a mechanism of immunological escape for NPC.
NKG2D is also an important receptor for NK cell activation, but the average percentage of NKG2D was not shown to be statistically different between NPC patients and healthy controls in our study. In fact, NKG2D acts as a costimulatory receptor delivered by many T lymphocytes, such as NKT cells, CD8+ T cells, and certain CD4+ T cells, to increase adaptive immunity activation (11). As expected, our study demonstrated that NKG2D on CD8+ T lymphocytes was markedly decreased in the study group as compared to that in the control group, but no marked difference was found in the proportion of NKG2D on CD4+ T lymphocytes. This finding suggests that the decreased expression of NKG2D on the membrane of CD8+ T cells may weaken the cytotoxic effect of T cells by affecting NKG2D-mediated costimulatory signals. Moreover, tumor cells have been shown to be able to release distinctly soluble ligands of NKG2D into circulating blood to bind to NKG2D, thus inducing the downregulated expression of NKG2D on CD8+ T lymphocytes and impair T cell activation (Lundholm et al., 2014). Therefore, the decreased expression of NKG2D on CD8+ T cells may represent a form of immune evasion in patients with NPC.
The interpretation of the current study was limited by the relatively insufficient number of cases, including only 31 healthy individuals and 64 NPC patients. In addition, other important subpopulations of T lymphocytes such as regulatory T cells and CD44 lymphocytes in NPC patients were not investigated.
In conclusion, our study, for the first time, reported on the downregulation of NKp30, NKp46, and NKG2D on CD8+ T lymphocytes in NPC. These findings may conjecture possible pathways of tumor escape and provide novel therapeutic strategies for NPC in the future.
Acknowledgements
This study was supported by the Joint Funds of the National Natural Science Foundation of China (Grant No. U1405221), the National Clinical Key Specialty Construction Program, P.R.C, the Key Clinical Specialty Discipline Construction Program of Fujian, P.R.C.
References
- 1.Brandt CS, Baratin M, Yi EC, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther. 2014;22:132–9. doi: 10.1038/mt.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clayton A, Mitchell JP, Court J, et al. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–58. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 4.De Maria A, Fogli M, Costa P, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–8. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 5.Fogg M, Murphy JR, Lorch J, et al. Therapeutic targeting of regulatory T cells enhances tumor-specific CD8+T cell responses in Epstein-Barr virus associated nasopharyngeal carcinoma. Virol J. 2013;441:107–13. doi: 10.1016/j.virol.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Iglesias T, Del Toro-Arreola A, Albarran-Somoza B, et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186. doi: 10.1186/1471-2407-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht ML, Rosental B, Horlacher T, et al. Natural cytotoxicity receptors NKp30, NKp44 and NKp46 bind to different heparan sulfate/heparin sequences. J Proteome Res. 2009;8:712–20. doi: 10.1021/pr800747c. [DOI] [PubMed] [Google Scholar]
- 8.Hu FJ, Ge MH, Li P, et al. Unfavorable clinical implications of circulating CD44+lymphocytes in patients with nasopharyngeal carcinoma undergoing radiochemotherapy. Clin Chim Acta. 2012;413:213–8. doi: 10.1016/j.cca.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Koch J, Steinle A, Watzl C, et al. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34:182–91. doi: 10.1016/j.it.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Konjevic G, Mirjacic Martinovic K, Vuletic A, et al. Distribution of several activating and inhibitory receptors on CD3-CD16+NK cells and their correlation with NK cell function in healthy individuals. J Membr Biol. 2009;230:113–23. doi: 10.1007/s00232-009-9191-3. [DOI] [PubMed] [Google Scholar]
- 11.Lee AW, Ng WT, Chan LL, et al. Evolution of treatment for nasopharyngeal cancer--success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110:377–84. doi: 10.1016/j.radonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Zeng XH, Mo HY, et al. Functional inactivation of EBV-specific T-lymphocytes in nasopharyngeal carcinoma: implications for tumor immunotherapy. PLoS One. 2007;2:e1122. doi: 10.1371/journal.pone.0001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin S, Pan J, Han L, et al. Update report of nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy and hypothesis of the optimal margin. Radiother Oncol. 2014;110:385–9. doi: 10.1016/j.radonc.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Lundholm M, Schroder M, Nagaeva O, et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+T cells: mechanism of immune evasion. PLoS One. 2014;9:e108925. doi: 10.1371/journal.pone.0108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan J, Xu Y, Qiu S, et al. A comparison between the Chinese 2008 and the 7th edition AJCC staging systems for nasopharyngeal carcinoma. Am J Clin Oncol. 2015;38:189–96. doi: 10.1097/COC.0b013e31828f5c96. [DOI] [PubMed] [Google Scholar]
- 16.Pardoll DM. Immunology, Stress, NK receptors, and immune surveillance. Science. 2001;294:534–6. doi: 10.1126/science.1066284. [DOI] [PubMed] [Google Scholar]
- 17.Pogge von Strandmann E, Simhadri VR, von Tresckow B, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–74. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Raulet DH, Gasser S, Gowen BG, et al. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–41. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110:398–403. doi: 10.1016/j.radonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Feng M, Fan Z, et al. Clinical outcomes and prognostic factors of 695 nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Biomed Res Int. 2014;2014:814948. doi: 10.1155/2014/814948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–54. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, Cao KY, Ng SP, et al. Complementary activation of peripheral natural killer cell immunity in nasopharyngeal carcinoma. Cancer Sci. 2006;97:912–9. doi: 10.1111/j.1349-7006.2006.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zong J, Lin S, Lin J, et al. Impact of intensity-modulated radiotherapy on nasopharyngeal carcinoma: Validation of the 7th edition AJCC staging system. Oral Oncol. 2015;51:254–9. doi: 10.1016/j.oraloncology.2014.10.012. [DOI] [PubMed] [Google Scholar]


