Abstract
Objective:
Dietary high fibre and calcium intake has been suggested to reduce colorectal cancer risk. However, there is limited information available regarding the potential of edible canna (Ganyong), with high dietary fibre and calcium content, to act as a preventive agent for colorectal cancer. This experimental study was conducted to investigate the preventive effect of Ganyong in reducing colorectal carcinogenesis with attention to effects on adenomatous polyposis coli (APC) and inducible nitric oxide synthase (iNOS) expression.
Methods:
Thirty male Wistar rats were divided into 5 equal groups; a normal control group without azoxymethane/dextran sodium sulphate (AOM/DSS) induction and Ganyong, a ‘cancer’ control group with AOM/DSS induction only, and three treatment groups with AOM/DSS induction and different percentages (5%, 10% and 20%) of Ganyong. Paraffin-embedded sections of rat colon tissue were analysed by haematoxylin-eosin and immunohistochemical staining against antibodies against APC and iNOS. Variation in rates of APC and iNOS expression were analyzed using the Kruskal-Wallis test followed by the Dunn’s test (SPSS statistic version 24). P<0.05 was considered statistically significant.
Results:
AOM/DSS induction increased the expression of APC (p=0.013) and iNOS (p=0.013) compared to the normal control group. APC expression in the treated groups was lower than in the ‘cancer’ control group (p=0.049), especially in the 10% Ganyong group (p=0.02). In contrast, there was no significant variation among the treated groups regarding iNOS expression. Histopathological features of the colon supported the data for APC and iNOS expression.
Conclusion:
This study indicated potential chemopreventive effects of Ganyong reducing expression of factors contributing to colorectal carcinogenesis.
Keywords: Canna edulis, adenomatous polyposis coli, inducible nitric oxide synthase, colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the Indonesian major health issues causing approximately 15.1 age-standardised mortality per 100,000 population a year (Ferlay et al., 2015). The aetiology of sporadic CRC is predominately rooted by dietary and lifestyle behaviours. Some studies revealed that high dietary intake of fibre and calcium have been associated with lower CRC risk (Haggar and Boushey, 2009; Murphy et al., 2012; Ahearn et al., 2012).
Edible canna or Ganyong (Canna edulis Kerr., family Cannaceae) is a crude herbaceous flowering plant with large small banana-like leave. It has a bright yellow, orange or red coloured flower. It grows from large, thick and underground rootstock that form a tuber and about 90 centimetres to 3 meters tall. This tuber contains various phytochemicals that has been used in traditional medicine for the treatment of many complaints. It also has high dietary fibre and calcium content (Al-Snafi, 2015). However, there was limited available information regarding the role of Ganyong as CRC preventing agent.
Dietary fibre has a protective effect against CRC by increasing faecal bulking, reducing inflammation and shortening the contact time between the carcinogenic agent and the gut (Lima and Gomes-da-Silva, 2005; Suckow et al., 2006; Kaczmarczyk et al., 2012). During the digestion process, the dietary fibre could be transformed to butyrate towards fermentation. Butyrate acts as histone deacetylase inhibitors agent (HDACi); a mechanism by which not only reducing proliferation and β-catenin expression but also increasing apoptosis and Adenomatous Polyposis Coli (APC) expression (Bordonaro at al., 2011; Zhao et al., 2014). In accordance, calcium reduces inflammation, β-catenin and increases E-cadherin, MutL Homolog 1 (MLH1) and MutS Homolog 2 (MLH2) expression (Sidelnikov et al., 2010; Yang et al., 2014).
Azoxymethane (AOM) is a carcinogenic compound. The combination of AOM and dextran sodium sulphate (DSS) has been widely used for CRC induction in vivo study. The pathogenesis of AOM/DSS-induced CRC often linked to APC and Inducible Nitric Oxide Synthase (iNOS). Adenomatous Polyposis Coli inhibits β-catenin thus reduces cell proliferation while iNOS acts as an inflammatory agent that can initiate tumour-associated inflammation (Saad et al., 2012; Chen et al., 2013). The aim of this study was to evaluate the role of APC and iNOS expression in AOM/DSS-induced rat treated with Ganyong.
Materials and Methods
Materials
Fresh Ganyong’s (Canna edulis Kerr.) tubers were supplied by local producers from Sukoharjo, Indonesia. A specimen voucher was deposited at herbarium unit in Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Samples were prepared by drying thick chips of the tubers in an oven (30 °C, 40 h). Subsequently, these dried chips were milled to produce powdered flours. The powdered Ganyong tubers were consumed as a food supplement.
Subject description
Thirty male Wistar rats were divided into 5 equal groups: (1) the control group, (2) positive control cancer group with AOM/DSS induction only, (3) treated with 5% Ganyong, (4) treated with 10% Ganyong and (5) treated with 20% Ganyong. Single 10 mg/kg BW dose of AOM in 10% buffer saline was given intraperitoneally to non-normal group in the fourth week of the experiment. Animals were also administered 2% DSS drink in the week of the experiment.
Wistar rats were obtained from Cancer Research Centre Laboratory, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia. All animals were placed individually and maintained under standard conditions of temperature (25 ± 1.0 °C), humidity (55 ± 10%), 12-h light/dark cycle with free access of rat’s standard food (Comfeed Industries Ltd) and water ad libitum. All equipment that were included in handling and sacrificing were in accordance to European Council Legislation for The Protection of Experimental Animals. All of the animal treatment procedures were approved by Medical and Health Research Ethics Committee (Faculty of Medicine, Universitas Gadjah Mada), decision number KE/FK/557/EC.
Tissue processing
After 14 weeks of treatment, colon tissue of Wistar rats were collected and embedded in paraffin wax using Swiss roll technique (Colnot et al., 2004). Sections were sliced for 5 and 4 µm of thickness used for histological and immunohistochemical staining respectively.
Histological staining
Five-micron paraffin sections were stained with Haematoxylin-Eosin for examining morphological changes of colon tissue according to previous study (Bourroul et al., 2013).
Immunohistochemical staining of APC and iNOS
Primary antibody anti-APC (Biorbyt, orb10109) 0.5 mg/ml and anti-iNOS (ABCAM, ab15323) 0.052 mg/ml in buffer saline were used in this study. Immunostaining was performed using Starr Trek™ Universal HRP Detection System kit (Biocare, Concord, CA, USA) at room temperature according to the manufacturer instruction. Peroxidase activity were detected by incubating samples with 3,3 Diaminobenzedine (DAB) chromogen substrate. It considered positive when there is a formation of a brown precipitate. Finally, sections were counterstained with haematoxylin and DPX mounted.
Evaluation of immunohistochemical staining
For evaluation, five and seven areas of each APC and iNOS section were selected randomly in 400X magnification, respectively. Each area was observed by two blinded observers. Data were collected from the modified proportion (Bourroul et al., 2013) sum of positive cell per total cell in the same area with given formula:
Proportion = (Total positive cells/ Total cells) x 100%
Data analysis
The data were shown in median (minimum-maximum) proportion. Data were collected from the mean of 5 and 7 images for APC and iNOS expression respectively. Normality test used Saphiro-Wilk test. APC and iNOS expression were tested by Kruskal-Wallis test followed by Dunn’s test for group comparison. A median difference considered significant when p<0.05 (SPSS statistics version 24).
Results
Histopathological Status of Rat Colon
Thirty paraffin sections of colon tissue from 30 male Wistar rats were used in this research. The histopathological status of CRC was divided into normal, colitis, dysplasia and adenocarcinoma and established under experienced pathologist supervision (Bourroul et al., 2013). Histopathological status of rat colon was summarized in Table 1.
Table 1.
The Profile of APC and iNOS Expression among Groups
| Groups | n | Histophatology (%) | APC Expression | iNOS Expression | |
|---|---|---|---|---|---|
| Normal | 6 | Normal | 100 | No expression | No expression |
| AOM/DSS | 6 | Normal | 16.7 | No expression | No expression |
| Colitis | 33.3 | Cytoplasmic staining of stromal | Cytoplasmic staining of stromal | ||
| Dysplasia | 33.3 | Cytoplasmic staining of stromal, stroma-epithelial border cell, and epithelial cell | Cytoplasmic staining of tumour cell | ||
| Adenocarcinoma | 16.7 | Cytoplasmic staining of epithelial and tumour cell | Cytoplasmic staining of tumour cell | ||
| 5% treated Ganyong | 6 | Normal | 16.7 | No expression | No expression |
| Colitis | 33.3 | Cytoplasmic staining of stromal | Cytoplasmic staining of stromal | ||
| Dysplasia | 16.7 | Cytoplasmic staining of stromal, stroma-epithelial border cell, and epithelial cell | Cytoplasmic staining of tumour cell | ||
| Adenocarcinoma | 33.3 | Cytoplasmic staining of epithelial and tumour cell | Cytoplasmic staining of tumour cell | ||
| 10% treated Ganyong | 6 | Normal | 50 | No expression | No expression |
| Colitis | 33.3 | Cytoplasmic staining of stromal | Cytoplasmic staining of stromal | ||
| Dysplasia | 16.7 | Cytoplasmic staining of stromal, stroma-epithelial border cell, and epithelial cell | Cytoplasmic staining of tumour cell | ||
| 20% treated Ganyong | 6 | Normal | 33.3 | No expression | No expression |
| Colitis | 33.3 | Cytoplasmic staining of stromal | Cytoplasmic staining of stromal | ||
| Dysplasia | 16.7 | Cytoplasmic staining of stromal, stroma-epithelial border cell, and epithelial cell | Cytoplasmic staining of tumour cell | ||
| Adenocarcinoma | 16.7 | Cytoplasmic staining of epithelial and tumour cell | Cytoplasmic staining of tumour cell |
Effect of Ganyong on APC and iNOS expressions
Both APC and iNOS were not expressed in normal control group. Meanwhile, APC was expressed in stromal, epithelial and tumour cells whereas iNOS was expressed in stromal and tumour cells (Figure 1) in non-normal control groups. The immunohistochemical staining profile of APC and iNOS were summarised in Table 1.
Figure 1.
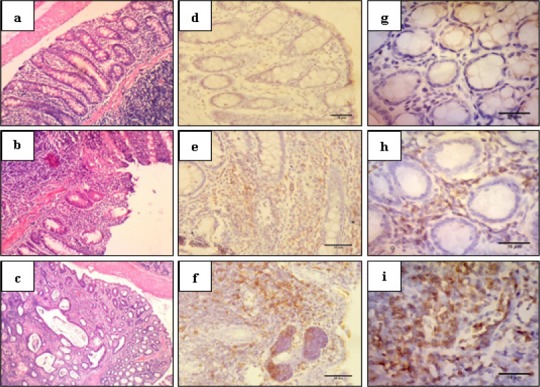
Haematoxylin-Eosin and Immunohistochemical Staining of Colon Tissue in 400X Magnification. The histopathological of colon was considered as normal, colitis, dysplasia and adenocarcinoma (a-c). APC localisation was shown in stromal, stromal-epithelial border, epithelial and tumour cells (d-f). iNOS localisation was shown in stromal and epithelial cells (g-i). Normal colon (a) was not expressed APC (d) and iNOS (g). Colitis colon (b) expressed APC and iNOS in the cytoplasm of stromal. Dysplasia and adenocarcinoma colon (c) expressed APC in the cytoplasm of stromal, stromal-epithelial border and epithelial cells (f), and expressed iNOS in tumour cell, whereas iNOS expression was in the cytoplasm of tumour cell (g).
The strongest expression of APC and iNOS were observed in cancer control group and it was significantly different compared to normal control group (p=0.01). Treated groups showed significantly lower expression of APC than cancer control (p=0.05), especially in the 10% Ganyong group (p=0.02). In contrast, there were no significant differences among all treated groups for iNOS expression compared to cancer control group. The result of Kruskal-Wallis test of both APC and iNOS expression can be seen in Table 2.
Table 2.
Expression of APC and iNOS in Colonic Rat Tissue
| Groups | n | APC | iNOS | ||
|---|---|---|---|---|---|
| Expression (%) | p value | Expression (%) | p value | ||
| Normal | 6 | 0 (0–0.02)a | P=0.01 | 0 (0–0.394)c | P=0.01 |
| AOM/DSS | 6 | 0.23 (0–0.68)a,b | 1.11 (0–9.74)c | ||
| 5% Treated Ganyong | 6 | 0.03 (0–0.34) | 0.2 (0–2.13) | ||
| 10% Treated Ganyong | 6 | 0 (0–0.05)b | p=0.02 | 1.57 (0–3.34) | |
| 20% Treated Ganyong | 6 | 0 (0–0.5) | 0.29 (0–1.67) | ||
Data distribution test with Saphiro-Wilk, p<0.05. Data showed in median (minimum-maximum); Kruskal-Wallis tests significant, p<0.05. Dunn’s test with significant result are marked with a superscript notation; a,b,c different subscript value in the same column indicates significant difference (p<0.05).
Discussion
The combination of AOM and DSS is commonly used for colon cancer induction in rodent model due to its fast and specific carcinogenic effect (Rosenberg et al., 2009; De Robertis et al., 2011; Zhao et al., 2014). Dextran Sodium Sulphate, an acute inflammatory agent, causes temporary epithelial damage, while the combination of AOM/DSS treatment leads to persistent inflammation (Schultz et al., 2004; Tanaka, 2012; Zeineldin et al., 2014). Furthermore, this combination induces APC mutation and nuclear β-catenin localisation leading to carcinogenesis. The localisation of nuclear β-catenin is correlated with iNOS expression (Takahashi et al., 2000; Neufert et al., 2007). Similar to previous studies, the present study demonstrated a significant difference of APC and iNOS expression between control group and AOM/DSS-induced group (cancer control group).
Generally, the expression of APC in Ganyong treated group was lower than cancer control group. However, the significant difference was only found in the group treated with 10% of Ganyong. The decreasing of CRC-incidence due to high dietary fibre intake was reported before (Chen et al., 2004). Butyrate, the major short chain fatty acid (SCFA) produced from Ganyong’s fibre fermentation, was able to inhibit the colorectal carcinogenesis and lowering the CRC risk (Chiaro et al., 2012). It modified any cellular processes through the activation of G-protein couple receptors (GPCRs), HDACi, stimulating the histone acyl transferase and stabilising hypoxia-inducible factor (HIF) (Corrêa-Oliviera et al., 2016). Butyrate also induced p21 expression and inhibited cancer cell proliferation (Chen et al., 2004). However, the fibre must reach the gut then fermented to produce SCFA to be functional as anti-carcinogenic agent (Toden et al., 2014). The role of microbial gut was also important in order to increase the rate of fermentation (Louis et al., 2014). On the other hand, butyrate from Anaerostipes hadrus BPB5 fermentation aggravated the colitis by increasing lipopolysaccharide producer (Zhang et al., 2015).
The dose dependent effect of Ganyong administration was implied in this study. The elevation of Ganyong administration decreased the mutant APC expression due to the increasing of butyrate fermentation rate (Chen et al., 2004). Nonetheless, the expression of APC in 20% Ganyong treated groups was tended to increase. It was assumed that the overproduction of butyrate might have led to butyrate resistance phenomenon (Garland et al., 2006). The cell resisted the anti-proliferative effect of HDACi from butyrate (Bordonaro et al., 2011). Moreover, the anti-proliferative effect has not been expressed by all types of butyrate. Iso-butyrate was reported to have a proliferative effect (Chen et al., 2004).
Ganyong is reported as a good source of calcium. One thousand eight hundred mg of calcium per day is needed to prevent CRC (Chiaro et al., 2011). Calcium was known as an anti-inflammatory agent through its binding to the bile acids. The present study showed that calcium only binds bile acids specifically from fat intake (Fedirko et al., 2015). Besides being as an anti-inflammatory agent, calcium also plays an important role as a cofactor. This study showed that the expression of iNOS was not significantly different between treated groups and cancer control group. Since iNOS are non-dependent-calcium enzyme (Kiemer and Vollmar, 2001), the non-significant effect of Ganyong in iNOS expression was reasonable.
This research only indicated the correlation between the elevation of mutant APC expression with bad prognosis. It detected in the colitis and increased along the transformation of histopathological status (Figure 1). Previous studies revealed that APC mutation could transformed normal epithelial cell to microadenoma, an early sign of dysplastic condition sequence of adenoma or adenocarcinoma (Fodde et al., 2001; Yamada and Mori, 2007; Harpaz and Polydorides, 2010). Moreover, the restoration of APC re-established the crypt homeostasis (Dow et al., 2015) was assumed that mutant APC expression could be used as a pre-neoplastic biomarker.
The present study shows that calcium and dietary fibre-rich Ganyong has been proved in reducing the CRC risk. Dietary fibre and calcium acts as anti-proliferative agent by reducing mutant APC expression. This present study highlighted the potency of Ganyong as chemopreventive agent for CRC through downregulating of mutant APC.
List of Abbreviations
AOM: Azoxymethane
APC: Adenomatous polyposis coli
BPB5: Butyrate-producing bacteria 5
CRC: Colorectal cancer
DSS: Dextran sodium sulphate
GPCRs: G-protein couple receptors
HDACi: Histone deacetylase inhibitors
HIF: Hypoxia-inducible factors
iNOS: Inducible nitric oxide synthase
MLH1: MutL homolog 1
MLH2: MutS homolog 2
SCFA: Short chain fatty acids
Funding Statement
The research was funded by a grant scholarship from the ministry of Research, Technology and Higher Education of Republic of Indonesia (BPPDN-DIKTI 2013).
Acknowledgements
This experiment was performed at Faculty of Medicine, Faculty of Pharmacy and Faculty of Veterinary Medicine, Universitas Gadjah Mada. The authors would like to thank Mr. Surono for his assistance in animal caring.
References
- 1.Ahearn TU, Shaukat A, Flanders WD, Rutherford RE, Bostick RM. A randomized clinical trial of the effects of supplemental calcium and vitamin D3 on the APC/β-catenin pathway in the normal mucosa of colorectal adenoma patients. Cancer Prev Res. 2012;5:1247–56. doi: 10.1158/1940-6207.CAPR-12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Snafi AE. Bioactive components and pharmacological effects of canna indica –an overview. Int J Pharm Toxicol. 2015;5:71–5. [Google Scholar]
- 3.Bordonaro M, Tewari S, Cicco CE, Atamna W, Lazarova DL. A switch from canonical to noncanonical wnt signaling mediates drug resistance in colon cancer cells. PLoS One. 2011;6:1–12. doi: 10.1371/journal.pone.0027308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourroul VS, Bourroul GM, Toloi GC, et al. APC Protein immunoexpression in colorectal adenoma and adenocarcinoma. J Coloproctol. 2013;33:118–25. [Google Scholar]
- 5.Chen HJ, Chen C, Sung M, et al. Canna indica L. attenuates high-glucose- and lipopolysaccharide-induced inflammatory mediators in monocyte/macrophage. J Ethnopharmacol. 2013;148:317–21. doi: 10.1016/j.jep.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZY, Rex S, Tseng C. Krüppel-like factor 4 Is transactivated by butyrate in colon cancer cells. J Nutr. 2004;134:792–8. doi: 10.1093/jn/134.4.792. [DOI] [PubMed] [Google Scholar]
- 7.Chiaro C, Lazarova DL, Bordonaro M. Tcf3 and cell cycle factors contribute to butyrate resistance in colorectal cancer cells. Biochem Biophys Res Commun. 2012;428:121–6. doi: 10.1016/j.bbrc.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Colnot S, Niwa-Kawakita M, Hamard G, et al. Colorectal cancers in a new mouse model of familial adenomatous polyposis: Influence of genetic and environmental modifiers. Lab Invest. 2004;84:1619–30. doi: 10.1038/labinvest.3700180. [DOI] [PubMed] [Google Scholar]
- 9.Corrêa-Oliviera R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5:1–8. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Robertis M, Massi E, Poeta ML, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:1–31. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dow LE, O'Rourke KP, Simon J, et al. APC restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell. 2015;161:1539–52. doi: 10.1016/j.cell.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedirko V, Bostick RM, Long Q, et al. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: A randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;9:280–91. doi: 10.1158/1055-9965.EPI-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: source, methods and major patterns in Globocan 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 14.Fodde R, Smits R, Clevers H. Apc, signal transduction and genetic instability in colorectal cancer. Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 15.Garland CF, Garland FC, Gorham ED. Calcium and vitamin D: their potential roles in colon and breast cancer prevention. Ann N Y Acad Sci. 2006;889:107–19. doi: 10.1111/j.1749-6632.1999.tb08728.x. [DOI] [PubMed] [Google Scholar]
- 16.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Col Rectal Surg. 2009;22:191–7. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harpaz N, Polydorides AD. Colorectal dysplasia in chronic inflammatory bowel disease: pathology, clinical implication, and pathogenesis. Arch Pathol Lab Med. 2010;134:876–95. doi: 10.5858/134.6.876. [DOI] [PubMed] [Google Scholar]
- 18.Kaczmarczyk MM, Miller MJ, Freund GG. The health benefits of dietary fibre: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism. 2012;61:1058–66. doi: 10.1016/j.metabol.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiemer AK, Vollmar AM. Elevation of intracellular calcium levels contributes to the inhibition of nitric oxide production by atrial natriuretic peptide. Immunol Cell Biol. 2001;79:11–7. doi: 10.1046/j.1440-1711.2001.00969.x. [DOI] [PubMed] [Google Scholar]
- 20.Lima MP, Gomes-da-Silva MH. Colorectal cancer: lifestyle and dietary factors. Nutr Hosp. 2005;20:235–41. [PubMed] [Google Scholar]
- 21.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–72. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 22.Murphy N, Norat T, Ferrari P, et al. Dietary fibre intake and risks of cancers of the colon and rectum in the European prospective investigation into cancer and nutrition (EPIC) PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0039361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg DW, Giardina C, Tanaka T. Mouse models for study of colon carcinogenesis. Carcinogenesis. 2009;20:183–96. doi: 10.1093/carcin/bgn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saad N, Esa NM, Ithnin H. Suppression of β-catenin and cyclooxygenase-2 expression and cell proliferation in azoxymethane-induced colonic cancer in rats by rice bran phytic acid (PA) Asian Pac J Cancer Prev. 2012;14:3093–9. doi: 10.7314/apjcp.2013.14.5.3093. [DOI] [PubMed] [Google Scholar]
- 26.Schultz M, Strauch UG, Linde H, et al. Preventive effects of Escherichia coli strain nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin Diagn Lab Immunol. 2004;11:372–8. doi: 10.1128/CDLI.11.2.372-378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidelnikov E, Bostick RM, Flanders WD, et al. Effects of calcium and vitamin D on MLH1 and MSH2 expression in rectal mucosa of sporadic colorectal adenoma patients. Cancer Epidemiol Biomarkers Prev. 2010;19:1022–32. doi: 10.1158/1055-9965.EPI-09-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suckow MA, Weisbroth SH, Franklin CL. chapter 9 - rat nutrition. 2nd ed. San Diego: Elsevier academic press; 2006. The laboratory rat; pp. 219–301. [Google Scholar]
- 29.Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of β-catenin, inducible nitric oxide synthase and cyclooxigenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21:1319–27. [PubMed] [Google Scholar]
- 30.Tanaka T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int J Inflam. 2012;8390:1–12. doi: 10.1155/2012/658786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toden S, Lockett TJ, Topping DL, et al. Butyrylated starch affects colorectal cancer marker beneficially and dose-dependently in genotoxin-treated rats. Cancer Biol Ther. 2014;15:1515–23. doi: 10.4161/15384047.2014.955764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada Y, Mori H. Multistep carcinogenesis of the colon in Apcmin/+mouse. Cancer Sci. 2007;98:6–10. doi: 10.1111/j.1349-7006.2006.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang B, McCullough ML, Gapstur SM, et al. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: the cancer prevention study-II nutrition cohort. J Clin Oncol. 2014;32:2335–50. doi: 10.1200/JCO.2014.55.3024. [DOI] [PubMed] [Google Scholar]
- 34.Zeineldin M, Miller MA, Sullivan R, Neufeld KL. Nuclear adenomatous polyposis coli suppresses colitis-associated tumorigenesis in mice. Carcinogenesis. 2014;35:1881–90. doi: 10.1093/carcin/bgu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Wu Y, Wang J, et al. Accelerated dysbiosis of gut microbiota during aggravation of DSS-induced colitis by a butyrate- producing bacterium. Sci Rep. 2015;6:1–11. doi: 10.1038/srep27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Ai Y, Li L, et al. Inhibition of azoxymethane-induced preneoplastic lesions in the rat colon by a stearic acid complexed high-amylose cornstarch using different cooking methods and assessing potential gene targets. J Funct Foods. 2014;6:499–512. [Google Scholar]


