Abstract
Background:
Prostate cancer (PCa) represents the second most commonly diagnosed malignancy and the sixth leading cause for cancer related death among men worldwide. Although use of the prostate specific antigen (PSA) as a diagnostic marker has improved the detection and management of PCa, low specificity and sensitivity has limited its clinical efficacy. Moreover, elevated PSA is frequently observed in benign prostate hyperplasia (BPH). Mean platelet volume (MPV) and platelet distribution width (PDW) are commonly used indicators of platelet activation. The purpose of current study was to investigate the ability of PSA, MPV, and PDW individually or in combination, to differentiate PCa from BPH.
Materials and Methods:
This study included 100 patients with PCa and 108 patients with BPH. We collected all participants’ clinical and laboratory characteristics. The benefit of adding MPV and PDW to a model with only PSA was evaluated as an increased in the area under the curve (AUC) obtained by receiver operating curve (ROC).
Results:
PCa patients had reduced MPV and elevated PSA and PDW levels compared to BPH patients. Single biomarkers had AUC values ranging from 0.683 for PDW to 0.865 for PSA. Moreover, the combination of PSA, MPV, and PDW increased the AUC to 0.935 (0.892-0.964) (p<0.0001), significantly higher than those of any single marker.
Conclusions:
The combined use of PSA, MPV, and PDW may be clinically useful in distinguishing between PCa and BPH.
Keywords: Prostate cancer, benign prostate hyperplasia-mean platelet volume, platelet distribution width-diagnosis
Introduction
Prostate cancer (PCa) represents the second most commonly diagnosed malignancy and the sixth leading cause for cancer related death among men worldwide(Jemal et al.,2010). Although use of the prostate specific antigen (PSA) as a diagnostic marker has improved the detection of PCa, its low sensitivity and specificity for PCa makes early finding of PCa difficult. More, PSA levels are frequently elevated in benign prostatic hyperplasia (BPH). There is, therefore, an urgent need for novel biomarkers that can effectively distinguish PCa from BPH.
Activated platelets play a critical role in cancer progression and metastasis (Bambace and Holmes, 2011; Goubran et al., 2014). Mean platelet volume (MPV) is a marker of activated platelets and is associated with gastric cancer, ovarian cancer, lung cancer, colon cancer, and breast cancer (Gu et al., 2015; Kemal et al., 2014; Kilincalp et al., 2014; Kumagai et al., 2015; Li et al., 2014). Platelet distribution width (PDW), another platelet parameter, indicates variation in platelet size and differentially diagnoses thrombocytopenia (Kaito et al., 2005).
Combination of several biomarkers for early detection may lead to enhanced sensitivities and specificities. The aim of the current study was to determine the ability of PSA, MPV, and PDW, individually or in combination, to distinguish PCa from BPH.
Materials and methods
Study population
100 patients with PCa and 108 patients with BPH were admitted to the third affiliated hospital, Harbin Medical University between Jan 2015 and Dec 2015. Patients meeting all of the following requirements were eligible for enrollment: (1) undergone surgical resection and diagnosis was confirmed by histology; (2) untreated before diagnosis; (3) measurement of PSA before surgery. Exclusion criteria included: hematological disorders, hypertension, diabetes mellitus, and medical treatment with anticoagulant, statins, and acetylic salicylic acid. Written informed consents were obtained from all patients. This study was approved by the Institutional Review Board of Harbin Medical University Cancer Hospital.
Clinical examination and biochemical measurements
All the subjects underwent physical examination. Clinical data including smoking status, medical history and medication use were recorded for each subject. Venous blood samples after a 10-hour overnight fasting were collected from the individuals within 1 week prior to surgery. The serum prostate specific antigen (PSA) was measured using an automatic electrochemistry luminescence immunoassay system (ROCHE cobas 8000; Roche, Germany). Platelet indices were measured by an autoanalyzer (Sysmex XE-2100, Kobe, Japan). The whole blood samples were collected in EDTA-containing tubes, and all samples were processed within 30 minutes after blood collection. The inter- and intra-assays coefficients of variation (CVs) of all these assays were below 5%.
Statistics
The descriptive statistics are presented as means ± SD or medians (interquartile range) for continuous variables and percentages of the number for categorical variables. Inter-group differences in categorical variables were assessed for significance using the Chi-square test; differences in continuous variables were assessed using the Mann-Whitney U test or t-test. ROC curves were calculated to analyze AUC values of measured serum markers, and the differences in the area under the curve (AUC) were detected by using MedCalc version 15.0. Two-tailed values of P <0.05 were considered statistically significant. Statistical analyses were performed using SPSS Statistics version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
The clinical characteristics of the subjects are shown in Table 1. The mean ages of the patients with BPH and PCa were 69.7 (8.1) years and 70.2 (5.4) years, respectively. Age, BMI, smoking, fasting plasma glucose, white blood cell, and platelet count in two groups had no difference. The levels of PSA and PDW are significantly increased, and MPV and haemoglobin are reduced in PCa patients compared with BPH patients.
Table 1.
Clinical and Laboratory Characteristics of the Participants
| Variables | Prostate cancer | Benign prostate hyperplasia | p-value |
|---|---|---|---|
| Number | 100 | 108 | |
| Age (years) | 69.7 (8.1) | 70.2 (5.4) | 0.618 |
| BMI (kg/m2) | 23.6 (3.2) | 23.2 (2.9) | 0.326 |
| Current smoker (n, %) | 10 (10.0) | 9 (8.3) | 0.677 |
| FPG (mmol/L) | 5.15 (4.82-5.86) | 5.04 (4.83-5.37) | 0.273 |
| WBC (×109/L) | 6.87 (2.37) | 6.70 (1.41) | 0.527 |
| Haemoglobin (g/dl) | 133.7 (16.6) | 139.3 (15.6) | 0.014 |
| Platelet (×109/L) | 214.6 (65.3) | 215.7 (63.6) | 0.898 |
| MPV (fL) | 8.5 (1.6) | 9.9 (1.6) | < 0.001 |
| PDW (%) | 16.7 (1.7) | 17.8 (1.3) | < 0.001 |
| PSA (ng/ml) | 65.0 (22.0–100.0) | 10.0 (5.0–14.0) | < 0.001 |
Data are presented as means (SD) or median (interquartile range) or percentage; BMI, body mass index; FPG, fasting plasma glucose; PSA, prostate specific antigen; WBC, white blood cells; MPV, mean platelet volume; PDW, platelet distribution width.
The relationships between MPV, PDW and PSA levels and clinicopathologic characteristics in PCa are listed in Table 2. PSA levels were associated with lymph node status and metastasis (p=0.002).
Table 2.
Correlations Between Clinic Pathological Features and Pre-operative PSA, MPV, and PDW in Prostate Cancer.
| Variables | N | PSA (ng/ml) | P value | MPV (fL) | P value | PDW (%) | P value |
|---|---|---|---|---|---|---|---|
| Age (years) | 0.429 | 0.839 | 0.763 | ||||
| ≤ 70 | 49 | 61.0 (16.5–100.0) | 8.4 (1.4) | 16.8 (1.6) | |||
| >70 | 51 | 67.0 (32.0–100.0) | 8.5 (1.9) | 16.7 (1.8) | |||
| PSA (ng/ml) | < 0.001 | 0.689 | 0.857 | ||||
| ≤ 10 | 26 | 4.0 (2.0–6.0) | 8.6 (2.2) | 16.7 (1.4) | |||
| > 10 | 74 | 100.0 (51.0–100.0) | 8.4 (1.4) | 16.7 (1.8) | |||
| Gleason score | 0.496 | 0.131 | 0.082 | ||||
| < 7 | 43 | 97.0 (18.5–100.0) | 8.2 (1.7) | 17.2 (1.4) | |||
| = 7 | 46 | 51.0 (24.0–100.0) | 8.8 (1.6) | 16.4 (1.9) | |||
| > 7 | 11 | 62.0 (10.0–100.0) | 8.2 (1.0) | 16.4 (1.7) | |||
| Clinical T stage | 0.277 | 0.181 | 0.189 | ||||
| T1-T2b | 71 | 60.0 (19.5–100.0) | 8.6 (1.8) | 16.6 (1.8) | |||
| ≥ T2c | 29 | 100.0 (24.0–100.0) | 8.1 (1.2) | 17.1 (1.4) | |||
| Lymph node status | 0.002 | 0.234 | 0.196 | ||||
| Negative | 87 | 57.0 (17.5–100.0) | 8.5 (1.6) | 16.6 (1.8) | |||
| Positive | 13 | 100.0 (85.8–100.0) | 8.0 (1.5) | 17.3 (1.0) | |||
| Metastasis | 0.002 | 0.132 | 0.155 | ||||
| No | 83 | 57.0 (17.0–100.0) | 8.4 (1.6) | 16.6 (1.8) | |||
| Yes | 17 | 100.0 (85.8–100.0) | 7.9 (1.6) | 17.3 (1.2) |
PSA, prostate specific antigen; MPV, mean platelet volume; PDW, platelet distribution width.
In Table 3, the sensitivity, specificity, positive predictive value, negative predictive value, and area under curve (AUC) values are presented for MPV, PDW, PSA, the combination of MPV, PDW, and PSA. PSA had the highest specificity (93.5%), but at the cost of an unsatisfactory low sensitivity (72.2%). The sensitivity of PSA markedly increased and the specificity of PSA did changed when the combination of PDW, MPV, and PSA were applied. Single biomarkers had AUC values ranging from 0.683 for PDW to 0.865 for PSA; the combination of PDW, MPV, and PSA increased the AUC to 0.935 (p < 0.0001) (Figure 1).
Table 3.
Receiver Operating Characteristic Curve Analyses Showing the Utility of Alone or Combined Markers for Differentiating Prostate Cancer from Benign Prostate Hyperplasia
| Marker | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|
| MPV (fL) | 69.0 | 71.3 | 69.0 | 71.3 | 0.742 (0.677-0.800) |
| PDW (%) | 61.0 | 67.6 | 63.5 | 65.2 | 0.683 (0.615-0.746) |
| PSA (ng/ml) | 72.2 | 93.5 | 90.9 | 78.9 | 0.865 (0.810-0.908) |
| PSA+ MPV | 81.4 | 87.0 | 84.9 | 83.9 | 0.901 (0.852-0.939) |
| PSA+ PDW | 80.4 | 92.6 | 90.7 | 84.0 | 0.905 (0.856-0.941) |
| PSA+MPV+PDW | 79.4 | 93.5 | 91.7 | 83.5 | 0.935 (0.892-0.964) |
PPV, positive predictive value; NPV, negative predictive value; AUC, area under curve; PSA, prostate specific antigen; MPV, mean platelet volume; PDW, platelet distribution width.
Figure 1.
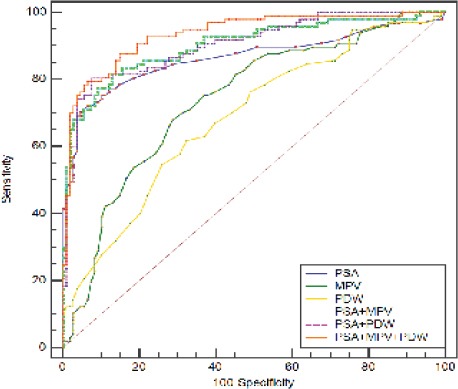
Receiver- Operator Characteristics (ROC) Curve for PSA, MPV, and PDW Combined Showing Sensitivity and 1-specificity of the Differential Diagnosis of Prostate Cancer Versus Benign Prostate Hyperplasia.
Discussion
Our study showed that PSA, and PDW are markedly higher and MPV is significantly reduced in PCa patients than in BPH patients. In addition, PSA, MPV, and PDW in combination significantly enhance the ability to distinguish PCa from BPH.
The mechanisms underlying the association of MPV and PDW with PCa are currently unclear. Numerous studies have identified enhanced platelet activation occurred in PCa. The platelet-derived growth factor (PDGF) proteins are potent stimulators of cell proliferation/transformation in PCa (Ustach et al., 2004). High expression of PDGF alpha-receptor activation is associated with bone metastases in castration-resistant PCa (Russell et al., 2010). Targeting PDGF alpha-receptor effectively counteracts skeletal metastases in PCa mice (Russell et al., 2010). These data are also in agreement with the current knowledge that anti-platelet is considered to be a part of cancer adjuvant therapy (Mezouar et al., 2016). MPV is an early marker of activated platelets. Lower MPV values could be suggestive of an enhanced consumption of large platelets in inflammatory states (Gasparyan et al., 2011). Recent studies confirmed that low levels of MPV are associated with high-grade inflammatory diseases and reverse in the course of anti-inflammatory therapy Gasparyan et al., (2011). Consistent with our results, Inagaki and Kumagai et al., (2014) reported that low MPV level was associated with poor prognosis in non-small-cell lung cancer. Aksoy et al., (2008) observed that solid tumors with bone marrow metastasis were more likely to have low MPV levels. In line with previous results, our study indirectly confirmed the findings using simple indicators of platelet activation.
Nearly 15–25% of PCa patients had normal levels of PSA (Greene et al., 2013). On the other hand, high PSA levels are frequently detected in BPH patients (Thompson et al., 2004). Therefore, identification of new biomarkers to correctly identify PCa patients would help to prevent individuals with BPH from getting unnecessary biopsies and from the side effects of overtreatment. Our study found that the AUC values for discriminating PCa from BPH range from 0.683 for PDW to 0.865 for PSA, respectively. The combination of PSA, MPV, and PDW increased the AUC to 0.935, significantly higher than those of any single marker. Furthermore, we found that PSA combined with MPV and PDW had higher sensitivity compared to PAS alone. Therefore, a combination of three serum markers is a more comprehensive parameter for cancer detection than single index in PCa patients.
In conclusion, a combined use of PSA, MPV, and PDW can be used as a surrogate for the presence of PCa. The results need to be validated with additional investigations.
Grant support
This work was supported by Science Foundation of Heilongjiang Academy of Medical Science (Grant No. 201714).
References
- 1.Aksoy S, Kilickap S, Hayran M, et al. Platelet size has diagnostic predictive value for bone marrow metastasis in patients with solid tumors. Int J Lab Hematol. 2008;30:214–9. doi: 10.1111/j.1751-553X.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 2.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–49. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 3.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation. Curr Pharm Des. 2011;17:47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 4.Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelet-cancer interactions. Semin Thromb Hemost. 2014;40:296–305. doi: 10.1055/s-0034-1370767. [DOI] [PubMed] [Google Scholar]
- 5.Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement:2009 update. J Urol. 2013;189:2–11. doi: 10.1016/j.juro.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Gu M, Zhai Z, Huang L, et al. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer. 2016;23:752–60. doi: 10.1007/s12282-015-0635-6. [DOI] [PubMed] [Google Scholar]
- 7.Inagaki N, Kibata K, Tamaki T, Shimizu T, Nomura S. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer. 2014;83:97–101. doi: 10.1016/j.lungcan.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 9.Kaito K, Otsubo H, Usui N, et al. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br J Haematol. 2005;128:698–702. doi: 10.1111/j.1365-2141.2004.05357.x. [DOI] [PubMed] [Google Scholar]
- 10.Kemal Y, Demirag G, Ekiz K, Yucel I. Mean platelet volume could be a useful biomarker for monitoring epithelial ovarian cancer. J Obstet Gynaecol. 2014;34:515–18. doi: 10.3109/01443615.2014.912620. [DOI] [PubMed] [Google Scholar]
- 11.Kilincalp S, Ekiz F, Basar O, et al. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014;25:592–4. doi: 10.3109/09537104.2013.783689. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai S, Tokuno J, Ueda Y, et al. Prognostic significance of preoperative mean platelet volume in resected non-small-cell lung cancer. Mol Clin Oncol. 2015;3:197–201. doi: 10.3892/mco.2014.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li JY, Li Y, Jiang Z, Wang RT, Wang XS. Elevated mean platelet volume is associated with presence of colon cancer. Asian Pac J Cancer Prev. 2014;15:10501–4. doi: 10.7314/apjcp.2014.15.23.10501. [DOI] [PubMed] [Google Scholar]
- 14.Mezouar S, Frere C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb Res. 2016;139:65–76. doi: 10.1016/j.thromres.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Russell MR, Liu Q, Fatatis A. Targeting the {alpha}receptor for platelet-derived growth factor as a primary or combination therapy in a preclinical model of prostate cancer skeletal metastasis. Clin Cancer Res. 2010;16:5002–10. doi: 10.1158/1078-0432.CCR-10-1863. [DOI] [PubMed] [Google Scholar]
- 16.Russell MR, Liu Q, Lei H, Kazlauskas A, Fatatis A. The alpha-receptor for platelet-derived growth factor confers bone-metastatic potential to prostate cancer cells by ligand- and dimerization-independent mechanisms. Cancer Res. 2010;70:4195–4203. doi: 10.1158/0008-5472.CAN-09-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–46. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 18.Ustach CV, Taube ME, Hurst NJ, et al. A potential oncogenic activity of platelet-derived growth factor d in prostate cancer progression. Cancer Res. 2004;64:1722–9. doi: 10.1158/0008-5472.can-03-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]


