Abstract
Purpose:
The work was a comparative study, the aim of which is to evaluate the impact of swallowing exercises on swallowing problems among head and neck cancer patients (HNCPs) after CRT.
Methods:
The sample of 60 HNCPs was equally divided into control and study groups. The investigators met all patients 3 times (before, during and after CRT); swallowing ability was assessed using the Sydney Swallowing Questionnaire (SSQ). The University of Texas, MD Anderson Cancer Center Swallowing Exercise Protocol was explained and demonstrated by the investigators to the study group. All tools used were translated into Arabic. Data analyses were carried out using the statistical package for social sciences (SPSS), program version 20.
Results:
Most of the patients from both groups experienced mild dysphagia during the 1st visit. By the 3rd visit, severe dysphagia (to thin and thick liquids, and soft and hard food) was higher in the control group (73.3%) compared to the study group (26.7%). By the third visit there was statistically significant difference between both groups in swallowing thin liquids (p = 0.01), as well as thick liquids (p = 0.01). At the 1st visit, there was no significant difference regarding swallowing soft food (p = 0.24), hard food (p = 0.17), dry food (p = 0.89) and swallowing Saliva (p = 0.28). While by the 3rd visit, there was significant difference between control and study groups in all parameters.
Conclusions:
Adequate prevention and treatment of dysphagia, with use of swallowing programs, is essential to plan a complete therapeutic programme.
Keywords: Dysphagia, exercises, quality of life
Introduction
Swallowing is a complicated process that needs coordination between more than 25 pairs of muscles in the oral cavity, pharynx, larynx, and esophagus (Kendall, 2008). Dysphagia has been reported in 30-50% of head and neck cancer patients (HNCPs) (Russi et al., 2012). It is defined as the difficulty or impossibility to swallow liquids, food, or medication, which can occur during the oropharyngeal or esophageal phase (Logermann and Larsen, 2012). The work was a comparative study, the aim of which is to evaluate the impact of swallowing exercises on swallowing problems among HNCPs after chemo-radiotherapy (CRT).
Materials and Methods
Between June 2014 and June 2016, 60 patients with locally advanced head and neck carcinoma (LA-HNCPs) were treated by intensity modulated radiation therapy (IMRT). The patients were equally divided into 2 groups, control and study groups. Both groups were treated at Kasr Al-Ainy Center of Clinical Oncology and Nuclear Medicine (NEMROCK).The two groups were treated concurrently with Cisplatin as a weekly sensitizer.
The study design was accepted from our institutional scientific and ethical committees. A written consent was taken from all patients before their recruitment in our study.
Radiation therapy technique and doses
All patients were planned using a seven fields IMRT technique using dynamic Multileaf collimator (MLC) delivery. A dedicated head and neck radiation oncologist delineated all cases on the Eclipse treatment planning system (TPS) (v 8.6). Three Planning target volume (PTV) were contoured: PTV70Gy (2.12Gy/fraction) denoting the gross tumor or lymph node plus a margin of 1 cm. PTV 59.4Gy (1.8Gy/fraction) which includes areas of high risk of harboring microscopic disease and PTV54Gy (1.6Gy/fraction) covering areas at low risk of microscopic disease. Simultaneous integrated boost (SIB) plans were done for all patients with a total of 33 fractions for each plan.
Tools of data collection
The data was collected by using the Sydney Swallow Questionnaire (SSQ) (Wallace et al., 2000). It was developed by Wallace, Middleton and Cook (2000) in order to measure swallowing problems. The questionnaire consists of 17 questions, in the form of visual analog scale. It is a line graded from zero to 100. Each question ranks from 0, 25, 50, 75 and 100 score. The total score was 1700. Each patient marks the grade which describe his/her degree of swallowing difficulty. The questionnaire was translated to the Arabic language. The investigators started to collect data from control group then study group. The investigators met the participating patients 3 times (1st day, day 23 and last day of CRT). During the first visit, the investigators explained the purpose and nature of the study.
Scoring system
Mild swallowing difficulty (0-566.66)
Moderate swallowing difficulty (566.67-1133.33)
Severe swallowing difficulty (1133.34-1700)
The University of Texas, MD Anderson Cancer Center Swallowing Exercise Protocol was explained and demonstrated by the investigators to the study group (Lewin, 2011). In the first visit, patients in the study group were encouraged to adhere to the swallowing exercises regularly. Participating patients were asked to re-demonstrate each exercise in front of the investigators. At the end of the 1st visit, a hard copy of the translated swallowing exercises was given to the patients in the study group. The first visit took between 30-45 minutes, but subsequent visits took around 20 minutes in the study group. Follow up was done by the investigators via the telephone on weekly basis to ensure that the participants were following the learnt exercises.
Statistical analysis
Data analyses were carried out using statistical package for social sciences (SPSS), program version 20. All data entries were checked for accuracy against the original raw data of each patient. Probability level of 0.01 and 0.05 was adopted as the level of significance for all statistical tests done. The significance level of all statistical analysis was at < 0.05 (P-value).
Results
Patients’ and tumor’s characteristics were recorded and all patients were staged using the revised 2002 American Joint Committee on Cancer (AJCC) criteria as shown in Table 1. The median age was 56 years in the control group, while it was 49 years in the study group. The number of illiterate participants was more or less equal in both groups, 19 vs 20 in the control and the study groups, respectively.
Table 1.
Patients’ Characteristics (N=60)
| Control group (N=30; (%)) | Study group (N=30; (%)) | |
|---|---|---|
| Age | ||
| ≤ 40 | 3 (10) | 6 (20) |
| 41-50 | 9 (30) | 12 (40) |
| 51-60 | 12 (40) | 9 (30) |
| > 60 | 6 (20) | 3 (10) |
| Median (range) | 56 (36-74) | 49 (17-68) |
| Gender | ||
| Male | 18 (60) | 15 (50) |
| Female | 12 (40) | 15 (50) |
| Educational level | ||
| Illiterate | 19 (63.33) | 20 (66.67) |
| Read and Write | 4 (13.33) | 2 (6.67) |
| Middle Education | 5 (16.67) | 6 (20) |
| Higher Education | 2 (6.67) | 2 (6.67) |
| T Stage | ||
| T1 | 0 (0) | 0(0) |
| T2a | 0 (0) | 0 (0) |
| T2b | 6 (20) | 3 (10) |
| T3 | 15 (50) | 18 (60) |
| T4 | 9 (30) | 9 (30) |
| N Stage | ||
| N0 | 0 (0) | 0 (0) |
| N1 | 0 (0) | 3 (10) |
| N2 | 9 (30) | 9 (30) |
| N3 | 21 (70) | 18 (60) |
| Tumor Grade | ||
| WHO 2 | 3 (10) | 6 (20) |
| WHO 3 | 27 (90) | 24 (80) |
| Primary Site | ||
| Nasopharynx | 20 (66.67) | 22 (73.33) |
Most of the patients from both groups experienced mild dysphagia during the 1st visit. By the 3rd visit, severe dysphagia (to thin and thick liquids, and soft and hard food) was higher in the control group (73.33%) compared to the study group (26.67%), as shown in Table 2.
Table 2.
The Degree of Dysphagia Among Control and Study Groups
| 1st visit | 2nd Visit | 3rd visit | ||||
|---|---|---|---|---|---|---|
| Control group (N=30; (%)) | Study group (N=30; (%)) | Control group (N=30; (%)) | Study group (N=30; (%)) | Control group (N=30; (%)) | Study group (N=30; (%)) | |
| Mild | 27 (90) | 29 (96.67) | 8 (26.67) | 18 (60) | 2 (6.67) | 10 (33.33) |
| Moderate | 3 (10) | 1 (3.33) | 20 (66.67) | 11 (26.67) | 6 (20) | 12 (40) |
| Severe | 0 | 0 | 2 (6.67) | 1 (3.33) | 22 (73.33) | 8 (26.67) |
Figure 1.
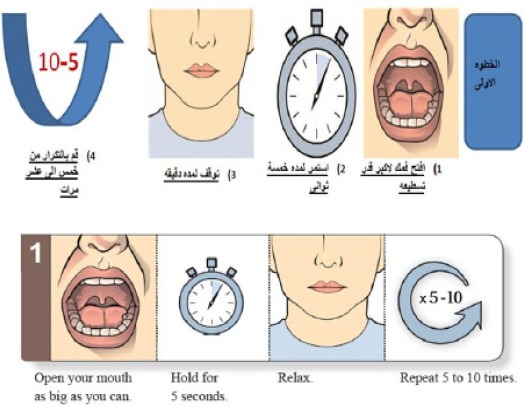
An example of the Translated MD Anderson Cancer Center Swallowing Exercise Protocol. $$$
Table 3 showed that during the 1st visit, there was no significant difference regarding swallowing soft food (p = 0.24), hard food (p = 0.17), dry food (p = 0.89) and swallowing Saliva (p = 0.28) between all patients. While by the 3rd visit, there was significant difference between control and study groups in all parameters. By the third visit there was a statistically significant difference between both groups in swallowing thin liquids (p = 0.01), as well as thick liquids (p = 0.01). This difference didn’t reach statistical significance in the 1st visit.
Table 3.
Comparison of the Swallowing Problems Between the Control and Study Groups, in the 3 Visits
| 1st visit (P-value) | 2nd Visit (P-value) | 3rd visit (P-value) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control Group X±SD | Study Group X±SD | P-value | Control Group X±SD | Study Group X±SD | P-value | Control Group X±SD | Study Group X±SD | P-value | |
| Difficulty in swallowing Liquids: | |||||||||
| a) Thin | 4.17±9.476 | 1.67±6.34 | 0.23 | 36.67±21.50 | 26.67±24.50 | 0.09 | 69.17±23.38 | 49.17±27.45 | 0.01 |
| b) Thick | 3.33±8.64 | 3.33±10.85 | 1.00 | 38.33±23.42 | 27.50±24.87 | 0.08 | 49.17±27.45 | 49.17±27.45 | 0.01 |
| Difficulty in swallowing Food: | |||||||||
| a) Soft | 9.17±17.96 | 4.17±14.80 | 0.24 | 52.50±30.336 | 35.00±31.894 | 0.03 | 81.67±29.312 | 56.67±32.783 | 0.02 |
| b) Hard | 6.67±25.70 | 8.33±21.10 | 0.17 | 68.33±31.44 | 40.00±33.86 | 0.03 | 88.33±24.33 | 65.83±34.41 | 0.03 |
| c) Dry | 10.00±16.86 | 13.33±26.85 | 0.89 | 65.83±29.71 | 42.50±37.22 | 0.01 | 90.00±22.36 | 63.33±34.57 | 0.02 |
| Difficulty in swallowing Saliva | 10.00±16.86 | 5.83±12.60 | 0.28 | 45.00±19.02 | 26.67±22.68 | 0.02 | 71.67±24.33 | 55.00±24.033 | 0.01 |
| Cough or Chocking during swallowing: | |||||||||
| a) Hard food | 3.33±10.85 | 3.33±8.64 | 1.00 | 30.83±29.85 | 14.17±23.38 | 0.02 | 64.17±30.57 | 27.50±30.33 | 0.01 |
| b) Thin liquids | 2.50±7.62 | 5.00±15.26 | 0.42 | 26.67±24.50 | 15.00±20.342 | 0.04 | 62.50±29.17 | 26.67±30.038 | 0.01 |
| Food or liquids come out of the nose | 1.67±6.34 | 0.83±4.56 | 0.56 | 11.67±19.402 | 5.83±15.652 | 0.20 | 35.83±30.57 | 15.83±20.21 | 0.01 |
| Swallowing problems affecting quality of life | 10.83±21.45 | 6.67±17.28 | 0.41 | 57.50±26.38 | 30.83±27.60 | 0.03 | 90.83±22.24 | 57.50±34.209 | 0.02 |
At the 1st visit, there was no statistical significant difference between control and study groups regarding cough or choking during swallowing hard food (p =1.00) and swallowing thin liquids (p = 0.42); yet the difference reached a significant level by the 3rd visit.
Discussion
It has been known that head and neck cancer (HNC) and its treatment lead to alterations in swallowing functioning. All treatment options, including surgery and chemo-radiation therapy (CRT) result in swallowing problems along with aspiration. The cause of dysphagia is clear for patients who underwent resection. Tissue loss after surgical excision, cutting through muscles and nerves, and resulting scar as well as loss of sensation result in severe alteration of swallowing. Factors that predict dysphagia include: Tumor size, nodal status, primary site, type of treatment, extension of treated region, patient characteristics (baseline swallowing function, performance status (PS), smoking and alcohol abuse, age, lean mass and gender) (Stamer et al., 2011).
Radiation therapy also leads to significant acute and late dysphagia, but in a different tissue damage process. Damage of the mucosa and soft tissue usually occurs within the treatment volume during the radiation sessions (Murphy, 2007). The production of reactive oxygen species is due to the inflammatory reaction that usually follows (Sonis and Keefe, 2004). Upon examination, the patients have mucositis, radiation dermatitis, and soft tissue edema. This contributes to acute dysphagia (Isitt et al., 2006). These clinical acute side effects usually subside within 3 months after the end of radiation for most of the patients. Nonetheless, some patients develop dysphagia years after ending their therapy (Rosenthal and Eisbruch, 2006). Late-effect lymphedema, fibrotic rigid tissues and radiation-induced damage to neural structures may explain late occurring dysphagia (Wynn, 2008).
Swallowing efficacy is the main driver for the quality of life (QoL) in HNC survivors (Hunter et al., 2012). It has been rated as the most important functional parameter in HNCPs during and after treatment; thus, it is very important to try to minimize dysphagia and its sequelae (Wilson et al., 2011). Although the use of swallowing exercises are generally believed to be of real benefit, unfortunately, prospective randomized trials addressing dysphagia and its prevention in HNCPs treated with CRT are very few (Murphy and Gilbert, 2009).
Swallowing exercises aiming at improving the motility and mobility of swallowing structures are usually used for HNCPs after finishing their CRT (Pauloski, 2008). These exercises improve tongue and jaw range of motion, as well as base of tongue to posterior wall contact, laryngeal elevation, and pharyngeal contraction (Logemann et al., 2008).
The optimal timing to start swallowing therapy has not been known yet. Logemann and his colleagues (2008) found that most of the patients have some degree of swallow dysfunction at the baseline. Moreover, the peak of swallowing disorder occurs at 3 months after CRT. They noticed that patients with significant swallowing difficulties after 3 months of treatment will not show significant improvement by 12 months after ending their treatment. In their study, they used motion exercises between 1 and 3 month after surgery for oral and oropharyngeal cancer. The researchers found significant link between range of motion exercises and the efficacy of swallowing liquids in oropharyngeal cancers. The study group showed significant improvement in all swallowing parameters. Aspiration rate was decreased by 50% to 75% as an effect of postural techniques. Although their patients were not primarily treated with CRT, they concluded that swallowing efficacy can be improved by swallowing exercises protocols. Denk et al., (1997) reported improved outcomes consistent with early therapy. Thus, speech and swallow rehabilitation preferably considered early after CRT. The use of prophylactic swallowing exercises has been recommended in some centers after the improvements in swallowing function seen in patients following post-treatment exercise protocols (Mittal et al., 2003).
Kulbersh and his colleagues (2006) retrospectively compared 25 patients who received pretreatment swallowing exercises with 12 patients who received standard care. Swallowing was found to be better in patients who started the pretreatment exercises, as assessed by the M. D. Anderson Dysphagia Inventory (MDADI) (70.4 vs 47.1, p-value = 0.0083). The MDADI is a self administrated survey that in particularly evaluates the impact of swallowing problems on the QoL in HNCPs after treatment. A main drawback of this study was that MDADI surveys were administrated to the study group (receiving pretreatment swallowing exercises protocol) years after it has been administrated to the control group, during this time treatment of HNC may have improved in their center. Carroll et al., (2008) also evaluated pretreatment therapy in 18 patients with advanced HNC who received CRT. Nine patients started swallowing exercises 2 weeks before CRT. They assessed swallowing physiologic mechanisms by videofluoroscopic swallowing evaluation. The authors found that patients who performed the swallowing exercises had better tongue base to posterior pharyngeal wall approximation as well as maintained epiglottic inversion, when assessed 3months after treatment. They concluded that these patients had a significantly improved swallowing over the control group, although there was no difference between both groups as regards laryngeal elevation, cricopharyngeal opening, evidence of aspiration and percutaneous endoscopic gastrostomy (PEG) removal rates. However, due to lack of randomization in both studies, it won’t be possible to standardize their results to all HNCPs receiving CRT (Kotz et al., 2012).
Kotz and his colleagues (2012) compared using prophylactic swallowing exercises before and during CRT to treatment as needed after completing CRT in HNCPs. In this study, they enrolled 26 patients with HNC receiving CRT. They assessed swallowing function with functional oral intake scale (FOIS) as well as patients’ PS. They found that patients who were randomized to do prophylactic exercises had statistically significant better swallowing function as well as swallowing-related QoL compared to the other group of patients at 3 and 6 months after CRT (median 3-month intervention score, 7 [range, 5-7], vs median control score, 5 [range, 3-7] [p=0.03]; median 6-month intervention score 7 [range, 6-7], vs median control score, 6 [range, 3-7] [p=0.009]), but not immediately after CRT (median intervention score, 3 [range, 1-7], vs median control group score, 4 [range, 1-6], p=0.88). They noticed that 69% of the patients (6 of 13) in the study group had discontinued exercises by the 5th week of CRT. These 6 patients discontinued exercises due to oral pain, throat discomfort and generalized fatigue accompanying CRT, not as an adverse effect of the exercises. They concluded that improvements in the swallowing function could be seen even without strict adherence to the exercise protocol.
In conclusion, HNC patients undergoing chemo-radiation are at high risk for acute and late-effect dysphagia. Dysphagia has been under-estimated and improperly treated in HNCPs. Adequate prevention and treatment of dysphagia is essential to plan a complete therapeutic programme, by reducing the side effects that may have negative impact on QoL and might affect the overall survival.
References
- 1.Carroll WR, Locher JL, Canon CL, et al. Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118:39–43. doi: 10.1097/MLG.0b013e31815659b0. [DOI] [PubMed] [Google Scholar]
- 2.Denk DM, Schima W, Eibenberger K. Prognostic factors for swallowing rehabilitation following head and neck cancer surgery. Acta Otolaryngol. 1997;117:769–74. doi: 10.3109/00016489709113476. [DOI] [PubMed] [Google Scholar]
- 3.Hunter KU, Schipper M, Feng FY, et al. Toxicities affecting quality of life after chemo-IMRT of oropharyngeal cancer: Prospective study of patient-reported, observer-rated, and objective outcomes. Int J Radiat Oncol Biol Phys. 2012;85:935–40. doi: 10.1016/j.ijrobp.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isitt J, Murphy BA, Beaumont JL, et al. Oral mucositis (OM) related morbidity and resource utilization is a prospective study of head and neck cancer (HNC) patients. Proc Am Soc Clin Oncol. 2006;24:289. (abstr 5539) [Google Scholar]
- 5.Kendall K. Anatomy and physiology of deglutition. In: Kendall LA, editor. Dysphagia assessment and treatment planning. San Diego, CA: Plural Publishing; 2008. pp. 27–34. [Google Scholar]
- 6.Kotz T, Federman AD, Kao J, et al. Prophylactic swallowing exercises in patients with head and neck undergoing chemoradiation. Arch Otolaryngol Head Neck Surg. 2012;138:376–82. doi: 10.1001/archoto.2012.187. [DOI] [PubMed] [Google Scholar]
- 7.Kulbersh BD, Rosenthal EL, McGrew BM, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope. 2006;116:883–6. doi: 10.1097/01.mlg.0000217278.96901.fc. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus CL, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phoniatr Logop. 2002;54:171–6. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
- 9.Lewin J. The University of Texas, MD Anderson Cancer Center Swallowing Exercise Protocol, by Dr. Jan Lewin. 2011. http://www.uhn.ca/PatientsFamilies/Health_Information/Health_Topics/Documents/Swallowing_Exercises_for_Patients_with_Head_and_Neck_Cancer_Receiving_Radiation_Treatment.pdf . [Google Scholar]
- 10.Logemann JA, Pauloski BR, Rademaker AW, et al. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck. 2008;30:148–58. doi: 10.1002/hed.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logemann JA, Larsen K. Oropharyngeal dysphagia: pathophysiology and diagnosis for the anniversary issue of diseases of the esophagus. Dis Esophagus. 2012;25:299–304. doi: 10.1111/j.1442-2050.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- 12.Mittal BB, Pauloski BR, Haraf DJ, et al. Swallowing dysfunction: preventative and rehabilitation strategies in patients with head-and-neck cancers treated with surgery, radiotherapy, and chemotherapy: a critical review. Int J Radiat Oncol Biol Phys. 2003;57:1219–30. doi: 10.1016/s0360-3016(03)01454-8. [DOI] [PubMed] [Google Scholar]
- 13.Murphy BA. Clinical and economic consequences of mucositis induced by chemotherapy and/or radiation therapy. J Support Oncol. 2007;5:13–21. [PubMed] [Google Scholar]
- 14.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;19:35–42. doi: 10.1016/j.semradonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Pauloski BR. Rehabilitation of dysphagia following head and neck cancer. Phys Med Rehabil Clin N Am. 2008;19:889–28. doi: 10.1016/j.pmr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal DI, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–43. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 17.Russi EG, Corvo R, Merlotti A, et al. Swallowing dysfunction in head and neck cancer patients treated by radiotherapy: review and recommendations of the supportive task group of the Italian association of radiation oncology. Cancer Treat Rev. 2012;38:1033–49. doi: 10.1016/j.ctrv.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Sonis ST, Keefe D. Perspectives on cancer therapy-induced mucosal injury pathogenesis, measurement, epidemiology, and consequences for patients. Cancer Supp. 2004;100:1995–25. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 19.Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck clinic and adherence with the speech pathology. Laryngoscope. 2011;121:2131–5. doi: 10.1002/lary.21746. [DOI] [PubMed] [Google Scholar]
- 20.Wallace KL, Middleton S, Cook IJ. Details of the development, validation and recommended analysis of the Sydney Swallow Questionnaire. Gastroeneterology. 2000;118:678–87. doi: 10.1016/s0016-5085(00)70137-5. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients'perspectives. Otolaryngol Head Neck Surg. 2011;145:767–71. doi: 10.1177/0194599811414506. [DOI] [PubMed] [Google Scholar]
- 22.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–10. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]


