Abstract
Purpose:
Curcumin (Cur), a herbal ingredient with anticancer properties, has been shown to inhibit growth of malignant cells in vivo and in vitro. However, studies on combination therapy of Cur with chemotherapeutic drugs have been limited. Here, effects of Cur on the cytotoxicity of 5-Fluorouracil (FU) were investigated with epithelial bladder cancer cells (EJ138) in vitro.
Methods:
EJ138 cells were treated with 5 and 15 µM of Cur and/ or 100 µM of FU. Cell viability was measured by sulforhodamine B colorimetric assay. The glucose concentration as an index of cell metabolism was evaluated by an enzymatic method. Total oxidant and antioxidant capacities were estimated by the ferrous oxidation-xylenol (FOX1) method and ferric reducing antioxidant power assay (FRAP), respectively.
Results:
Combination of 5 µM Cur with FU significantly reduced its cytotoxicity in EJ138 cells, while 15 µM Cur caused an opposite increase. Significant increase in glucose concentration at 24 h and decrease in the FRAP value at 48 h incubation was observed in cells treated with FU in combination with Cur. There were no significant changes in total oxidant capacity with the combination therapy.
Conclusion:
Our findings suggest a crucial role of Cur concentration in regulating chemotherapeutic agent-induced cytotoxicity. Further investigations are needed to understand the precise mechanisms of action of Cur and determine appropriate doses with combination therapy for clinical application against human cancers.
Keywords: Curcumin, 5-fluorouracil, bladder cancer
Introduction
Bladder carcinoma is the second most common cancer of the genitourinary tract worldwide (Sahin et al., 2016). This disease is the fourth most frequent cancer among males and the ninth most frequent among females (Jemal et al., 2006). Despite the advances in the management of bladder carcinoma, this cancer has a high rate of recurrence and progression. The recurrence rates of 50-90% have been reported at the first year after transurethral resection of bladder tumor (Manikandan et al., 2017). Accordingly, there are ongoing investigations to optimize the diagnostic and therapeutic strategies for bladder cancer.
5-Fluorouracil (FU) is a chemotherapeutic drug which is used alone or in combination with other drugs with or without radiation to treat bladder cancer (El-Taji et al., 2016). This pyrimidine analogue is similar in structure to uracil and acts as an antimetabolite agent. After intracellular conversion of FU to active metabolites, they interfere with the synthesis of DNA through blocking the conversion of deoxyuridylic acid to thymidylic acid by the enzyme thymidylate synthetase. FU can also interfere with synthesis of various forms of RNA (Reynolds and Parfitt, 1996).
Curcumin (Cur), a yellow-colored phytochemical constituent which is derived from the root of turmeric (Curcuma longa), is known to have antineoplastic effect. This nontoxic natural agent has antioxidant, anti-inflammatory and anti-microbial properties (Bengmark, 2006). Administration of Cur in diets of experimental animals has shown the chemo-preventive effect on the formation of various cancers including skin, mouth, stomach, duodenum, colon, tongue, lung, breast and pituitary cancers (Azuine and Bhide, 1992; Azuine and Bhide, 1994; Huang et al., 1994; Rao et al., 1995). Cur induces apoptosis in human leukemia (Kuo et al., 1996), bladder (Chadalapaka et al., 2008), colon (Hanif et al., 1997) and breast (Ramachandran and You, 1999) cancer cells. However, it inhibits apoptosis in T lymphocyte cells (Sikora et al., 1997) and protects cardiac cells against the toxic effects of Adriamycin (Bachmeier et al., 2007).
Several mechanisms have been proposed for the chemo-preventive and antineoplastic effects of Cur. It has been effective in cancer prevention and increasing the therapeutic responses in cancer patients partly through the inhibition of nuclear factor kappa B (NF-κB) (Feng et al., 2005). This factor is responsible for the induction and progression of some cancers and also in the resistance of some cancer cells to chemotherapy (Luo et al., 2005). Cur has reduced the rate of cancer cells proliferation and cancer metastasis by inhibiting the expression of cyclooxygenase-2 (COX-2) (Claria and Romano, 2005) and matrix metalloproteinase-9 (John and Tuszynski, 2001), (Notarbartolo et al., 2005). Cur has also decreased the activity of telomerase enzyme in some drug-resistant cancer cells leading to the induction of apoptosis in these cells (Ramachandran et al., 2002). Telomerase activity as an important target in cancer researches is involved in almost 85% of human cancers (Kim et al., 1994; Ramachandran et al., 2002). An interesting point about Cur is its safety and tolerability even at high doses (12 grams per day) (Maheshwari et al., 2006).
Some studies have shown that Cur is able to inhibit bladder cancer cells proliferation in cellular and animal models (Sindhwani et al., 2001; Kamat et al., 2007). Cur has revealed inhibitory activities against human bladder cancer cells which were stronger than those of cisplatin and inhibited bladder tumor progression in a rat model of bladder carcinoma (Tian et al., 2008).
Since the effect of Cur in combination with FU for treatment of bladder cancer has not been previously studied, this investigation was aimed to evaluate the possible beneficial effects of this combination therapy to establish more efficient and less toxic therapeutic strategy for bladder cancer.
Materials and Methods
Materials
Malignant epithelial bladder cell line (EJ138) was obtained from Pasteur Institute (national cell bank of Pasteur Institute, Iran). Sulforhodamine B (SRB) colorimetric assay kit, glucose concentration assay kit, total oxidant capacity (TOC) assay kit and ferric reducing antioxidant power (FRAP) assay kit were purchased from East Sage Research Co., Iran. Cur, FU and all other ingredients were of analytical grade and were procured from Sigma-Aldrich Co., USA.
Cell culture and treatment
EJ138 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin and 100 mg/ml streptomycin in a humidified 95% air/5% CO2 incubator at 37°C. Cells were allowed to attach for 12 h before treatment. Cur (98% purity) was dissolved in dimethyl sulfoxide (DMSO) as a 10 mg/ml stock solution and stored at -20°C in a light protected cover. Cur was diluted in complete medium exactly before experiments in a way that the final concentration of DMSO was not more than 1.5%. DMSO concentration was the same in all groups. The final concentrations of Cur were 5 and 15 μM in cell culture (Motterlini et al., 2000). FU was used as the final concentration of 100 μM. Briefly, cells were incubated with FU, Cur or combination of FU and Cur. Control group contained cells without any treatment. Cell viability, glucose concentration, TOC and FRAP were assayed after 24, 48, and 72 h incubation (Zhang et al., 2013).
Particle size analysis
Particle size of the Cur in cell culture medium was determined using a Zetasizer Nano ZS analyzer (Malvern Instruments, Malvern, UK).
Cell viability assay
The SRB assay kit was used for cell viability and cytotoxicity screening based on the measurement of cellular protein content.
Glucose concentration assay
GLU assay kit was provided for measurement of glucose concentration in cells medium. Glucose concentration was measured by enzymatic colorimetric method.
FRAP assay
Total antioxidant capacity was evaluated using FRAP assay kit. This method uses antioxidants as reducing agents in a redox-linked colorimetric method. In this assay, FRAP value was estimated by the reduction of ferric–2, 4, 6-tripyridyl-s-triazine complex at low pH to the ferrous form, which was blue-colored and its absorbance was measured at 593 nm (Safaeian et al., 2016).
TOC assay
TOC was measured by TOC assay kit based on ferrous oxidation-xylenol (FOX1) method. In this method, oxidants present in the sample oxidize the ferrous ion-O-dianisidine complex to ferric ion. The ferric ion makes a colored complex with xylenol orange. The color intensity at 560 nm was measured as the total amount of oxidant capacity in the medium (Safaeian et al., 2015).
Statistical analysis
Data were presented as mean ± standard deviation (SD). For statistically analysis, SPSS version 23.0 was used (SPSS Ltd, Quarry Bay, Hong Kong). Differences between groups were evaluated using a one-way analysis of variance (ANOVA) followed by Tukey post-hoc. A p-value less than 0.05 was considered as significant.
Results
Cur particle size
In particle size analysis, cell culture containing 5 µM Cur did not show any distinct peak compared to untreated cells indicating the solubility of this concentration of Cur While 15 µM Cur displayed some particles with diameter 80-400 nm (average size of 180 nm).
Effect of Cur and/or FU on cell viability
Figure 1 shows the effect of Cur or FU alone or combination of them on viability of EJ138 cells.
Figure 1.
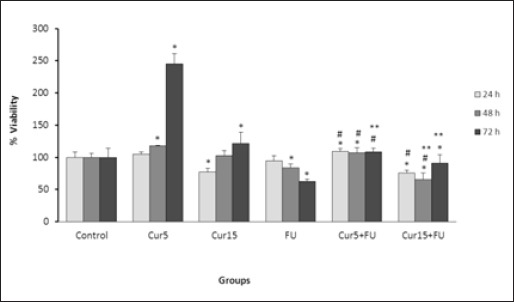
Effect of Cur (5 and 15 µM), FU (100 µM) and Cur+FU on viability of EJ138 cells after 24, 48 and 72 h incubation by sulforhodamine B colorimetric assay. Values are means ± SD from at least three independent experiments. *p < 0.05 versus control (untreated cells), #p < 0.05 versus cells treated with FU alone and **p < 0.05 versus cells treated with Cur alone.
Cell viability was significantly increased in the cells treated with Cur at 5 µM concentration at 48 and 72 h compared with control cells. However, treatment of EJ138 cells with 15 µM of Cur resulted in significant cell growth inhibition after 24 h while increased the cell viability after 72 h incubation.
Treatment with FU caused a significant reduction in cell viability at 48 and 72 h. Generally, the cell viability was significantly higher in cells treated with Cur than in FU treated cells at almost all times of incubation except for 24 h treatment with 15 µM of Cur.
Combination of FU with 5 µM of Cur led to the significant increase in cell viability at 24 and 48 h while the opposite results were observed at the combination of FU with of 15 µM of Cur compared to the control cells. When compared with cells treated with FU alone, combination of Cur 5 µM and FU resulted in a significant reduction in cytotoxicity of FU at all times of incubation while a significant increase in FU cytotoxicity was observed in combination of Cur 15 µM with FU at 24 and 48 h then the trend changed at 72h. When compared with cells treated with Cur alone, combination therapy caused a significant reduction in cell viability. Greater reduction in cell viability was observed with higher concentrations of Cur.
Effect of Cur and/or FU on glucose concentration
Significant increase in medium glucose concentration as an index of cellular metabolism was observed in the cells treated with FU in combination with Cur only after 24 h incubation compared to the untreated cells (Figure 2). No significant difference was found between other groups.
Figure 2.
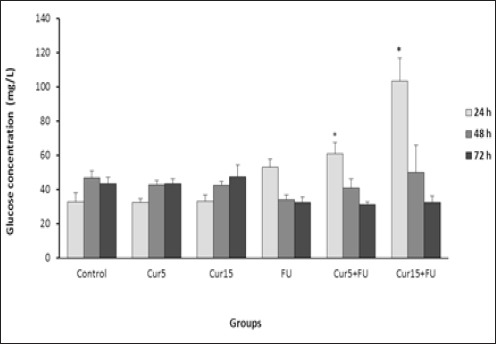
Effect of Cur (5 and 15 µM), FU (100 µM) and Cur+FU on medium glucose concentration (GLU) as an index of EJ138 cells metabolism after 24, 48 and 72 h incubation by enzymatic colorimetric assay. Values are means ± SD from at least three independent experiments. *p < 0.05 versus control (untreated cells)
Effect of Cur and/or FU on total antioxidant capacity
As shown in Figure 3, treatment of EJ138 cells with FU caused a significant decrease in total antioxidant capacity compared to the control cells after 24 and 48 h incubation. Combination of FU with Cur also led to the significant reduction in FRAP value at 48 h. There was not any significant difference in total antioxidant capacity between other groups.
Figure 3.
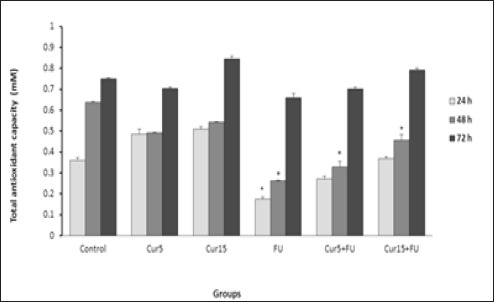
Effect of Cur (5 and 15 µM), FU (100 µM) and Cur+FU on total antioxidant capacity of EJ138 cell medium after 24, 48 and 72 h incubation by colorimetric assay (FRAP). Values are means ± SD from at least three independent experiments. *p < 0.05 versus control (untreated cells)
Figure 4.
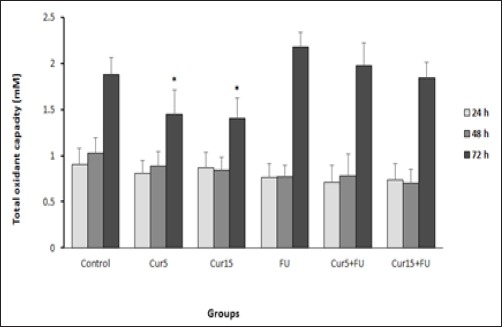
Effect of Cur (5 and 15 µM), FU (100 µM) and Cur+FU on total oxidant capacity of EJ138 cell medium after 24, 48 and 72 h incubation by colorimetric assay (FOX1). Values are means ± SD from at least three independent experiments. *p < 0.05 versus control (untreated cells)
Effect of Cur and/or FU on total oxidant capacity
After treatment of EJ138 cells with Cur, a significant decrease in total oxidant capacity was observed after 72 h incubation compared to the untreated cells. There was not any significant difference in total oxidant capacity between other groups (Figure 3).
Discussion
The present study investigated the efficacy of Cur as the major bioactive compound of turmeric on cytotoxicity induced by FU on bladder cancer cells. The results of this study led to novel findings about contrast result reported regarding effect of Cur in combination chemotherapeutic drugs.
FU is a chemotherapeutic agent widely used for the treatment of many types of cancers including bladder cancer. However, its usage has been somewhat limited due to the progressive tumor cells resistance and drug toxicity on healthy cells (Benson, 2006; Ohtsu, 2008). Many studies have shown the synergistic anticancer activities of polyphenols such as Cur in combination with antineoplastic agents (Patel et al., 2008; González-Vallinas et al., 2013; Vinod et al., 2013; Sivanantham et al., 2015). In spite of protective and proliferative effects in healthy cells, Cur sensitizes the tumor cells to chemotherapeutics with unclear mechanisms (Verma et al., 1998; Aggarwal et al., 2005; Zhou et al., 2017). Some studies have shown that Cur in combination with conventional chemotherapeutic drugs has led to improving the efficacy of chemotherapy and overcoming the drug resistance through various mechanisms including inhibition of cycloxygenase-2, lipoxygenase, ornithine decarboxylase (Gafner et al., 2004), c-Jun/AP-1, c-Jun N-terminal kinase, protein kinase C (Cho et al., 2005) and NF-κB (Bharti et al., 2003; Shakibaei et al., 2013). Recently, it has been proven that Cur chemosensitizes colorectal cancer cells to FU by suppressing the epithelial–mesenchymal transition (EMT) through upregulation of EMT-suppressive miRNAs in FU resistant cell lines (Toden et al., 2015). However, there are some contradictory reports in which Cur has reduced the cytotoxicity of FU in human breast cancer cells (Ferguson and Orlando, 2015).
The present study revealed some novel findings about the role of Cur in cancer treatment. The results of particle size analysis showed that Cur in concentrations higher than 5 µM may be agglomerated to particles with nano-diameters. Treatment of EJ138 cells with 15 µM Cur increased cytotoxicity of 100 µM FU in human bladder cancer cells. The same results were reported about the synergic effect of Cur and FU on head and neck squamous cell carcinoma (Sivanantham et al., 2015), breast cancer (Vinod et al., 2013) and gastrointestinal carcinomas including gastric (Pandey et al., 2015) and colorectal cancers (Shakibaei et al., 2014; Shakibaei et al., 2015).
Our results also showed the increasing in cell viability in cells treated with 5 µM Cur compared to the normal untreated cells. Surprisingly, this concentration of Cur reduced the cytotoxicity of 100 µM FU in human bladder cancer cells. These observations are consistent with some reports about the combined effect of Cur (6 µM) with FU on breast cancer cells (Ferguson and Orlando, 2015). They suggested that different signaling pathways targeted by Cur in various tumors may be the reason for these contradictory results (Koo et al., 2004).
Therefore, our data demonstrated the role of different concentrations of Cur in changing the cytotoxicity of FU in bladder cancer cells. Since these observations happened in one kind of cell line and additionally, considerable changes were not observed in glucose consumption as an index of cell metabolism or in oxidant/antioxidant balance as a possible marker of cellular response to drugs, these contradictory observations are not probably related to signaling pathways targeted by Cur.
In conclusion, the results of this study showed the role of different concentrations of Cur in increasing or decreasing the cytotoxicity of FU in human bladder cancer cells. However, further investigations are required for understanding the exact mechanism of action of Cur and determining its appropriate doses in combination chemotherapy for clinical usage in human cancers.
Acknowledgments
This study was financially supported by Vice Chancellor of Research of Isfahan University of Medical Sciences. The authors would like to thank East Sage Research Corporation for their support and assistance with this project.
Statement conflict of Interest
The authors declare that they have no conflict of interest.
References
- Aggarwal BB, Shishodia S, Takada Y, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-κB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–8. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- Azuine M, Bhide S. Adjuvant chemoprevention of experimental cancer:catechin and dietary turmeric in forestomach and oral cancer models. J Ethnopharmacol. 1994;44:211–7. doi: 10.1016/0378-8741(94)01188-5. [DOI] [PubMed] [Google Scholar]
- Azuine MA, Bhide SV. Chemopreventive effect of turmeric against stomach and skin tumors induced by chemical carcinogens in Swiss mice. Nutr Cancer. 1992;17:77–83. doi: 10.1080/01635589209514174. [DOI] [PubMed] [Google Scholar]
- Bachmeier BE, Nerlich AG, Iancu CM, et al. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol Biochem. 2007;19:137–52. doi: 10.1159/000099202. [DOI] [PubMed] [Google Scholar]
- Bengmark S. Curcumin, an atoxic antioxidant and natural NFκB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor:a shield against acute and chronic diseases. J Parenter Enteral Nutr. 2006;30:45–51. doi: 10.1177/014860710603000145. [DOI] [PubMed] [Google Scholar]
- Benson AB. New approaches to the adjuvant therapy of colon cancer. Oncologist. 2006;11:973–80. doi: 10.1634/theoncologist.11-9-973. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Singh S, et al. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor–κB and IκBαkinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–62. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- Chadalapaka G, Jutooru I, Chintharlapalli S, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–54. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J-W, Park K, Kweon GR, et al. Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT) by inhibiting activation of AP-1:p38 MAP kinase and JNK as potential upstream targets. Exp Mol Med. 2005;37:186. doi: 10.1038/emm.2005.25. [DOI] [PubMed] [Google Scholar]
- Claria J, Romano M. Pharmacological intervention of cyclooxygenase-2 and 5-lipoxygenase pathways. Impact on inflammation and cancer. Curr Pharm Des. 2005;11:3431–47. doi: 10.2174/138161205774370753. [DOI] [PubMed] [Google Scholar]
- El-Taji OM, Alam S, Hussain SA. Bladder Sparing Approaches for Muscle-Invasive Bladder Cancers. Curr Treat Option On. 2016;17:1–14. doi: 10.1007/s11864-016-0390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, Lu Y, Bowman LL, et al. Inhibition of activator protein-1, NF-κB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J Biol Chem. 2005;280:27888–95. doi: 10.1074/jbc.M503347200. [DOI] [PubMed] [Google Scholar]
- Ferguson JE, Orlando RA. Curcumin reduces cytotoxicity of 5-Fluorouracil treatment in human breast cancer cells. J Med Food. 2015;18:497–502. doi: 10.1089/jmf.2013.0086. [DOI] [PubMed] [Google Scholar]
- Gafner S, Lee S-K, Cuendet M, et al. Biologic evaluation of curcumin and structural derivatives in cancer chemoprevention model systems. Phytochemistry. 2004;65:2849–59. doi: 10.1016/j.phytochem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- González-Vallinas M, Molina S, Vicente G, et al. Antitumor effect of 5-fluorouracil is enhanced by rosemary extract in both drug sensitive and resistant colon cancer cells. Pharmacol Res. 2013;72:61–8. doi: 10.1016/j.phrs.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Hanif R, Qiao L, Shiff SJ, et al. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med. 1997;130:576–84. doi: 10.1016/s0022-2143(97)90107-4. [DOI] [PubMed] [Google Scholar]
- Huang M-T, Lou Y-R, Ma W, et al. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–7. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Patho Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-κB and nuclear factor-κB–regulated gene products in IFN-α–sensitive and IFN-α–resistant human bladder cancer cells. Mol Cancer Ther. 2007;6:1022–30. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Koo JY, Kim HJ, Jung K-O, et al. Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J Med Food. 2004;7:117–21. doi: 10.1089/1096620041224229. [DOI] [PubMed] [Google Scholar]
- Kuo M-L, Huang T-S, Lin J-K. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. BBA Mol Basis Dis. 1996;1317:95–100. doi: 10.1016/s0925-4439(96)00032-4. [DOI] [PubMed] [Google Scholar]
- Luo J-L, Kamata H, Karin M. IKK/NF-κB signaling:balancing life and death–a new approach to cancer therapy. J Clin Invest. 2005;115:2625–32. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari RK, Singh AK, Gaddipati J, et al. Multiple biological activities of curcumin:a short review. Life Sci. 2006;78:2081–7. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Manikandan R, Rodriguez O, Parada R, et al. Nonmuscle-invasive bladder cancer:what's changing and what has changed. Urologia. 2017;84 doi: 10.5301/uro.5000213. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–12. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- Notarbartolo M, Poma P, Perri D, et al. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- Ohtsu A. Chemotherapy for metastatic gastric cancer:past, present, and future. J Gastroenterol. 2008;43:256–64. doi: 10.1007/s00535-008-2177-6. [DOI] [PubMed] [Google Scholar]
- Pandey A, Vishnoi K, Mahata S, et al. Berberine and curcumin target survivin and STAT3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-fluorouracil. Nutr Cancer. 2015;67:1295–306. doi: 10.1080/01635581.2015.1085581. [DOI] [PubMed] [Google Scholar]
- Patel BB, Sengupta R, Qazi S, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–73. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- Ramachandran C, Fonseca HB, Jhabvala P, et al. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002;184:1–6. doi: 10.1016/s0304-3835(02)00192-1. [DOI] [PubMed] [Google Scholar]
- Ramachandran C, You W. Differential sensitivity of human mammary epithelial and breast carcinoma cell lines to curcumin. Breast cancer Res Treat. 1999;54:269–78. doi: 10.1023/a:1006170224414. [DOI] [PubMed] [Google Scholar]
- Rao CV, Rivenson A, Simi B, et al. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–66. [PubMed] [Google Scholar]
- Safaeian L, Hajhashemi V, Javanmard SH, Naderi HS. The effect of protocatechuic acid on blood pressure and oxidative stress in glucocorticoid-induced hypertension in rat. Iran J Pharm Res. 2016;15:83–91. [PMC free article] [PubMed] [Google Scholar]
- Safaeian L, Ghasemi-Dehkordi N, Javanmard ShH, Namvar H. Antihypertensive and antioxidant effects of a hydroalcoholic extract obtained from aerial parts of Otostegia persica (Burm.) Boiss Res Pharm Sci. 2015;10:192–9. [PMC free article] [PubMed] [Google Scholar]
- Sahin AF, Altok M, Akdeniz F, Yıldız G, Divrik RF. Second primary cancers in patients with urothelial cancers. Investig Clin Urol. 2016;57:330–5. doi: 10.4111/icu.2016.57.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaei M, Buhrmann C, Kraehe P, et al. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS One. 2014;9:e85397. doi: 10.1371/journal.pone.0085397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaei M, Kraehe P, Popper B, et al. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer. 2015;15:250. doi: 10.1186/s12885-015-1291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaei M, Mobasheri A, Lueders C, et al. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS One. 2013;8:e57218. doi: 10.1371/journal.pone.0057218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora E, Bielak-Zmijewska A, Piwocka K, et al. Inhibition of proliferation and apoptosis of human and rat T lymphocytes by curcumin, a curry pigment. Biochem Pharmacol. 1997;54:899–907. doi: 10.1016/s0006-2952(97)00251-7. [DOI] [PubMed] [Google Scholar]
- Sindhwani P, Hampton JA, Baig MM, et al. Curcumin prevents intravesical tumor implantation of the MBT-2 tumor cell line in C3H mice. J Urol. 2001;166:1498–501. [PubMed] [Google Scholar]
- Sivanantham B, Sethuraman S, Krishnan UM. Combinatorial effects of curcumin with an anti-neoplastic agent on head and neck squamous cell carcinoma through the regulation of EGFR-ERK1/2 and apoptotic signaling pathways. ACS Comb Sci. 2015;18:22–35. doi: 10.1021/acscombsci.5b00043. [DOI] [PubMed] [Google Scholar]
- Tian B, Wang Z, Zhao Y, et al. Effects of curcumin on bladder cancer cells and development of urothelial tumors in a rat bladder carcinogenesis model. Cancer Lett. 2008;264:299–308. doi: 10.1016/j.canlet.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Toden S, Okugawa Y, Jascur T, et al. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015;36:355–67. doi: 10.1093/carcin/bgv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SP, Goldin BR, Lin PS. The inhibition of the estrogenic effects of pesticides and environmental chemicals by curcumin and isoflavonoids. Environ Health Perspect. 1998;106:807. doi: 10.1289/ehp.106-1533252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod B, Antony J, Nair H, et al. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013;4:505. doi: 10.1038/cddis.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cao H, Yu Z, Peng H, Zhang C. Curcumin inhibits endometriosis endometrial cells by reducing estradiol production Iran. Iran J Reprod Med. 2013;11:415–22. [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Zhang S, Shen H, et al. Curcumin inhibits cancer progression through regulating expression of microRNAs. Tumor Biol. 2017;39:1–12. doi: 10.1177/1010428317691680. [DOI] [PubMed] [Google Scholar]


