Abstract
Objective:
This study was designed to visually represent postoperative recurrence patterns using event dynamics and to assess sex-based differences in the timing of recurrence for non-small cell lung cancer.
Methods:
We studied 829 patients (538 men, 291 women) with NSCLC who underwent complete pulmonary resection in 9 hospitals. Event dynamics with the use of life-table methods were evaluated, and only first events (distant metastases or local recurrence) were considered. The effects of sex, histological type, pathological stage, and smoking history were studied.
Result:
The resulting smoothed hazard rate curves indicated that the recurrence risk pattern definitely correlated with sex, with a sharp peak in the first year in men and a broad peak during the first 2 to 3 years in women. These findings were also confirmed by analyses according to pathological stage, histological type, and smoking history.
Conclusion:
The peak times of recurrence differed considerably between men and women. The delayed time of peak recurrence in women, associated with a longer disease-free interval within subsets of patients with similar disease stage, histological type, and smoking status, might account for the better survival in women.
Keywords: Gender, non-small cell cancer, recurrence, event dynamics
Introduction
Lung cancer is the leading cause of cancer-related mortality in men as well as women worldwide. Although the incidence of lung cancer in men has been declining for years, the overall incidence of lung cancer has increased steadily owing to the marked increase in women (Thomas et al., 2005). Several studies of patients with non-small-cell lung cancer (NSCLC) have reported that women live significantly longer than men after treatment (Ferguson et al., 1990; Albain et al., 1991; Paesmans et al., 1995; Ouellette et al., 1998). At present, however, the reasons for the better survival of women with NSCLC are not completely understood, and few studies have focused on gender-related disparities in the timing of recurrence. Our study was designed to visually represent postoperative recurrence patterns using event dynamics and to analyze sex-related differences in the timing of recurrence for NSCLC.
Materials and Methods
Between January 2005 and December 2007, we studied 829 patients (538 men, 291 women) with NSCLC who underwent complete pulmonary resection in 9 hospitals affiliated with the Yokohama Consortium of Thoracic Surgeons (Yokohama City University Hospital and affiliated hospitals). Preoperative staging investigations were performed with the use of routine computed tomography (CT) of the chest and abdomen, and brain CT or magnetic resonance imaging (MRI), according to UICC TNM classification. Bone scintigraphy was performed in patients with suspicious symptoms. Positron emission tomography (PET) was performed along with the standard examinations in selected patients. Patients who died during the initial hospitalization or within 30 days after surgery were excluded. A single primary tumor was diagnosed in all patients, and no patient had a prior history of lung cancer (excluding those with multicentric cancers). The postoperative follow-up schedule consisted of a clinic visit every 3 to 6 months through the fifth year, and annually thereafter. In general, chest X- ray was done every 3 to 6 months, CT scans were performed every 6 months in the first 3 years after resection and annually thereafter during follow-up period. Disease recurrence was diagnosed histologically, cytologically, and radiologically. The date of recurrence was defined as the date of confirmation of recurrence based on clinical and radiological findings. Local recurrence was defined as reappearance of cancer at the surgical margin, ipsilateral hilum or mediastinum and all other sites of failure was defined as distant metastasis.Second primary lung cancers were excluded. Disease-free interval (DFI) was defined as the interval from the date of surgery to the date of recurrence. Only first events were considered in this study.
Statistical analysis
Chi-square tests and Student’s t-tests were used to evaluate the statistical significance of differences in categorical variables and continuous variables, respectively. Survival curves were estimated by the Kaplan-Meier method, and differences in survival were assessed by the log-rank test. P values of less than 0.05 were considered to indicate statistical significance.
Event dynamics using life-table methods were studied to estimate the discrete hazard rate for the considered event, i.e., the conditional probability of the event occurring within a defined time interval, given that the patient did not previously have the event at the beginning of the interval (Demicheli et al., 2012). Because the hazard rate estimates showed some instability owing to random variation, a kernel-like smoothing procedure was used, and the smoothed curve was graphically represented to facilitate understanding of the underlying pattern (Ramlau-Hansen, 1983).
Results
Patient characteristics
The patients’ characteristics are shown in Table 1. Women were more likely than men to have adenocarcinoma (p<0.001), to have early stage disease (p<0.001), and to be nonsmokers (p<0.001). The median follow-up after complete resection was 68.6 months (range, 3 to 121 months). A total of 283 (34%) of the 829 patients had recurrence. The patients with recurrence comprised 199 men (37%) and 84 women (29%).
Table 1.
Patient, Treatment, and Tumor Characteristics (n=829)
| Characteristic | Male | Female | p value |
|---|---|---|---|
| (n=538) | (n=291) | ||
| Age, years | 0.169 | ||
| Mean | 67.7±9.3 | 66.7±9.9 | |
| Histology | <0.001 | ||
| Adenocarcinoma | 273 (51%) | 245 (84%) | |
| Squamous cell | 178 (33%) | 30 (10%) | |
| Others | 87 (16%) | 16 (6%) | |
| Surgical procedure | 0.237 | ||
| Segmentectomy | 57 (11%) | 23 (8%) | |
| Lobectomy | 453 (84%) | 259 (89%) | |
| Bi-lobectomy | 6 (1%) | 3 (1%) | |
| Pneumonectomy | 22 (4%) | 6 (2%) | |
| Pathological stage | <0.001 | ||
| IA | 218 (41%) | 174 (60%) | |
| IB | 151 (28%) | 60 (20%) | |
| IIA | 19 (4%) | 6 (2%) | |
| IIB | 63 (12%) | 10 (3%) | |
| IIIA | 63 (12%) | 31 (10%) | |
| IIIB | 24 (5%) | 10 (3%) | |
| Smoking status | <0.001 | ||
| Current smoker/ ex-smoker | 483 (90%) | 95 (33%) | |
| Nonsmoker | 36 (7%) | 175 (60%) | |
| NOS | 19(4%) | 21(7%) | |
| Adjuvant chemotherapy | 0.003 | ||
| Yes | 147 (27%) | 53 (18%) | |
| No/ NOS | 391 (73%) | 238 (82%) |
NSCLC, non-small cell lung cancer; NOS, not otherwise specified
Disease- free survival curves
Disease-free survival (DFS) curves estimated by the Kaplan- Meier method showed that women had significantly better rates of DFS than men (p=0.001) (Figure 1a).
Figure 1.
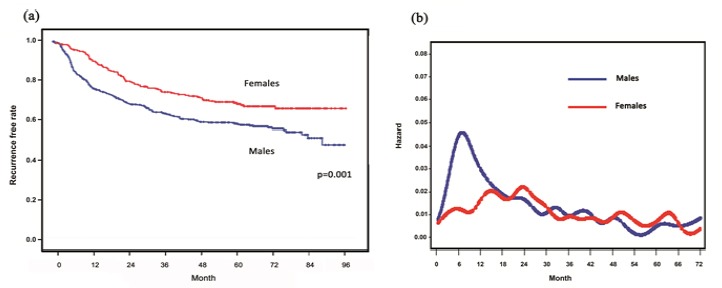
(a) Disease-free survival curves according to sex ; (b) Smoothed hazard rate estimates for first event according to sex
Event dynamics
The resulting smoothed hazard rate curve displayed an initial surge during the first year after surgery in men (Figure 1b). On the other hand, the maximum peak occurred between second and third year after surgery in women, which was about 16 months later than the peak in men.
As for pathological stage, the peaks of the hazard rate curves displayed increasing height with increasing pathological stage (Figure 2a). In both the stage IB group and stage IIA to IIIB group, the maximum peak of recurrence was found 6 to 8 months after surgery, whereas stage IA patients lacked such a large, sharp peak.
Figure 2.
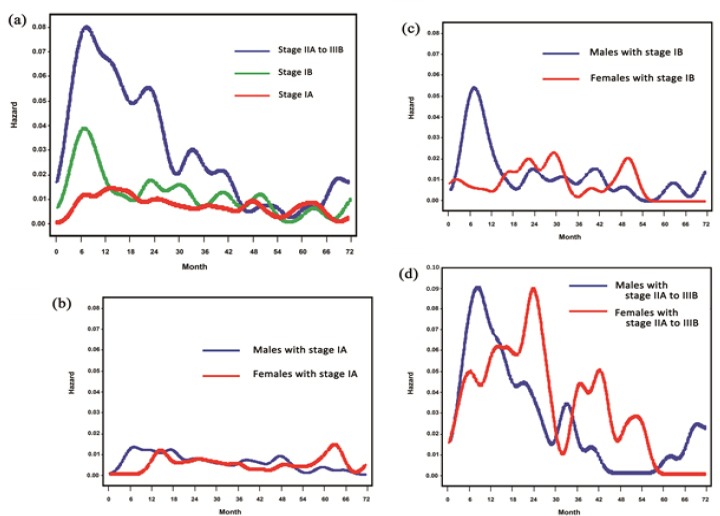
(a) Smoothed hazard rate estimates for first event according to pathological stage; (b) Smoothed hazard rate estimates for first event by sex in pathological stage: Stage IA; (c) Smoothed hazard rate estimates for first event by sex in pathological stage: Stage IB; (d) Smoothed hazard rate estimates for first event by sex in pathological stage: Stage IIA to IIIB
When the hazard rate curves were compared between men and women in the stage IA group, the recurrence risk remained low in both sexes during the follow-up period, as expected (Figure 2b). In the stage IB group, the recurrence risk had a quite narrow early peak, with a very steep rise to a maximum at 6 to 8 month in men. In women, the peak was considerably broader, reaching its maximum at 28 to 30 months (Figure 2c). In the stage IIA to IIIB group, the first narrow peak in men showed a very steep rise to a maximum 6 to 8 months after surgery. The recurrence risk for women increased gradually to reach a maximum 24 to 26 months after surgery (Figure 2d).
As for histological type, men with squamous cell carcinoma showed a sharp peak in the hazard rate in the first year. In contrast, only a small peak was noted at 22 to 24 months in women with squamous cell carcinoma (Figure 3a). Men with adenocarcinoma displayed a wide high peak with maximum risk at 6 to 8 months. In contrast, after a gradual increase, the highest peak was noted at 22 to 24 months in women with adenocarcinoma. The hazard rate then moderately decreased subsequently (Figure 3b).
Figure 3.
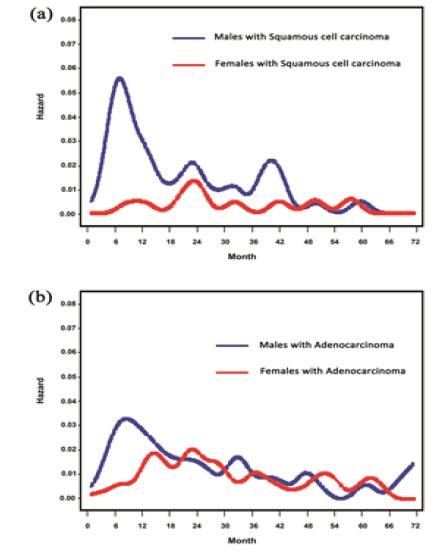
(a) Smoothed hazard rate estimates for first event by sex in histology: Squamous cell carcinoma; (b) Smoothed hazard rate estimates for first event by sex in histology: Adenocarcinoma
As for smoking history in women, the hazard rate curves, displaying a broad peak without an early sharp peak, were quite similar in smokers and non-smokers. However, the timing of maximum peak was 16 to 18 months after surgery in female smokers, which was about 6 months earlier than the peak in female non-smokers (Figure 4). In men, no noticeable peak was found during the follow-up period owing to the small number of non-smokers.
Figure 4.
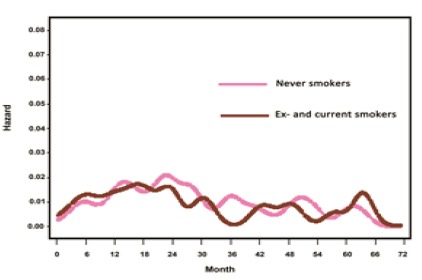
Smoothed Hazard Rate Estimates for First Event by Sex in Smokers
Discussion
A number of studies have reported that female patients with NSCLC live significantly longer than male patients after surgical or non-surgical treatment. Several factors might account for the better survival of women. Mc Govern et al., (2009) reported that a survival advantage for women was confirmed only in stage I patients. Cardarella et al., (2007) reported that women with adenocarcinoma had better outcomes than women with squamous cell carcinoma. Ulas et al., (2015) showed that survival duration was longer and chemotherapy response was better in women without having anemia or comorbidities. On the other hand, Nakamura et al., (2011) demonstrated that survival was significantly better in women than in men, suggesting that women’s survival benefits were not limited to patients with stage I disease or adenocarcinoma, even after adjusting for the effects of life expectancy. At present, the reason why women with NSCLC live significantly longer than men remains elusive. While a number of studies have shown differences in overall survival between the sexes, few studies have focused on the timing of recurrence after surgery. Nearly all previous studies analyzed the risks of recurrence in relation to the time after surgery with the use of cumulative incidence curves plotted by the Kaplan-Meier method. However, such curves do not provide direct information on changes in event-specific hazard rates during the follow-up period. Moreover, it is very difficult to evaluate postoperative recurrence patterns on the basis of the shapes of cumulative incidence curves. We therefore studied event dynamics, estimated by calculating event-specific hazard rates during the follow-up period (Simes and Zelen, 1985), to evaluate sex-related disparities in the timing of recurrence after surgery.
By using this method, we previously reported that the recurrence patterns were suggested to differ between the sexes (Watanabe et al., 2016). The present study showed the hazard rate in the stage IA group remained low during the follow-up period in both sexes. Moreover, the times with the highest risks of recurrence after surgery in the stage IB group and stages IIA to IIIA group were suggested to differ between men and women, with a sharp peak in the first year in men and a broad peak from 2 to 3 years after surgery in women. These sex-dependent findings were also confirmed in the analyses according to histological type and smoking history. New evidence from our study suggests that the reason why women have good outcomes is not necessarily attributed to the high rates of adenocarcinoma or stage IA disease among women. The delayed times of peak recurrence in women within subsets of patients with the same pathological stage, histological type, and smoking history might result in better outcomes.
The most important question is ‘why does the timing of recurrence after surgery differ between the sexes?’ One reason for the difference might be that a higher proportion of women than men had histological subtypes associated with a long DFI, particularly adenocarcinoma, which was present in a higher proportion of female patients. The International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) jointly issued a new tumor classification system in 2011 which characterized lung adenocarcinomas as a mixture of five histopathological subtypes (Travis et al., 2011). Several studies demonstrated significant differences in outcomes among the histopathological subtypes (Woo et al., 2012; Urer et al., 2012; Mansuet-Lupo et al., 2014). Among the invasive adenocarcinoma subtypes, micropapillary (MIP) and solid (SOL) predominant subtypes had poorer outcomes than the other subtypes. In contrast, lepidic predominant invasive adenocarcinoma (LEP) had better outcomes than the other subtypes. Moreover, adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) had a DFS rate of 100% in these studies. Unfortunately, the information about the pathological findings in our database was not sufficient, because the subtype of adenocarcinoma was not recorded at the time of surgery. Therefore, the lack of information on the subtype of adenocarcinoma prevented us from clarifying the role of subtype in sex-related differences in outcomes. In addition, the hypothesis about histological subtype cannot explain why sex-related differences in the timing of recurrence were found even in patients with squamous cell carcinoma.
The second possible reason for the difference in the time of recurrence is sex-related disparities in the dormant state of cancer cells after surgery. To date, many concepts have been proposed to convincingly explain the clinical behavior of cancer, such as tumor homeostasis, tumor dormancy, and surgery-related enhancement of metastatic growth (Hedley and Chambers, 2009). The fact that the first peak of recurrence occurs within 1 year after surgery suggests that surgical invasion disrupts homeostasis, accelerating the proliferation of dormant tumor cells. Sex-dependent differences in the recurrence patterns found in our study can be explained by the gender difference in the interval during which residual tumor cells proliferated and micrometastases developed after entering a transient state of dormancy. The sex-related inner milieu of the host might act differently on residual tumor cells in men and women, resulting in different durations of the micrometastatic dormancy phase and the different timing of the recurrence peak. At present, however, the detailed mechanisms underlying the hypothesis of tumor dormancy remain to be clarified.
The third reason for the different times of recurrence might be related to hormonal status. The exact mechanism by which estrogens may be involved in lung carcinogenesis is not clear, but some investigators have reported that estrogens and estrogen receptors may play an important role in the pathogenesis of lung cancer (Seow et al., 2002; Siegfried et al., 2009). In addition, different concentrations of sex hormones have been proposed to be a major factor underlying the gender-related disparities in lung cancer susceptibility (Patel, 2005). However, Stabile et al., (2002) has offered conflicting data regarding the expression and potential influence of hormonal factors in the development of lung cancer.
Another notable result of our study was that despite the similar hazard rate curves of smokers and non-smokers among women, the peak timing of recurrence appeared to be about 6 months earlier in smokers than in non-smokers. The correlation between smoking and lung cancer is very obvious. Smoking accounts for about 90% of global lung cancer deaths in men and 70% of such deaths in women (Ezzati and Lopez, 2003). Henschke et al., (2006) reported that as compared with males, lung cancer developed in females with a lower amount of smoking, and the average age of female patients tended to be much younger. Several explanations for the sex-related differences in lung cancer have been proposed, and females have been reported to have higher activities of specific enzymes such as Cytochrome P450 that react with carcinogen (Mollerup et al., 1999), greater formation of DNA adducts that are built up by the direct action of carcinogen on DNA (Kure et al., 1996), and less DNA repair capacity (Spitz et al, 2003; Planchard et al., 2009). On the other hand, lung cancer in never-smokers is more common in women. Several studies have demonstrated that lung cancer in never-smokers is associated with better survival than that in smokers (Tammemmagi et al., 2004; Zell et al., 2005). As major clinicopathological differences and differences at the molecular level have been observed between never-smokers and smokers, these studies imply that lung cancer in never-smokers is a separate entity from lung cancer in smokers (Yano et al., 2008). Differences in the timing of recurrence in women in our study might again suggest that lung cancer in never-smokers had a longer DFI than lung cancer in smokers.
An important limitation of our study is that it was retrospective and therefore subject to the effects of lead-time and length-time bias. In this study, we measured all hazard rate levels at 2- month intervals. As the timing of events depends primarily on the timing of clinic visits or imaging studies, follow-up and analysis at shorter assessment intervals would be needed to more precisely estimate the risk of recurrence. Another limitation of the study is the relatively small number of female smokers. Actually, the sample size can markedly affect the hazard rate and the peak times of recurrence. Even though recurrence dynamic of smokers and non-smokers may different in our study, further studies with larger sample sizes would be required to confirm this finding. Despite its limitations, however, our study provides a preliminary insight into sex-related differences in the timing of recurrence after surgery for NSCLC. Lung cancer appears to be a heterogeneous disease associated with remarkable differences in outcomes among individuals. As the methodology for the personalized treatment of lung cancer rapidly becomes accepted, individually designed follow-up programs will most likely be required. There is no doubt that a randomized, prospective study in a larger number of subject is needed to determine risk factors that affect the timing of recurrence. If confirmed in a prospective setting, our findings might have some impact on the future design of follow-up strategies for NSCLC.
In conclusion, we found that the hazard rate and the peak times of recurrence after resection of NSCLC differed considerably between men and women. Our results indicate that the delayed time of peak recurrence in women, as represented by women having a longer DFI than men within subsets of the same disease stage, histological type, and smoking status, might account for the better survival in women.
Statement conflict of Interest
We have no conflict of interest to declare.
References
- 1.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the southwest oncology group experience. J Clin Oncol. 1991;9:1618–26. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 2.Cardarella A, Crocetti E, Comin CE, et al. Gender differences in non-small cell lung cancer: A population-based study. Eur J Surg Oncol. 2007;33:763–8. doi: 10.1016/j.ejso.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Demicheli R, Fornili M, Ambrogi F, et al. Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol. 2012;7:723–30. doi: 10.1097/JTO.0b013e31824a9022. [DOI] [PubMed] [Google Scholar]
- 4.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;13:847–52. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson MK, Skosey C, Hoffman PC, Golomb HM. Sex-associated differences in presentation and survival in patients with lung cancer. J Clin Oncol. 1990;8:1402–7. doi: 10.1200/JCO.1990.8.8.1402. [DOI] [PubMed] [Google Scholar]
- 6.Hedley BD, Chambers AF. Tumor dormancy and metastasis. Adv Cancer Res. 2009;102:67–101. doi: 10.1016/S0065-230X(09)02003-X. [DOI] [PubMed] [Google Scholar]
- 7.Henschke CI, Yip R, Miettinen OS. Women's susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. 2006;296:180–4. doi: 10.1001/jama.296.2.180. [DOI] [PubMed] [Google Scholar]
- 8.Kure EH, Ryberg D, Hewer A, et al. p53 mutations in lung tumours: relationship to gender and lung DNA adduct levels. Carcinogenesis. 1996;17:2201–5. doi: 10.1093/carcin/17.10.2201. [DOI] [PubMed] [Google Scholar]
- 9.Mansuet-Lupo A, Bobbio A, Blons H, et al. The new histological classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: the experience of a French cohort. Chest. 2014;146:633–43. doi: 10.1378/chest.13-2499. [DOI] [PubMed] [Google Scholar]
- 10.McGovern SL, Liao Z, Bucci MK, et al. Is sex associated with the outcome of patients treated with radiation for nonsmall cell lung cancer? Cancer. 2009;115:3233–42. doi: 10.1002/cncr.24361. [DOI] [PubMed] [Google Scholar]
- 11.Mollerup S, Ryberg D, Hewer A. Sex differences in lung CYP1a1 expression and DNA adduct levels among lung cancer patients. Cancer Res. 1999;59:3317–20. [PubMed] [Google Scholar]
- 12.Nakamura H, Ando K, Shinmyo T, et al. Female gender is an indepent prognostic factor in non-small-cell lung cancer: a meta-analysis. Ann Thorac Cardiovasc Surg. 2011;17:469–80. doi: 10.5761/atcs.oa.10.01637. [DOI] [PubMed] [Google Scholar]
- 13.Ouellette D, Desbiens G, Emond C, Beauchamp B. Lung cancer in women compared with men: stage, treatment, and survival. Ann Thorac Surg. 1998;66:1140–3. doi: 10.1016/s0003-4975(98)00557-8. [DOI] [PubMed] [Google Scholar]
- 14.Paesmans M, Sculier JP, Libert P, et al. Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients the European lung cancer working party. J Clin Oncol. 1995;13:1221–30. doi: 10.1200/JCO.1995.13.5.1221. [DOI] [PubMed] [Google Scholar]
- 15.Patel JD. Lung cancer in women. J Clin Oncol. 2005;23:3212–8. doi: 10.1200/JCO.2005.11.486. [DOI] [PubMed] [Google Scholar]
- 16.Planchard D, Loriot Y, Goubar A, Commo F, Soria JC. Differential expression of biomarkers in men and women. Semin Oncol. 2009;36:553–65. doi: 10.1053/j.seminoncol.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Ramlau-Hansen H. Smoothing counting process intensities by means of kernel functions. Ann Statist. 1983;11:453–66. [Google Scholar]
- 18.Seow A, Poh WT, Teh M, et al. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: evidence for a protective effect of soy in nonsmokers. Int J Cancer. 2002;97:365–71. doi: 10.1002/ijc.1615. [DOI] [PubMed] [Google Scholar]
- 19.Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol. 2009;36:524–31. doi: 10.1053/j.seminoncol.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simes RJ, Zelen M. Exploratory data analysis and the use of the investigator's primer. J Clin Oncol. 1985;3:1418–31. doi: 10.1200/JCO.1985.3.10.1418. [DOI] [PubMed] [Google Scholar]
- 21.Spitz MR, Wei Q, Dong Q, Amos CI, Wu X. Genetic susceptibility to lung cancer: the role of DNA damage and repair. Cancer Epidemiol Biomarkers Prev. 2003;12:689–98. [PubMed] [Google Scholar]
- 22.Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–50. [PubMed] [Google Scholar]
- 23.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest. 2004;125:27–37. doi: 10.1378/chest.125.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest. 2005;128:370–81. doi: 10.1378/chest.128.1.370. [DOI] [PubMed] [Google Scholar]
- 25.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/ American thoracic society/ European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulas A, Tokluoglu S, Kos M, et al. Lung cancer in women, a different disease: survival differences by sex in Turkey. Asian Pac Cancer Prev. 2015;16:815–22. doi: 10.7314/apjcp.2015.16.2.815. [DOI] [PubMed] [Google Scholar]
- 27.Urer HN, Kocaturk CI, Gunluoglu MZ, et al. Relationship between lung adenocarcinoma histological subtype and patient prognosis. Ann Thorac Cardiovasc Surg. 2014;20:12–8. doi: 10.5761/atcs.oa.12.02073. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe K, Tsuboi M, Sakamaki K, et al. Postoperative follow-up strategy based on recurrence dynamics for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2016;49:1624–31. doi: 10.1093/ejcts/ezv462. [DOI] [PubMed] [Google Scholar]
- 29.Woo T, Okudela K, Mitsui H, et al. Prognostic value of the IASLC/ ATS/ ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int. 2012;62:785–91. doi: 10.1111/pin.12016. [DOI] [PubMed] [Google Scholar]
- 30.Yano T, Miura N, Takenaka T, et al. Never-smoking nonsmall cell lung cancer as a separate entity: clinicopathologic features and survival. Cancer. 2008;113:1012–8. doi: 10.1002/cncr.23679. [DOI] [PubMed] [Google Scholar]
- 31.Zell JA, Ou SH, Ziogas A, Anton-Culver H. Epidemiology of bronchioloalveolar carcinoma: improvement in survival after release of the 1999 WHO classification of lung tumors. J Clin Oncol. 2005;23:8396–8405. doi: 10.1200/JCO.2005.03.0312. [DOI] [PubMed] [Google Scholar]


