Abstract
Background:
Despite angiogenesis, many tumours remain hypovascular and starved of nutrients while continuing to grow rapidly. The specific biochemical mechanisms associated with starvation resistance, austerity, may be new biological characters of cancer that are critical for cancer progression.
Objective:
This study aim was to investigate the effect of nutrient starvation on HeLa cells and the possible mechanism by which the cells are able to tolerate nutrient-deprived conditions.
Methods:
Nutrient starvation was achieved by culturing HeLa cells in nutrient-deprived medium (NDM) and cell survival was estimated by using cell counting kit-8. The effect of starvation on cell cycle distribution and the quantitative analysis of apoptotic cells were investigated by flow cytometry using propidium iodide staining. Western blotting was used to detect the expression levels of Akt and phosphorylated Akt at Ser473 (Ser473p-Akt) proteins.
Results:
HeLa cells displayed extremely long survival when cultured in NDM. The percentage of apoptotic HeLa cells was significantly increased by starvation in a time-dependent manner. A significant increase in the expression of Ser473p-Akt protein after starvation was also observed. Furthermore, it was found that Akt inhibitor III molecule inhibited the cells proliferation in a concentration- and time-dependent manner.
Conclusion:
Results of the present study provide evidence that Akt activation may be implicated in the tolerance of HeLa cells for nutrient starvation and may help to suggest new therapeutic strategies designed to prevent austerity of cervical cancer cells through inhibition of Akt activation.
Keywords: HeLa cells, starvation, austerity, Akt-Akt inhibitor III molecule
Introduction
Rapidly growing solid tumours are often inherently hypovascular, thus exhibiting reduced oxygen and nutrient supply (Sutherland, 1988; Vaupel et al., 1989). Rather than impeding cancer progression, such poor metabolic conditions can contribute to genomic instability, impaired cellular repair, mutagenesis, and resistance to chemotherapy, thus worsening prognoses for patients (Yun et al., 1995; Reynolds et al., 1996; Tomida et al., 1996; Yuan et al., 2000). These rapidly growing tumour cells outgrow their blood supply resulting in a reduced nutrients microenvironment. Tumour cells by altering metabolic strategies and inducing angiogenesis can adapt to this stressful environment, thus ensuring survival and proliferation (Izuishi et al., 2000; Awale et al., 2006; Awale et al., 2008; Wek and Staschke, 2010; Calastretti et al., 2014; Jones et al., 2014; Md Tohid et al., 2014; Kim et al., 2015; Farley et al., 2016). Therefore, angiogenesis is regarded as the key step in progression of tumor, and antiangiogenic therapy is the most promising cancer treatment, with extensive studies conducted to prevent tumor angiogenesis (Bergers et al., 1999).
Despite considerable evidence of angiogenesis (Fisher and Berger, 2003; Fleming and Brekken, 2003; Thorpe, 2004; Masamune et al., 2008), many tumours remain hypovascular, and starved of nutrients while continuing to grow rapidly. The therapeutic strategies of angiogenesis inhibition and vascular targeting (Richard et al., 1999; Thorpe, 2004) endeavour to kill tumour cells by selectively depriving them of nutrients. In this light, aggressive tumours, that thrive despite being chronically nutrient-deprived, present a serious therapeutic challenge.
It is well known that tumor cells have high glycolytic activity (Dang and Semenza, 1999). This is because the multiple steps of carcinogenesis expose the tumor cells to insufficient nutrient supply because of increasing demand and insufficient vascularization. Even after the size of tumor increases, the cancer cells’ immediate environment often becomes heterogeneous. In addition, microenvironmental niches often present in some regions of large tumors, displaying a significant gradient of critical metabolites including oxygen, glucose, other nutrients, and growth factors (Helmlinger et al., 1997; Dang and Semenza, 1999).
In 2000, It was shown that certain cancer cell lines demonstrate an extraordinary capacity for survival in nutrient-deprived medium (NDM) (Izuishi et al., 2000). Specific biochemical mechanisms associated with starvation resistance, termed austerity, continue to be elucidated (Magolan and Coster, 2010). Therefore, it is hypothesized that some cancer cells through their progression, in addition to their ability to stimulate angiogenesis, may acquire a tolerance for nutrient deficiency (Calastretti et al., 2014; Jones et al., 2014; Farley et al., 2016).
Since its discovery, the phosphoinositol-3-kinase (PI3K)-Akt pathway has been found to have key regulatory roles in many cellular processes, including proliferation, cell survival and differentiation (Wymann and Marone, 2005). PI3Ks are heterodimeric lipid kinases composed of catalytic and regulatory subunits. The main function of PI3Ks is to phosphorylate the second messenger phosphotidylinositol-4,5-bisphosphate (PI-4,5-P2) to phosphotidylinositol-3,4,5-triphosphate (PI-3,4,5-P3). Through this enzymatic function, PI3K signaling pathway plays an important role in regulating cell responses to external stimuli. Accordingly, PI3K and signaling regulated by PI3K has been targets of therapeutic strategies for a wide range of diseases (Van Meter et al., 2006).
Akt is involved in the pathways responsible for cellular survival through the inhibition of apoptotic processes. It can induce protein synthesis pathways, so it is considered as a key signaling protein in the cellular pathways that lead to general tissue growth. Since it can promote cell survival, Akt has been implicated in many types of cancer as a major factor. It plays a role in the cell cycle and regulates cellular survival by regulating many effectors, e.g. Nuclear Factor-kappa B (NFκB). Under various circumstances, cell cycle arrest can be overcomed by the activation of Akt. Moreover, Akt has also been implicated in tumor development and angiogenesis. Although deficiency of Akt in mice inhibited angiogenesis, its activation enhanced tumor growth and pathological angiogenesis associated with matrix abnormalities in blood vessels and skin (Soroceanu et al., 2007). High expression of Akt was involved in the metabolic adaptation of pancreatic, gastric and colorectal cancer cells subjected to extreme nutrient deprivation (Calastretti et al., 2014; Jones et al., 2014).
Affecting women worldwide, cervical cancer is the fourth most common malignancy. In developing countries, it remains a leading cause of cancer-related death for women (Forouzanfar et al., 2011; Siegel et al., 2016). In this study, HeLa cells, human cervical carcinoma cells, were examined if they are able to survive even under complete nutrient deficiency conditions or not. These investigations raise the possibility that, in addition to angiogenesis, the tolerance for nutrient deficiency might be critical for cancer progression and a new biological character of cancer. Recent advances in identification of molecular signals that mediate the tolerance of cancer cells to nutrient deprived conditions are necessary for the development of new anticancer strategies. In addition, because normal tissues are rarely exposed to extreme deficiency of nutrients, tolerance for nutrient deprived conditions might serve as a new goal for anticancer drugs.
Materials and Methods
Cell culture and nutrient starvation
HeLa cells, obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), were maintained in fresh Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich Co., St Louis, MO, USA) supplemented with 10% Fetal Bovine Serum (FBS, Biosolutions International, Melbourne, Australia), 1% L-glutamine (Sigma-Aldrich Co.), and 1% Penicillin-Streptomycin mixture (Invitrogen, Grand Island, NY, USA). Nutrient starvation was achieved by using NDM which was prepared following the method of Kim et al., (2015). NDM was composed of 265 mg/L CaCl2(2H2O), 0.1 mg/L Fe(NO3)(9H2O), 400 mg/L KCl, 200 mg/L MgSO4(7H2O), 6400 mg/L NaCl, 3500 mg/L NaHCO3, 125 mg/L NaH2PO4, 15 mg/L phenol red, and 25 mmol/L HEPES buffer (pH 7.4), supplemented with MEM vitamin solution (Life Technologies. Rockville, MD, USA). Cells were plated on microplates or culture dishes using either of both media and incubated at 37 ºC in a 5% CO2 humidified incubator.
Cell viability assay
Cell viability assay was done with cell counting kit-8 from Dojindo Molecular Technologies (Dojindo Co., Kumamoto, Japan). Briefly, the cells were seeded in sextuplicate at a density of 5×103 cells per well in 96-well plates and allowed to grow in fresh DMEM medium for 24 h. The cells were then washed with Phosphate Buffered Saline (PBS, pH = 7.4, Sigma-Aldrich, Inc) and the medium was changed to either DMEM or NDM. After the specified time of incubation, the cells were washed again with PBS, then 100 µL of DMEM medium with 10% WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium) solution was added to the wells, and the plate was incubated for a further 2 hours. Then, the absorbance of the wells at 450 nm was measured using HTS Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT). The absorbance is proportional to the number of viable cells in the medium. Cell viability at different time points was expressed as percentage relative to that at the start of starvation (0 hr) which is considered as 100%.
Assessment of cell cycle and apoptosis by flow cytometry
The cells were seeded in triplicate at a density of 2×105 cells per well in 6-well plates and allowed to grow in fresh DMEM medium for 24 h. The cells were then washed with PBS and the medium was changed to either DMEM or NDM. The protocol for the flow cytometric analysis of cell cycle distributions using propidium iodide was used (Sophonnithiprasert et al., 2017). At different time points (24 hr, or 48 hr) of incubation, the whole cells (Pooled, free and adherent cells) were collected, fixed and permeabilized with cold 70% ethanol. The fixed cells were washed with PBS and resuspended in RNAse A (180 μg/mL) and incubated at room temperature for 30 min. Then cells were stained with 50 µg/ml propidium iodide solution (Invitrogen™; Thermo Fisher Scientific, Inc.). The stained cells were incubated at least for 20 minutes at 4 °C in the dark, and the percentage of cells with different DNA content and cell apoptosis were quantified by using the FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) using Cell Quest software, and data were analyzed using WinMDI ver 2.9.
Analysis of Akt protein expression via immunoblotting
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were used to detect the expression levels of Akt and phosphorylated Akt at Ser473 (Ser473p-Akt) proteins. The cells were seeded in triplicate at a density of 2×105 cells per well in 6-well plates and allowed to grow in fresh DMEM medium for 24 h. The cells were then washed with PBS and the medium was changed to either DMEM or NDM. The procedures for SDS-PAGE and immunoblotting were modified from Taylor and Posch (2014). After the specified period of incubation, the whole cells were then collected for protein extraction in RIPA lysis buffer, containing 50 mM Tris–Cl, pH 7.5; 150 mM NaCl, 0.1% SDS, 1 mM PMSF, 0.5% sodium deoxycholate, and 1% Nonidet P-40, supplemented with the complete protease inhibitor cocktail (Roche, Mannheim, Germany). Cell lysates containing 50 µg of protein were separated by SDS-PAGE (12–14% acrylamide) and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA). The membranes were blocked with 5% skimmed-milk for 30 min at room temperature, washed with PBS containing 0.3% Tween 20 (Sigma-Aldrich, Inc) then incubated overnight at room temperature with Akt and Ser473p-Akt antibodies (New England Biolabs, Ipswich, MA) diluted (1:1000) with PBS. Bound primary antibodies was detected using a horseradish peroxidase-conjugated anti-rabbit secondary antibody (Dako, Glostrup, Denmark) by chemiluminescence using an enhanced chemiluminescence kit (GE Healthcare, Little Chalfont, UK) according to the manufacturer’s instructions. Immunoreactive proteins were visualized using a luminescent image analyzer (LAS-4000, Fujifilm Co., Tokyo, Japan). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Santa Cruz Biotechnology, Santa Cruz, Calif, USA) was used (1:1000) as a loading control to ensure equal loading and even transfer from the gel to the membrane across the whole gel. Electrophoresis and electroblotting were carried out in a Bio-Rad Trans-Blot SD Cell apparatus (Bio-Rad, Hercules, CA) using a discontinuous buffer system. Bands corresponding to proteins expression were analyzed densitometrically, using The Image Processing and Analysis Java (ImageJ) program, relative to that of cells cultured in DMEM. Data were normalized to GAPDH levels.(Taylor and Posch, 2014)
Assessment of Akt inhibitor III molecule effect on cell viability
The cells were seeded in triplicate at a density of 5×103 cells per well in 96-well plates and allowed to grow in fresh DMEM medium for 24 h. The cells were then washed with PBS (pH=7.4, Sigma-Aldrich, Inc) and the medium was changed to NDM containing different concentrations (0, 6.25, 12.5, or 25 µM) of Akt inhibitor III molecule (Calbiochem, CA, USA). After the specified time of incubation, the cells were washed again with PBS, then 100 µL of DMEM medium with 10% WST-8 solution was added to the wells, and the plate was incubated for a further 2 hours. Then, the absorbance of the wells at 450 nm was measured using HTS Multi-Mode Microplate Reader (BioTek Instruments). The absorbance is proportional to the number of viable cells in the medium. Cell viability was expressed as percentage relative to cells treated with 0 μM of Akt inhibitor III molecule.
Statistical analysis
All results were obtained from at least three independent experiments and were expressed as mean ± standard deviation. Statistical comparison was conducted using Student’s t-test after one-way analysis of variance (ANOVA) using GraphPad Prism 5 statistical software (GraphPad, La Jolla, CA) and Excel software (Microsoft, Redwood, WA). The results were considered to be significant when the probability values (P) were less than 0.001.
Results
Survival of HeLa cells under nutrient-deprived conditions
To examine the tolerance for nutrient deprivation, cell survival under extreme nutrient starvation was examined by using medium containing only vitamins and electrolytes for culturing. After 24 hr of starvation more than 84% of the cells could survive under these extreme conditions, as shown in Figure 1. More interestingly, more than 12% of the cells tolerated the starvation for 48 hr.
Figure 1.
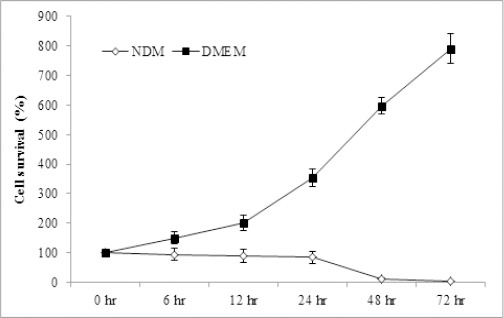
Survival of HeLa Cells under Nutrient-Deprived Conditions. Survival of cells after different time points of culturing the cells in fresh DMEM or NDM was expressed as percentage relative to that at 0 hr which is considered as 100%. Data represent mean ± SD. DMEM, Dulbecco’s Modified Eagle’s Medium; NDM, nutrient-deprived medium.
Effect of starvation on cell cycle and apoptosis of HeLa cells
The effect of starvation on cell cycle distribution and the quantitative analysis of apoptotic cells were investigated using propidium iodide staining to observe DNA content in the cells, as shown in Figure 2. The results showed that starvation, when compared to cells cultured in DMEM, significantly increased (P < 0.001) the percentage of apoptotic HeLa cells (sub-G1 phase) to 20 ± 0.77% and 67.3 ± 4.29% after 24 hr and 48 hr of starvation, respectively, indicating apoptosis in a time-dependent manner.
Figure 2.
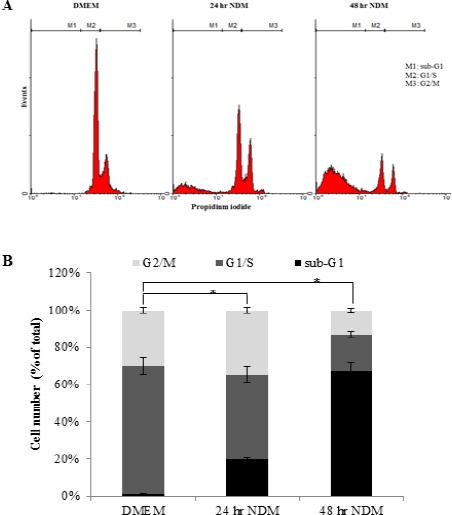
Effect of Starvation on Cell Cycle and Apoptosis of HeLa Cells. (A) Flow cytometric analysis of cell cycle distribution using propidium iodide staining after culturing the cells in DMEM or NDM for 24 hr and 48 hr. M1, sub-G1 (apoptotic) phase; M2, G1/S; M3, G2/M. (B) Cell number of each cell cycle phase was expressed as percentage of total cells. Bars represent mean ± SD. Significant difference between percentages of apoptotic cells (sub-G1 phase) is analyzed by one-way ANOVA test, where: *; P < 0.001 compared to apoptotic cells cultured in DMEM. DMEM, Dulbecco’s Modified Eagle’s Medium; NDM, nutrient-deprived medium.
Effect of starvation on Akt protein expression
To check the relationship between adaptation of cancer cells to starvation and the level of Akt activation, Akt activation was examined, by immunoblotting with Ser473p-Akt antibody, in HeLa cells after nutrient deprivation. As shown in Figure 3, there is a significant increase (P < 0.001) in the expression of Ser473p-Akt protein after starvation in a time-dependent manner when compared to that of cells cultured in normal medium (DMEM). This effect of starvation on Ser473 phospho-Akt protein expression in HeLa cells might explain the high percentage of cell survival.
Figure 3.
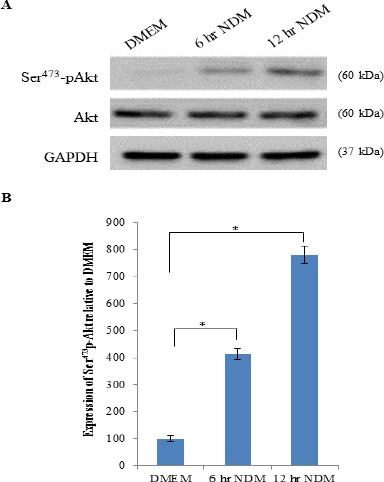
Effect of Starvation on Akt Protein Expression. (A) Representative immunoprecipitation blots of Akt and Ser473p-Akt proteins expression of cells cultured in DMEM or NDM for 6 hr and 12 hr. (B) Expression of Ser473p-Akt protein of cells cultured in NDM for 6 hr and 12 hr was expressed densitometrically as percentage relative to that of cells cultured in DMEM using bands in A. Expression was normalized to the corresponding internal control GAPDH protein expression. Bars represent mean ± SD. Significant difference is analyzed by one-way ANOVA test, where: *; P < 0.001. DMEM, Dulbecco’s Modified Eagle’s Medium; NDM, nutrient-deprived medium; kDa, kilo Dalton; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Effect of Akt inhibitor III molecule on survival of HeLa cells under nutrient-deprived conditions
To clarify the involvement of Akt activation in tolerance, HeLa cells were treated with different concentrations of Akt inhibitor III in NDM for 24 hr and the percentage of viable cells compared to cells cultured in NDM only (untreated cells) was determined by cell counting kit-8 assay. It was found that Akt inhibitor III, in a concentration-dependent manner, inhibited the cells proliferation, as shown in Figure 4A. The number of viable cells was decreased to 90 ± 4%, 30.3 ± 2.5%, and 0.4 ± 0.05% when treated with 6.25, 12.5, and 25 µM of Akt inhibitor III molecule, respectively, for 24 hr.
Figure 4.
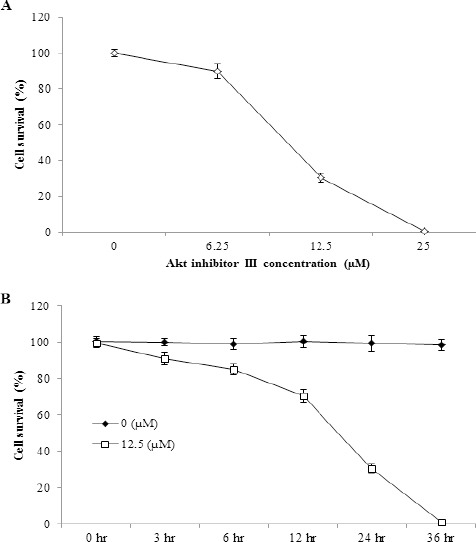
Effect of Akt Inhibitor III Molecule on HeLa Cells Under Nutrient-Deprived Conditions is Concentration- and Time-Dependent. (A) Survival of HeLa cells treated with different concentrations (6.25, 12.5, and 25 μM) of Akt inhibitor III molecule in NDM for 24 hr expressed as percentage relative to that of cells cultured in NDM only (0 μM of Akt inhibitor III molecule). Data represent mean ± SD. (B) Survival of HeLa cells treated with 0 and 12.5 μM of Akt inhibitor III molecule in NDM at different time intervals expressed as percentage relative to cells treated with 0 μM of Akt inhibitor III molecule. Data represent mean ± SD. NDM, nutrient-deprived medium.
Figure 4 III Molecule on HeLa Cells Under Nutrient-Deprived Conditions is Concentration- and Time-Dependent. (A) Survival of HeLa cells treated with different concentrations (6.25, 12.5, and 25 µM) of Akt inhibitor III molecule in NDM for 24 hr expressed as percentage relative to that of cells cultured in NDM only (0 µM of Akt inhibitor III molecule). Data represent mean ± SD. (B) Survival of HeLa cells treated with 0 and 12.5 µM of Akt inhibitor III molecule in NDM at different time intervals expressed as percentage relative to cells treated with 0 µM of Akt inhibitor III molecule. Data represent mean ± SD. NDM, nutrient-deprived medium.
For more clarification of the involvement of Akt activation in tolerance, the cells were treated with 0 and 12.5 µM of Akt inhibitor III in NDM and the percentage of viable cells compared to cells treated with 0 µM of Akt inhibitor III was determined at different time intervals, Figure 4B. The number of viable cells was decreased in a time-dependent manner. These results suggest that the decay of viable cells number and the increase of the percentage of apoptotic cells following starvation might be dependent on Akt activation status.
Discussion
Because of their intrinsic unregulated growth program due to genetic alterations, tumors continuously grow. In general, tumors are always exposed to poor nutrition because of insufficient vascularization (Helmlinger et al., 1997; Dang and Semenza, 1999). Generally without neovascularization or angiogenesis, tumor size is limited (Helmlinger et al., 1997). Metabolic stress triggers gene expression and signal transduction, causing the synthesis of vascular endothelial growth factors and angiogenic factors, resulting in greater metastatic potential, neovascularization, invasion and tumor growth (Holash et al., 1999; Rofstad and Danielsen, 1999; Shaheen et al., 1999). This is the reason why angiogenesis is considered one of the indicators for tumor progression. There are two ways of adaptation to nutrient insufficiency that could be interlinked but distinct from each other. The first way, widely accepted, is by increasing the supply. The second way is austerity, tolerating insufficiency. By angiogenesis, tumors grow beyond their ability to improve nutrient supplies. That is why necrotic foci are often associated with tumors. The tumor cells that have the ability to survive unfavorable conditions might be malignant.
Although neovascularization is important for tumor progression as suggested by most studies, less attention has been directed to the tolerance for poor nutritional conditions. It was observed that short periods of glucose and glutamine starvation for breast cancer cells made the cells rounded and induced apoptosis by mitochondrial dysfunction and generation of reactive oxygen species (Visagie et al., 2015). Also, it was found that Warburg effect was increased in cancer cells, cultured under low nutrient conditions, through activation of pyruvate dehydrogenase kinase (Wu et al., 2013). Our results showed that HeLa cells had acquired tolerance for nutrient deprivation and could survive for long periods, more than 48 hours, even under extremely nutrient-starved conditions.
In the present work, tolerance was closely associated with the expression of high levels of Ser473p-Akt protein. Recent investigations suggested that Akt is highly expressed in pancreatic and ovarian cancers, and the high level of expression was associated with increased tumorigenicity, poor prognosis and tolerance to nutrient-deprived conditions (Cheng et al., 1996; Miwa et al., 1996; Moore et al., 1998; Ruggeri et al., 1998; Shayesteh et al., 1999; Izuishi et al., 2000; Awale et al., 2006; Awale et al., 2008; Calastretti et al., 2014; Jones et al., 2014). Moreover, expression of constitutively active forms of Akt could prevent cell death upon growth factors removal (Song et al., 2005). Furthermore, it was reported that a site-specific phosphorylation of Akt, Ser473p-Akt and Thr308p-Akt, was observed in ovarian, breast, lung, colon, and kidney cancer cell lines subjected to transient and prolonged glucose deprivation, respectively (Gao et al., 2014). These observations lead us to hypothesize that HeLa cells sensitivity to starvation is, in part, dependent on Akt activation status as tolerance for nutrient starvation was found to be associated with high levels of p-Akt.
In addition, the tolerance to nutrient starvation, as shown in the present study, can remarkably be inhibited by Akt inhibitor III in a concentration-dependent as well as time-dependent manner. These results are consistent with others who used MK-2206, an AKT inhibitor, to inhibit the tolerance and decrease the survival of cancer cells subjected to glucose deprivation (Gao et al., 2014).
In conclusion, the modulation of Akt activity was found to affect the ability of HeLa cells to tolerate nutrient starvation. Our present findings might be able to provide a possible understanding and may help to suggest new approaches designed to prevent cancer cells’ metabolic adaptation. We believe that our results have important implications for new cancer therapies and open a new area of research on the biological nature of cervical cancer. Further understanding of these stress pathways and their regulation is necessary to provide new therapeutic strategies preventing austerity.
References
- Awale S, Li F, Onozuka H, et al. Constituents of Brazilian red propolis and their preferential cytotoxic activity against human pancreatic PANC-1 cancer cell line in nutrient-deprived condition. Bioorg Med Chem. 2008;16:181–9. doi: 10.1016/j.bmc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Awale S, Nakashima EM, Kalauni SK, et al. Angelmarin, a novel anti-cancer agent able to eliminate the tolerance of cancer cells to nutrient starvation. Bioorg Med Chem Lett. 2006;16:581–3. doi: 10.1016/j.bmcl.2005.10.046. [DOI] [PubMed] [Google Scholar]
- Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–12. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- Calastretti A, Gatti G, Quaresmini C, Bevilacqua A. Down-modulation of Bcl-2 sensitizes PTEN-mutated prostate cancer cells to starvation and taxanes. Prostate. 2014;74:1411–22. doi: 10.1002/pros.22857. [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, et al. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–41. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- Farley CM, Dibwe DF, Ueda JY, et al. Evaluation of synthetic coumarins for antiausterity cytotoxicity against pancreatic cancers. Bioorg Med Chem Lett. 2016;26:1471–4. doi: 10.1016/j.bmcl.2016.01.054. [DOI] [PubMed] [Google Scholar]
- Fisher WE, Berger DH. Angiogenesis and antiangiogenic strategies in pancreatic cancer. Int J Gastrointest Cancer. 2003;33:79–88. doi: 10.1385/IJGC:33:1:79. [DOI] [PubMed] [Google Scholar]
- Fleming JB, Brekken RA. Functional imaging of angiogenesis in an orthotopic model of pancreatic cancer. J Cell Biochem. 2003;90:492–501. doi: 10.1002/jcb.10644. [DOI] [PubMed] [Google Scholar]
- Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010:a systematic analysis. Lancet. 2011;378:1461–84. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- Gao M, Liang J, Lu Y, et al. Site-specific activation of AKT protects cells from death induced by glucose deprivation. Oncogene. 2014;33:745–55. doi: 10.1038/onc.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo:high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–82. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–8. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H. Remarkable tolerance of tumor cells to nutrient deprivation:possible new biochemical target for cancer therapy. Cancer Res. 2000;60:6201–7. [PubMed] [Google Scholar]
- Jones DR, Keune WJ, Anderson KE, et al. The hexosamine biosynthesis pathway and O-GlcNAcylation maintain insulin-stimulated PI3K-PKB phosphorylation and tumour cell growth after short-term glucose deprivation. FEBS J. 2014;281:3591–608. doi: 10.1111/febs.12879. [DOI] [PubMed] [Google Scholar]
- Kim SE, Park HJ, Jeong HK, et al. Autophagy sustains the survival of human pancreatic cancer PANC-1 cells under extreme nutrient deprivation conditions. Biochem Biophys Res Commun. 2015;463:205–10. doi: 10.1016/j.bbrc.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Magolan J, Coster MJ. Targeting the resistance of pancreatic cancer cells to nutrient deprivation:anti-austerity compounds. Curr Drug Deliv. 2010;7:355–69. doi: 10.2174/156720110793566272. [DOI] [PubMed] [Google Scholar]
- Masamune A, Kikuta K, Watanabe T, et al. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2008;295:709–17. doi: 10.1152/ajpgi.90356.2008. [DOI] [PubMed] [Google Scholar]
- Md Tohid SF, Ziedan NI, Stefanelli F, Fogli S, Westwell AD. Synthesis and evaluation of indole-containing 3,5-diarylisoxazoles as potential pro-apoptotic antitumour agents. Eur J Med Chem. 2014;56:263–70. doi: 10.1016/j.ejmech.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Miwa W, Yasuda J, Murakami Y, et al. Isolation of DNA sequences amplified at chromosome 19q13.1-q13.2 including the AKT2 locus in human pancreatic cancer. Biochem Biophys Res Commun. 1996;225:968–74. doi: 10.1006/bbrc.1996.1280. [DOI] [PubMed] [Google Scholar]
- Moore SM, Rintoul RC, Walker TR, et al. The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70s6k-dependent pathway. Cancer Res. 1998;58:5239–47. [PubMed] [Google Scholar]
- Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996;56:5754–7. [PubMed] [Google Scholar]
- Richard DE, Berra E, Pouyssegur J. Angiogenesis:how a tumor adapts to hypoxia. Biochem Biophys Res Commun. 1999;266:718–22. doi: 10.1006/bbrc.1999.1889. [DOI] [PubMed] [Google Scholar]
- Rofstad EK, Danielsen T. Hypoxia-induced metastasis of human melanoma cells:involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer. 1999;80:1697–707. doi: 10.1038/sj.bjc.6690586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri BA, Huang L, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21:81–6. [PubMed] [Google Scholar]
- Shaheen RM, Davis DW, Liu W, et al. Antiangiogenic therapy targeting the tyrosine kinase receptor for vascular endothelial growth factor receptor inhibits the growth of colon cancer liver metastasis and induces tumor and endothelial cell apoptosis. Cancer Res. 1999;59:5412–6. [PubMed] [Google Scholar]
- Shayesteh L, Lu Y, Kuo WL, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sophonnithiprasert T, Mahabusarakam W, Nakamura Y, Watanapokasin R. Goniothalamin induces mitochondria-mediated apoptosis associated with endoplasmic reticulum stress-induced activation of JNK in HeLa cells. Oncol Lett. 2017;13:119–28. doi: 10.3892/ol.2016.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L, Kharbanda S, Chen R, et al. Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proc Natl Acad Sci U S A. 2007;104:3466–71. doi: 10.1073/pnas.0611271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RM. Cell and environment interactions in tumor microregions:the multicell spheroid model. Science. 1988;240:177–84. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- Taylor SC, Posch A. The design of a quantitative western blot experiment. Biomed Res Int. 2014;2014:361590. doi: 10.1155/2014/361590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res. 2004;10:415–27. doi: 10.1158/1078-0432.ccr-0642-03. [DOI] [PubMed] [Google Scholar]
- Tomida A, Yun J, Tsuruo T. Glucose-regulated stresses induce resistance to camptothecin in human cancer cells. Int J Cancer. 1996;68:391–6. doi: 10.1002/(SICI)1097-0215(19961104)68:3<391::AID-IJC19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Van Meter TE, Broaddus WC, Cash D, Fillmore H. Cotreatment with a novel phosphoinositide analogue inhibitor and carmustine enhances chemotherapeutic efficacy by attenuating AKT activity in gliomas. Cancer. 2006;107:2446–54. doi: 10.1002/cncr.22248. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors:a review. Cancer Res. 1989;49:6449–65. [PubMed] [Google Scholar]
- Visagie MH, Mqoco TV, Liebenberg L, et al. Influence of partial and complete glutamine-and glucose deprivation of breast-and cervical tumorigenic cell lines. Cell Biosci. 2015;5:37. doi: 10.1186/s13578-015-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Staschke KA. How do tumours adapt to nutrient stress? EMBO J. 2010;29:1946–7. doi: 10.1038/emboj.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CA, Chao Y, Shiah SG, Lin WW. Nutrient deprivation induces the Warburg effect through ROS/AMPK-dependent activation of pyruvate dehydrogenase kinase. Biochim Biophys Acta. 2013;1833:1147–56. doi: 10.1016/j.bbamcr.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Marone R. Phosphoinositide 3-kinase in disease:timing, location, and scaffolding. Curr Opin Cell Biol. 2005;17:141–9. doi: 10.1016/j.ceb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Yuan J, Narayanan L, Rockwell S, Glazer PM. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60:4372–6. [PubMed] [Google Scholar]
- Yun J, Tomida A, Nagata K, Tsuruo T. Glucose-regulated stresses confer resistance to VP-16 in human cancer cells through a decreased expression of DNA topoisomerase II. Oncol Res. 1995;7:583–90. [PubMed] [Google Scholar]


