Abstract
Background
Breast cancer is affected by the immune system in that different cytokines play roles in its initiation and progression. Interleukin-10 (IL-10), an anti-inflammatory cytokine, is an immunosuppressive factor involved in tumorigenesis. The present study was conducted to investigate the gene silencing effect of a small interference RNA (siRNA) targeting IL-10 on the apoptotic pathway in breast cancer cell line.
Methods
The siRNA targeting IL-10 and a glyceraldehyde 3-phosphate dehydrogenase (GAPDH) clone were introduced into MDA-MB-231 cells. Real-time PCR assays were used to determine IL-10 and GAPDH gene expression levels, in addition to those for protein kinase B (AKT), phosphoinositide 3-kinase (PI3K), B-cell lymphoma 2 (Bcl2), caspase-3 and caspase-9 genes related to apoptosis.
Results
Inhibition of IL-10 by the siRNA accelerated apoptosis and was accompanied by significant increase in caspase-3 and caspase-9 and a significant decrease in PI3K, AKT and Bcl2 expression levels compared to the non-transfected case.
Conclusions
In conclusion, the production of IL-10 may represent a new escape mechanism by breast cancer cells to evade destruction by the immune system. IL-10 gene silencing causes down regulation of both PI3K/AKT and Bcl2 gene expression and also increases the Bbc3, BAX caspase3, and caspase 3 cleavage expression levels. IL–10 might represent a promising new target for therapeutic strategies.
Keywords: Interleukin 10, small interference RNA, real time PCR-caspase-3, caspase-9
Introduction
Breast cancer is one of the most common cancers worldwide (Torre et al., 2015). It can be divided into different subtypes based on molecular classification and on the gene expression profiles (Rychly et al., 2008). Triple-negative breast cancer (TNBC) is an aggressive subtype, and is occurred in approximately 20-25% of patients (Korlimarla et al., 2016) resulting in poorer outcomes than other subtypes (Simon et al., 2016). The TNBC is characterized by lack of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) expressions (Korlimarla et al., 2016; Lasham et al., 2016). Patients with TNBC lack the appropriate targets for targeted treatments such as endocrine therapy or trastuzumab (Liedtke et al., 2008).
The regulation of apoptotic and anti-apoptotic factors by protective cytokines has been well documented (Puthier et al., 1999) for instance Interleukin(IL)-10, IL-6 and tumor necrosis factor-α (TNF-α) have anti-apoptotic effect on a variety of tumors (Borsellino N1, 1995; Alas and Bonavida, 2003). Several reports suggest that modulation of the immune response is vital in initiation and progression of breast cancer (Hamidullah et al., 2012). Immuno-regulatory cytokines are important components of biological situation associated with breast cancer (Rao et al., 2006). Cytokines including IL-2, IL-6, IL-8, IL-10, and TNF-α are known to play important role in synchronized way in breast carcinogenesis (Carpi et al., 2009).
The IL-10 is immunosuppressive and anti-inflammatory cytokine. It inhibits antigen presentation to T cells via other cytokines (Ralph et al., 1992) and its up-regulation is associated with tumorigenesis, autoimmunity, and transplantation resistance (Katsikis et al., 1994; Yang et al., 2003). The IL-10 is produced mostly by monocytes, to some degree by lymphocytes (Fiorentino et al., 1989; Reddy et al., 2001) and by human tumor cells (Chen et al., 1994; Heckel et al., 2011). However, other cancer cells such as ovarian cancer did not express IL-10 transcripts (Merogi et al., 1997). High serum levels of IL-10, IL-6 and TNF-α in the cancer patients are correlated with their survival outcome, resistant to the cytotoxicity of the chemotherapeutic drugs.
The apoptotic/anti-apoptotic B-cell lymphoma 2 (Bcl-2) family members have been involved in the drug-resistant phenotype of many malignancies (Reed, 1995). The serine/threonine protein kinase AKT, a member of the phosphoinositide 3-kinase (PI3K) pathway, is involved in different cellular processes including cell proliferation and apoptosis (Franke et al., 2003). The activation of PI3K/Akt stimulates the cell survival via the phosphorylation and inactivation of several pro-apoptotic proteins, including Glycogen synthase kinase 3 (GSK-3), Bcl-2-associated death promoter (BAD) and caspase-9 (Cardone et al., 1998; Szanto et al., 2009; Lee et al., 2013). The hyper activation of PI3K/AKT is occurred in cancer (Sliva et al., 2002), therefore the inhibition of PI3K/Akt pathway may provide a new therapeutic approach for cancer treatment.
Small interference RNA (siRNA) is a genetic tool to facilitate the post-transcriptional sequence specific gene silencing (Okamoto and Murawaki, 2012). Therefore, this technique might be used for the inhibition of a specific gene in vitro and in vivo in order to understand its function. In siRNA process, a double stranded RNA combines with its homologous mRNA leading to mRNA degradation or silence of the translation process (Elbashir et al., 2001). This study aimed to evaluate the apoptotic gene regulation in human TNBC cell lines via knockdown IL-10 using siRNA.
Materials and Methods
IL-10 and GAPDH SiRNA Transfection
Transient transfection of siRNA was performed using lipofectamine transfection 3000 reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instruction. In brief, triple negative human breast cancer (MDA-MB -231) and human breast epithelial cell lines (MCF10A) were plated in triplicates with cells density of 2 × 104 per well in 12-well plates for 16 hours until cells reached approximately 70% confluence. One hour before transfection the cells were cultured in antibiotic-free medium. The cells were incubated with transfection mixtures containing 20 nM of GAPDH- or IL-10-siRNA for 8 hours, and then the medium was replaced with full culture medium and the cells incubated for 48 hours. Control cells (un-transfected cells) were transfected with lipofectamine 3000 reagent only. The RNA was extracted, reverse transcriptase polymerase chain reaction (RT-PCR) assay and the real time RT-PCR assay were done.
Detection of IL-10 by RT-PCR
To confirm the detection of IL-10 in both MDA-MB-231 and MCF-10 A, total RNA was extracted from non-transfected cell lines. The cDNA was generated from 1μg total RNA using High capacity cDNA reverse transcriptase kit (Applied Biosystem, CA, USA). The PCR reaction was done in 50 μl, contained 20-50ng cDNA, 1X PCR Master Mix (Promega, Madison, USA), 3 mM MgCl2, 300nM of forward primer GTGATGCCCCAAGCTGAGA and reverse primer CCCCCAGGGAGTTCACATG. The PCR was performed with the following cycling profile: incubation at 94°C for 5 min followed by 40 cycles of 1 min denaturation at 95°C, 1 min annealing at 55°C, and 1 min elongation at 72°C. The last cycle was followed by a final extension of 10 min at 72°C. Polymerase chain reaction master mix without cDNA was used as negative control. The PCR products were analyzed on a 2% agarose gel stained with ethidium bromide and visualized by UV-transillumination.
Measurement of caspase-3 activity
The caspase-3 activity was measured in transfected- and nontranfected-MDA-MB-231 cells by using colorimetric assay ab39401 (Abcam, Cambridge, MA 02139-1517, USA) kit according to the manufacturer instructions. In brief, transfected MDA-MB -231 cells were incompletely homogenized in 50 µl cell lysis buffer on ice for 10 minutes. After centrifugation, the protein concentrations were adjusted to 50–200 μg protein per reaction. Fifty microliter of caspase reaction mix containing 10 mM DTT was added to each well. N-Acetyl-Asp-Glu-Val-Asp p-nitroanilide (DEVD-p-NA) (200µM) substrate was added, then after 120 min incubation at 37ºC the plate was measured at 405 nm. Fold-increase in caspase 3 activity can be determined by comparing treated cells results with the level of the untreated control.
Real time RT-PCR
Quantitative real-time PCR reaction was carried out to evaluate the expression levels of GAPDH, IL-10, PI3K, AKT, Bcl2, BAX, caspase9 and caspase3. Total RNA from MDA-MB-231 and MCF10A cells were extracted using Axygen total RNA extraction kit (Axygen, Corning GmbH, Germany) according to the manufacturer’s instruction. The concentration and purity of the extracted total RNA were determined by Nanodrop (Thermo Scientific, USA) in triplicates. The extracted RNA had a 260/280 ratio of 1.9–2.1. Total RNA concentration was then adjusted to 250 µg/μl before storing at –80°C immediately after extraction. The cDNA was synthesized from 1 µg RNA using high capacity cDNA reverse transcription kit (life technology, Applied biosystem, USA) according to the manufacturer’s instruction. Quantitative real-time PCR reaction was used 1μl of cDNA, 300 nM of forward and reverse primers Table 1 in 20 ul of 1X PCR master mix with SYBR Green (Applied biosystem, life technology, USA). All reactions were run in triplicate with reaction profile as pre-denaturation for 2 min at 95°C followed by 40 cycles with 95°C for 10 secs and 60°C for one min. The gene expression results were analyzed using 2-ΔΔCT method using MCF10A as a control and GAPDH as endogenous control (Hafez et al., 2014). The data were expressed as mean fold changes ± standard error for three independent amplifications.
Table 1.
The Primers Sequence Used in This Study
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| PI3K | 5’-CTGTTGAACTGCAGTGCACC-3’ | 5’-CAATCGGTGACTGTGTGGGA-3’ |
| AKT | 5’-CGACCGCACATCATCTCGTA-3’ | 5’-GGACAAGGACGGGCACATTA-3’ |
| Bcl2 | 5’-ACCTACCCAGCCTCCGTTAT-3’ | 5’-GAACTGGGGGAGGATTGTGG-3’ |
| Bax | 5’-GAGCTAGGGTCAGAGGGTCA-3’ | 5’-CCCCGATTCATCTACCCTGC-3’ |
| Caspase3 | 5’-GCTGGATGCCGTCTAGAGTC-3’ | 5’-ATGTGTGGATGATGCTGCCA-3’ |
| Caspase-9 | 5’-ATTGCACAGCACGTTCACAC-3’ | 5’-TATCCCATCCCAGGAAGGCA-3’ |
| GAPDH | 5’-GAA GGT GAA GGT CGG AGT C-3’ | 5’-GAA GAT GGT GAT GGG ATT TC-3’ |
Statistical analyses
Each experiment was performed in triplicat on three independent cluture. Data were reported as means ± SEM. Student’s t-test (two-tailed) was used to compare differences between groups. P < 0.05 was considered statistically significant. Using Graph pad prism 5 software (GraphPad Software, CA, USA) The differences were considered statistically significant when p < 0.05.
Results
Transfection efficiency
To determine the transfection efficiency and the successful delivery of siRNA in MDA-MB-231 cells we used GAPDH as a positive control for gene silencing. The results showed a significant inhibition of GAPDH and IL-10 mRNA expression levels after transfection compared to the non-transfected cells (P<0.05) Figure 1.
Figure 1.
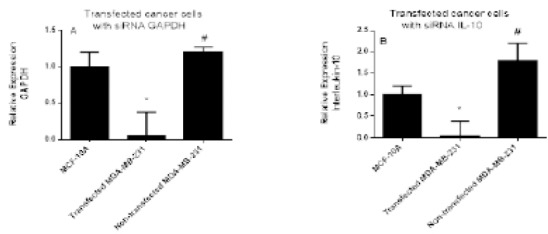
The GAPDH and IL-10 mRNA Expression Levels in Transfected and Non-transfected Cells Measured After Transfection with 20 nM siRNA Targeting GAPDH and IL-10. GAPDH siRNA was used as a positive control for transfection efficiency. Data were presented as mean ±SEM, n = 3. * and # indicated significant difference from control and transfected cells, respectively.
The effect of IL-10 gene silencing on the expression levels of PI3K in non-transfected and transfected MDA-231 cell line was shown in Figure 2A. The silencing of IL-10 induced by siRNA resulted in a significant downregulation of PI3K by 4-fold compared to the non-transfected cells (P<0.002) and by 5-fold compared to MCF10A cells.
Figure 2.
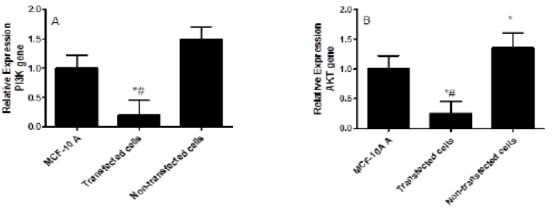
The Effect of IL-10 Gene Silencing on the PI3K (A) and AKT (B) Expression Levels in MDA-231 Cell Line Compared to MCF-10 A and Non-transfected Cells. Data were presented as mean ±SEM, n =3. * indicated significant changes from non-transfected cell line.
The effect of IL-10 knockdown on the AKT expression levels in non-transfected and transfected MDA-231 cell line compared to MCF10A was shown in Figure 2B. The siRNA silencing of IL-10 resulted in significant down regulation of AKT by 5.44-fold compared to the non-transfected cells and by 4-fold compared to MCF10A cells (P<0.002). Whereas, the AKT expression levels was significantly increased in the untreated cells compared to the MCF10A cells (P<0.005).
Figure 3A showed the effect of IL-10 gene silencing on the Bcl2 expression levels in non-transfected and transfected MDA-231 cell line. The silencing of IL-10 induced by siRNA resulted in a significant decrease in the Bcl2 expression level by 3.5-fold compared to non-transfected cells and by 2.5-fold compared to the MCF10A cells (P<0.02). In the non-transfected cells, the high expression levels of Bcl2 was observed compared to MCF10A cells (P<0.002).
Figure 3.
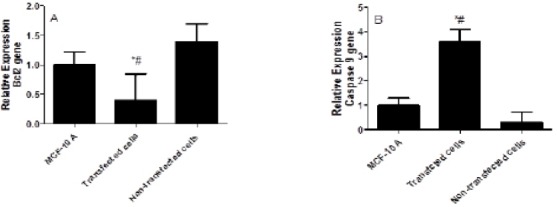
The Effect of IL-10 Gene Silencing on the Bcl2 and Caspase 9 Expression Levels in MDA-231 Cell Line Compared to MCF-10 A and Non-transfected Cells. Data were presented as mean ±SEM, n=3. * indicated significant changes from non-transfected cell line.
The effect of IL-10 gene silencing on the caspase 9 expression levels in non-transfected and transfected MDA-231 cell line was shown in Figure 3B. The silencing of IL-10 resulted in up regulation of caspase 9 by 12-fold compared to the non-transfected cells and by 3.6-fold compared to the MCF10A cells (P<0.005). In the non-transfected cells, the expression levels of caspase 9 was decreased by 70% compared to the MCF10A cells (P<0.001).
The effect of IL-10 silencing on the expression levels of caspase 3 and caspase 3 cleavages were shown in Figure 4 A and B. Gene silencing induced by siRNA lead to increase in the expression levels of caspase 3 mRNA by 31-fold compared to the non-transfected cells and by 6.3-fold compared to the MCF10A cells (P<0.001). The increase in the caspase3 gene expression was associated with the significant increase in its cleavages in transfected cells compared to non-transfected cells.
Figure 4.
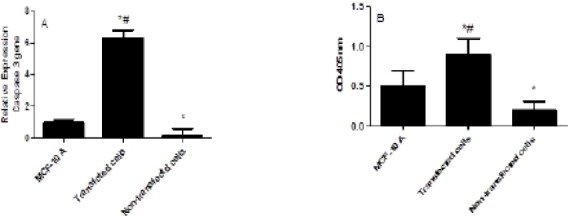
The Effect of IL-10 Silencing on the Expression Levels of Caspase 3 (A) and Cleavage Caspase 3 (B) in MDA-231 Cell Line Compared to MCF-10 A and Non-transfected Cells. Data were presented as mean ±SEM, n = 3. * indicated significant changes from non-transfected cell line.
Figure 5 (A and B) shows the effect of silencing IL-10 on the Bbc3 and BAX genes expression levels. The silencing of IL-10 increased the Bbc3 and BAX expression levels in the transfected cells compared to the non-transfected cells. The knockdown of IL-10 was resulted in increasing in the BbC3 expression levels by 5.4-fold compared to both the MCF10A cells and to the non-transfected cells (P<0.002). In the same manner, the knockdown of IL-10 lead to significant increase in the BAX expression levels by 4.2-fold compared to MCF10A and by 9.3-fold compared to the non-transfected cells (P<0.005). On the other hand, the expression levels of BAX gene in the non-transfected cells was significantly decreased by 2-fold compared to the MCF10A cells (P<0.05).
Figure 5.
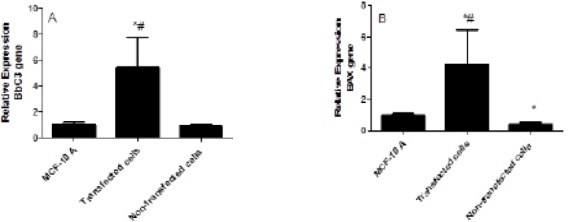
The Effect of IL-10 Silencing on the Expression Levels of BbC3 (A) and BAX (B) in MDA-231 Cell Line Compared to MCF-10 A and Non-transfected Cells. Data were presented as mean ±SEM, n = 3. * indicated significant changes from non-transfected cell line.
Discussion
The expression of cytokines within tumor microenvironment plays an important role in the cancer development and progression. Most tumor cells produce immunosuppressive cytokines which lead to their escape from the host immune response (Yanaihara et al., 2012), therefore the regulation of apoptosis in malignant cells is a vital area in cancer research. Also different cytokines play an important role in the breast cancer initiation and progression.
Anti-inflammatory cytokine such as Interleukin-10 induces immunosuppression and assists the escape mechanism of tumor cells from tumor immune surveillance. Human cancer cells can produce IL-10 but it is mainly produced by monocytes (Heckel et al., 2011). Like several other cytokines, IL-10 can exert dual proliferative and inhibitory effect on breast cancer cells showing its complex role in the cancer initiation and progression. Some studies reported the high IL-10 expression levels in breast cancer paraffin section and its expression is correlated with worse outcome in patients with malignant tumor (Li et al., 2014; Zhao et al., 2015). The IL-10 suppresses the production of other cytokines and inhibits IFN-γ synthesis. It also inhibits nuclear factor-κB (NF-κB) translocation by activating Th-cells and peripheral blood mononuclear cells that considered as a mechanism for inhibiting immediate-early pro-inflammatory response (Hamidullah et al., 2012).
In the present study, IL-10 gene silencing induced by siRNA lead to down regulation of PI3K/AKT gene expression. The PI3K activity is associated with a variety of human tumors as breast cancer (Fry, 2001), lung cancer (Lin et al., 2001), melanomas (Krasilnikov et al., 1999), and leukemia (Martinez-Lorenzo et al., 2000). The PI3K can be activated after the cytokine activation for a specific receptor. The inhibition of PI3K/AKT pathway can be used as new chemotherapeutic targets for different types of cancer. The Akt (protein kinase B, PKB), a downstream kinase of PI3K, is involved in the cancer transformation and has some downstream substrates that may also contribute to malignant transformation (Nicholson and Anderson, 2002). Some of these substrates are Bad, procaspase-9, I-kB kinase (IKK), cAMP response element binding protein (CREB), the fork head family of transcription factors (FKHR/AFX/FOX), glycogen synthase kinase-3 (GSK-3), cyclin-dependent kinase inhibitor 1 (p21Cip1) and Rapidly Accelerated Fibrosarcoma (Raf) (Nicholson and Anderson, 2002).
The Bcl2 is a family of proteins includes the anti-apoptotic and pro-apoptotic molecules. Its members are important regulators for apoptosis and inflammation. The Bcl2 family is classified into three subfamilies (depending on the homology and the function of each protein: The first subfamily has anti-cell death activity e.g. Bcl2, B-cell lymphoma-extra-large (Bcl-xL) and B-cell lymphoma-2-like protein 2 (Bcl2L2). The second subfamily has pro-apoptotic activity e.g. Bax and B-cell lymphoma -2 antagonist killer 1 (Bak). The third subfamily includes pro-apoptotic protein e.g. Bcl2-Interacting Killer (Bik) and BH3 interacting-domain death agonist (Bid) (Tsujimoto, 1998). In the current study, the silencing of IL-10 lead to downregulation of the Bcl2 gene expression. The IL-10 is a known promoter of Bcl-2 expression in the hematopoietic cells (Levy and Brouet, 1994; Cohen et al., 1997). Therefore, the down-regulation of Bcl-2 expression may serve as a possible mechanism for the sensitization of tumor cells to a variety of chemotherapeutic drugs.
The Bcl-2-binding component 3 (Bbc3), also known as p53 upregulated modulator of apoptosis (PUMA), gene encodes a member of the Bcl-2 family of proteins. It can bind to the anti-apoptotic Bcl-2 family members to induce mitochondrial dysfunction and caspase activation (Nakano and Vousden, 2001; Gomez-Lazaro et al., 2005; Belle et al., 2016). In the current study, silencing the IL-10 gene increased the Bbc3 and BAX expression levels in the transfected cells compared to the non-transfected cells. Similarly, PUMA can promote apoptosis either by the direct inhibition of the anti-apoptotic molecules or the direct activation of Bax and Bak (Nakano and Vousden, 2001). The PUMA is involved in various apoptotic responses (Akhtar et al., 2006; Hikisz and Kilianska, 2012), and can play a major role in the control of memory T- and B-lymphocyte survival (Akhtar et al., 2006). Because of Bbc3 gene pro-apoptotic role, it may be a possible drug target for tumor treatment.
The phosphorylation of several substrates by Akt increases the cell resistance to apoptosis. The Akt activation enhances the expression of Bcl-2 anti-apoptotic gene via phosphorylation of cyclic AMP response binding protein (Pugazhenthi et al., 2000). Synergy or additive effects between PI3K/AKT pathway and Bcl-xL in controlling apoptosis were reported in lung cancer (Qian J1, 2009). The mechanism by which IL-10 induces its protective effects against apoptosis are still unknown, but it may be involved in common pathways of diverse drugs use to induce apoptosis. The Bcl-2 plays an important role in the cancer cells survival via increasing their ability to resist different apoptotic stimuli (Alas et al., 2001).
The caspases are a family of genes broadly classified by their known roles in apoptosis (caspase-3, -6,-7, -8, and -9 in mammals), and in inflammation (caspase-1, -4, -5, -12 in humans and caspase-1, -11, and -12 in mice) (McIlwain et al., 2015). The caspases involved in apoptosis can be sub-classified by their mechanism of action as initiator (caspase-8 and -9) or killer caspases (caspase-3, -6, and -7). In the present study, caspase3 (and its cleavage) and caspase9 were up-regulated resulted in silencing of IL-10. Similarly, other study found that the increase levels of IL-10 leads to caspase 3 inactivation via NF-kB inactivation (Bachis et al., 2001). Thus, IL-10 has an intrinsic ability to inhibit directly or indirectly caspase-3 activity, and this mechanism can explain the protective effect of IL-10 on neurons cells (Grilli et al., 2000). Caspase-9 is activated by the dimerization induced when the caspase-9 CARD domain binds to the adapter protein apoptotic protease-activating factor-1 (APAF1) (Shiozaki et al., 2002). Both APAF1 and caspase-9 exist in an inactive form that could be activated by cytochrome c released from the mitochondria after the cells exposed to the stress.
In conclusion, the production of IL-10 may represent a new escape mechanism by breast cancer cells to evade the destruction of cancer cells by the immune system. The IL-10 gene silencing causes down regulation of both PI3K/AKT and Bcl2 genes expression and also increases the Bbc3, BAX, caspase3, and caspase 3 cleavage expression levels. Therefore, IL–10 may be used as a good target for breast cancer treatment.
Acknowledgments
The authors thank the Deanship of Scientific Research at KSU for funding this work (research group project no. RGP-142).
References
- 1.Akhtar RS, Geng Y, Klocke BJ, et al. BH3-only proapoptotic Bcl-2 family members Noxa and Puma mediate neural precursor cell death. J Neurosci. 2006;26:7257–64. doi: 10.1523/JNEUROSCI.0196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9:316–26. [PubMed] [Google Scholar]
- 3.Alas S, Emmanouilides C, Bonavida B. Inhibition of interleukin 10 by rituximab results in down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin's lymphoma to apoptosis. Clin Cancer Res. 2001;7:709–23. [PubMed] [Google Scholar]
- 4.Bachis A, Colangelo AM, Vicini S, et al. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci. 2001;21:3104–12. doi: 10.1523/JNEUROSCI.21-09-03104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belle JI, Petrov JC, Langlais D, et al. Repression of p53-target gene Bbc3/PUMA by MYSM1 is essential for the survival of hematopoietic multipotent progenitors and contributes to stem cell maintenance. Cell Death Differ. 2016;23:759–75. doi: 10.1038/cdd.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borsellino NBA, Bonavida B. Endogenous interleukin 6 is a resistance factor for cis-diamminedichloroplatinum and etoposide-mediated cytotoxicity of human prostate carcinoma cell lines. Cancer Res. 1995;15:4633–9. [PubMed] [Google Scholar]
- 7.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 8.Carpi A, Nicolini A, Antonelli A, et al. Cytokines in the management of high risk or advanced breast cancer: an update and expectation. Curr Cancer Drug Targets. 2009;9:888–903. doi: 10.2174/156800909790192392. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Daniel V, Maher DW, et al. Production of IL-10 by melanoma cells: examination of its role in immunosuppression mediated by melanoma. Int J Cancer. 1994;56:755–60. doi: 10.1002/ijc.2910560524. [DOI] [PubMed] [Google Scholar]
- 10.Cohen SB, Crawley JB, Kahan MC, et al. Interleukin-10 rescues T cells from apoptotic cell death: association with an upregulation of Bcl-2. Immunology. 1997;92:1–5. doi: 10.1046/j.1365-2567.1997.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franke TF, Hornik CP, Segev L, et al. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–98. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 14.Fry MJ. Phosphoinositide 3-kinase signalling in breast cancer: how big a role might it play? Breast Cancer Res. 2001;3:304–12. doi: 10.1186/bcr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Lazaro M, Galindo MF, Fernandez-Gomez FJ, et al. Activation of p53 and the pro-apoptotic p53 target gene PUMA during depolarization-induced apoptosis of chromaffin cells. Exp Neurol. 2005;196:96–103. doi: 10.1016/j.expneurol.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Grilli M, Barbieri I, Basudev H, et al. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci. 2000;12:2265–72. doi: 10.1046/j.1460-9568.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- 17.Hafez MM, Al-Shabanah OA, Al-Harbi NO, et al. Association between paraoxonases gene expression and oxidative stress in hepatotoxicity induced by CCl4. Oxid Med Cell Longev. 2014;2014:893212. doi: 10.1155/2014/893212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamidullah Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat. 2012;133:11–21. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 19.Heckel MC, Wolfson A, Slachta CA, et al. Human breast tumor cells express. IL-10 and IL-12p40 transcripts and proteins, but do not produce IL-12p70. Cell Immunol. 2011;266:143–53. doi: 10.1016/j.cellimm.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Hikisz P, Kilianska ZM. PUMA, a critical mediator of cell death--one decade on from its discovery. Cell Mol Biol Lett. 2012;17:646–69. doi: 10.2478/s11658-012-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsikis PD, Chu CQ, Brennan FM, et al. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994;179:1517–27. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korlimarla A, Prabhu JS, Remacle J, et al. Identification of BRCA1 deficiency using multi-analyte estimation of BRCA1 and its repressors in FFPE tumor samples from patients with triple negative breast cancer. PLoS One. 2016;11:e0153113. doi: 10.1371/journal.pone.0153113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krasilnikov M, Adler V, Fuchs SY, et al. Contribution of phosphatidylinositol 3-kinase to radiation resistance in human melanoma cells. Mol Carcinog. 1999;24:64–9. doi: 10.1002/(sici)1098-2744(199901)24:1<64::aid-mc9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Lasham A, Mehta SY, Fitzgerald SJ, et al. A novel EGR-1 dependent mechanism for YB-1 modulation of paclitaxel response in a triple negative breast cancer cell line. Int J Cancer. 2016;139:1157–70. doi: 10.1002/ijc.30137. [DOI] [PubMed] [Google Scholar]
- 25.Lee YC, Liao PC, Liou YC, et al. Glycogen synthase kinase 3 beta activity is required for hBora/Aurora A-mediated mitotic entry. Cell Cycle. 2013;12:953–60. doi: 10.4161/cc.23945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424–8. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Yu H, Jiao S, et al. Prognostic value of IL-10 expression in tumor tissues of breast cancer patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014;30:517–20. [PubMed] [Google Scholar]
- 28.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 29.Lin X, Bohle AS, Dohrmann P, et al. Overexpression of phosphatidylinositol 3-kinase in human lung cancer. Langenbecks Arch Surg. 2001;386:293–301. doi: 10.1007/s004230100203. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Lorenzo MJ, Anel A, Monleon I, et al. Tyrosine phosphorylation of the p85 subunit of phosphatidylinositol 3-kinase correlates with high proliferation rates in sublines derived from the Jurkat leukemia. Int J Biochem Cell Biol. 2000;32:435–45. doi: 10.1016/s1357-2725(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 31.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2015;7:1–26. doi: 10.1101/cshperspect.a026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merogi AJ, Marrogi AJ, Ramesh R, et al. Tumor-host interaction: analysis of cytokines, growth factors, and tumor-infiltrating lymphocytes in ovarian carcinomas. Hum Pathol. 1997;28:321–31. doi: 10.1016/s0046-8177(97)90131-3. [DOI] [PubMed] [Google Scholar]
- 33.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–94. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto K, Murawaki Y. The therapeutic potential of RNA interference: novel approaches for cancer treatment. Curr Pharm Biotechnol. 2012;13:2235–47. doi: 10.2174/138920112802501944. [DOI] [PubMed] [Google Scholar]
- 36.Pugazhenthi S, Nesterova A, Sable C, et al. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–6. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 37.Puthier D, Derenne S, Barille S, et al. Mcl-1 and Bcl-xL are co-regulated by IL-6 in human myeloma cells. Br J Haematol. 1999;107:392–5. doi: 10.1046/j.1365-2141.1999.01705.x. [DOI] [PubMed] [Google Scholar]
- 38.Qian J1 ZY, Rahman JS, Lu B, Massion PP. Synergy between phosphatidylinositol 3-kinase/Akt pathway and Bcl-xL in the control of apoptosis in adenocarcinoma cells of the lung. Mol Cancer Ther. 2009;8:101–9. doi: 10.1158/1535-7163.MCT-08-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ralph P, Nakoinz I, Sampson-Johannes A, et al. IL-10T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J Immunol. 1992;148:808–14. [PubMed] [Google Scholar]
- 40.Rao VS, Dyer CE, Jameel JK, et al. Potential prognostic and therapeutic roles for cytokines in breast cancer (Review) Oncol Rep. 2006;15:179–85. doi: 10.3892/or.15.1.179. [DOI] [PubMed] [Google Scholar]
- 41.Reddy RC, Chen GH, Newstead MW, et al. Alveolar macrophage deactivation in murine septic peritonitis: role of interleukin 10. Infect Immun. 2001;69:1394–401. doi: 10.1128/IAI.69.3.1394-1401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed JC. Bcl-2 family proteins: regulators of chemoresistance in cancer. Toxicol Lett. 1995;82-83:155–8. doi: 10.1016/0378-4274(95)03551-6. [DOI] [PubMed] [Google Scholar]
- 43.Rychly B, Sidlova H, Danis D. The 2007 World Health Organisation classification of tumours of the central nervous system, comparison with 2000 classification. Cesk Patol. 2008;44:35–6. [PubMed] [Google Scholar]
- 44.Shiozaki EN, Chai J, Shi Y. Oligomerization and activation of caspase-9, induced by Apaf-1 CARD. Proc Natl Acad Sci U S A. 2002;99:4197–202. doi: 10.1073/pnas.072544399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon N, Antignani A, Sarnovsky R, et al. Targeting a cancer-specific epitope of the epidermal growth factor receptor in triple-negative breast cancer. J Natl Cancer Inst. 2016;108:1–8. doi: 10.1093/jnci/djw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sliva D, Rizzo MT, English D. Phosphatidylinositol 3-kinase and NF-kappaB regulate motility of invasive MDA-MB-231 human breast cancer cells by the secretion of urokinase-type plasminogen activator. J Biol Chem. 2002;277:3150–7. doi: 10.1074/jbc.M109579200. [DOI] [PubMed] [Google Scholar]
- 47.Szanto A, Bognar Z, Szigeti A, et al. Critical role of bad phosphorylation by Akt in cytostatic resistance of human bladder cancer cells. Anticancer Res. 2009;29:159–64. [PubMed] [Google Scholar]
- 48.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics 2015. CA Cancer J Clin. 2012;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 49.Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 50.Yanaihara N, Anglesio MS, Ochiai K, et al. Cytokine gene expression signature in ovarian clear cell carcinoma. Int J Oncol. 2012;41:1094–100. doi: 10.3892/ijo.2012.1533. [DOI] [PubMed] [Google Scholar]
- 51.Yang BC, Lin HK, Hor WS, et al. Mediation of enhanced transcription of the IL-10gene in T cells, upon contact with human glioma cells, by Fas signaling through a protein kinase A-independent pathway. J Immunol. 2003;171:3947–54. doi: 10.4049/jimmunol.171.8.3947. [DOI] [PubMed] [Google Scholar]
- 52.Zhao S, Wu D, Wu P, et al. Serum IL-10 predicts worse outcome in cancer patients: A meta-analysis. PLoS One. 2015;10:e0139598. doi: 10.1371/journal.pone.0139598. [DOI] [PMC free article] [PubMed] [Google Scholar]


