Abstract
Background:
Aloe-emodin belongs to the group of anthraquinones having extremely high biological activity. The aim of this study was to evaluate the range of morphological and biochemical changes in HeLa cells treated with aloe-emodin, especially with regard to the lysosomal compartment.
Methods:
Marking of lysosomes was performed with neutral red staining for conventional light microscopy and acridine orange staining for confocal microscopy. To evaluate ctivity of lysosomal enzymes and permeability of the lysosomal membrane, spectrophotometric techniques were employed.
Results:
Aloe-emodin caused increased permeability of lysosomal membranes in HeLa cells, expressed inter alia by extinction of the orange color of acridine orange (lysosomal marker) and in reduction of neutral red uptake by lysosomes. These changes are accompanied by release of cathepsins from the interior of the lysosomes with a simultaneous highly significant increase in their activity in the cytoplasm.
Conclusion:
The results indicate that aloe-emodin can activate lysosomal pathway-dependent apoptosis in HeLa cells.
Keywords: Anthraquinones, aloe, emodin, lysosomal compartment, cathepsins, apoptosis
Introduction
Research indicates that the use of new compounds of plant origin may be important for clinical medicine, especially when used in chemotherapy. This may be the case for the anthraquinones present in Rhamnus frangulaL. (Kovacevic et al., 2002), Aloe barbadensisMill. (Zhong et al., 2013), Aloe arborescensMill. (Choi and Chung, 2003) and Rheum palmatumL. (Yang at al., 1999). An example of one of the oldest and best-known herbs still used in various herbal remedies in Chinese medicine for diverse therapeutic indicationsis is Rheum palmatum. Among anthraquinones, the greatest biological activity is shown by aloe-emodin, emodin, chrysophanol, fiscion, and rhein (Zhang at al., 2010; Hsu and Chung, 2012; Wang at al., 2014). Numerous in vitro and in vivo studies have shown that aloe-emodin (1,8-dihydroxy-3-hydroxymethyl-9,10-anthrachinon) has antibacterial (Tian at al., 2003; Coopoosamy and Magwa, 2006), antiviral (Sydiskis at al., 1991; Lin at al., 2008) antifungal (Agarwal at al., 2000), hepatoprotective (Arosio at al., 2000) and antioxidant action (Yen et al., 2000). In studies on different tumor cell lines it has been shown that aloe-emodin can modulate cell cycle and induce apoptosis, suggesting that the anthraquinone may have potential anti-cancer properties (Pecere at al., 2002, 2003; Lee, 2001; Kuo at al., 2002; Mijatovic at al., 2004, 2005; Lin at al., 2006; Chen at al., 2007; Guo at al., 2007; Chiu at al., 2009). According to the available literature in spite of numerous studies, its anticancer mechanism of action is still not fully understood.
The aim of this study is to assess the biochemical and morphological changes in cancer cells exposed to aloe-emodin, with particular attention paid to the lysosomal system, which plays an important role in the proper functioning of the cell.
Materials and Methods
In vitro culture conditions
The HeLa cell line (human cervix carcinoma) was cultured in Nunc plates at a temperature of 37 °C and in a 5% carbon dioxide atmosphere in a CO2 DirectHeat incubator (Thermo Fisher Scientific). Cells came from the Department of Radiobiology and Immunology, UJK Kielce. Cell culture was carried out in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic mixture from Thermo Fisher. Aloe-emodin (C15H10O5) was purchased from Sigma-Aldrich (USA). Cells were exposed to the test anthraquinone in concentration ranges of 1 μM to 100 μM.
Analysis of activity of the lysosomal system-optical method
To visualize the lysosomes, their absorption of neutral red (NR) was determined using a methodology modified from that of Michalik et al., (2003). Cells were grown on sterile cover slips in tissue culture dishes. After 48 hours of incubation, the control cells and cells treated with anthraquinone were incubated with NR (50 mg/ml) in DMEM for a period of 3 hours at a temperature of 37 °C. The process of endocytosis was then stopped by washing the cells in PBS, which at the same time removed excess dye from the cell surface. The activity of the lysosomes was examined using a Nikon Eclipse 80i optical microscope.
Neutral red uptake assay (NR) by lysosomes
The degree of cytotoxicity of aloe-emodin to HeLa cells was determined by the modified Borenfreund and Puerner method (1985). Cells were plated in 96-well plates (Nunc) and incubated at 37 °C for 24 hours. The culture medium was then removed and replaced by a new medium containing the appropriate doses of test agent and reincubated for a period of 48 hours. In a next step, after removing the medium with a test agent, the cells were incubated with neutral red. The red solution was then removed by washing with PBS while blocking the process of endocytosis. In a next step the solvent was added in order to release the absorbed red by cells and extracted on a microplate shaker. The amount of dye bound in the cells measured spectrophotometrically was directly proportional to the number of cells with intact membranes. The absorbance value was read at wavelengths of 540 nm and 690 nm using a Synergy 2 multimode microplate reader (Biotek) and GEN5 software, that determines the degree of toxicity of anthraquinone. The experiment was performed in independent triplicate.
Marking lysosomes using acridine orange
In order to label the lysosomes using acridine orange (C17H19N3) (according to the modified method of Harhaji et al.) (2007), cells were grown on sterile cover slips in tissue culture dishes. After 48 hours of incubation with basal medium (for control cells) and medium with the test agent (for the test cells), the pooled culture medium and the cells were washed in PBS and incubated with a solution of acridine orange dissolved in PBS (50 mg/ml) under culture conditions. The samples thus prepared were examined with a confocal Nikon A1R microscope using lasers of wavelength 488 nm and 561 nm.
Enzymatic method for the assessment of lysosomal membrane permeability
After 48 hours of incubation with aloe-emodin, the cells were trypsinized and resuspended in sucrose solution. The resulting cell suspension was subjected to a homogenization process. Next, the resulting homogenate was subjected to differential centrifugation to obtain a lysosomal fraction, according to the modified methodology of Marzella and Glauman (1980). In the lysosomal fractions, as well as in the extralysosomal fractions, the activity of the lysosomal enzymes cathepsin-D and L (EC. 3.4.23.5, EC. 3.4.22.15.) was determined using a UV/VIS Nicolet Evolution spectrophotometer (Thermo Scientific), following to the method of Langer et al., (1973). In addition, the total protein content was determined according to the method of Lowry in the modification of Kirschke and Wiederanders (1984). The activity of the enzymes is shown in μmol/mg protein/hr. The biochemical results are the average of three independent experiments, in each of which analyses were replicated three times.
Visualization of apoptosis in HeLa cells-DAPI staining
For evaluation of apoptosis after administration of the test compound, cells were stained with a fluorescent dye with 4’, 6-diamidino-2-phenylindole (DAPI) to detect nuclear condensation and fragmentation. HeLa cells were cultured in medium with aloe-emodin (100 µM) or without the test compound (control cells) for 48 h. Then the cell culture medium was discarded and 10 μg/ml of DAPI solution (Sigma, USA) was added to the cell culture and incubated for 15 min. The cells were then washed with PBS and analyzed under Nikon Eclipse 80i fluorescence microscope.
Annexin V/PI double cell staining
Apoptotic cells were quantified using annexin V-FITC assay (BD Pharmingen) and analyzed by flow cytometry. For this purpose, cells were treated with aloe-emodin at concentrations of 1 μM and 100 μM for 48 hours. Cells were then stained with V-FITC and propidium iodide and incubated for 15 minutes at 37°C in the dark. Apoptotic cells were analyzed using FACSCanto II flow cytometer and FACSDiva software (BD Biosciences).
Statistical analysis
The results were evaluated using the statistical analysis with Statistica 10.0 (StatSoft, Poland).
The assessment of changes in cytotoxicity was supported by an analysis of cell viability, using analysis variance with Tukey’s test. Tukey’s test was also used to assess changes in the activity of lysosomal enzymes.
Results
Aloe-emodin induces an increase in the number of lysosomes and vacuoles
An activity of the lysosomal system is expressed as the number of red-stained lysosomes in the control HeLa cells. The changes obtained after applying the NR uptake assay are shown in Figure 1A. In cells exposed to aloe-emodin at concentrations of 1-15 µM, its stimulatory effect on the growth in the number of lysosomes densely arranged around the nucleus was seen (Figure 1B) and the presence of cells with cytoplasmic vacuolation (Figure 1C). A concentration of 30 µM led to increasing activation of the lysosomal system characterized by an increase in the number of lysosomes and the presence of numerous cells with a clear cytoplasmic vacuolation (Figure 1D). Following the incubation of the cells with 60 µM aloe-emodin, increased cytoplasmic vacuolization was observed, as was the presence of cells with autophagic vacuoles containing a substantial amount of the absorbed dye (Figure 1E). After exposing cells to 100 µM aloe-emodin, cells with a few red lysosomes were observed but the vast majority of cells were apoptotic (Figure 1F).
Figure 1.
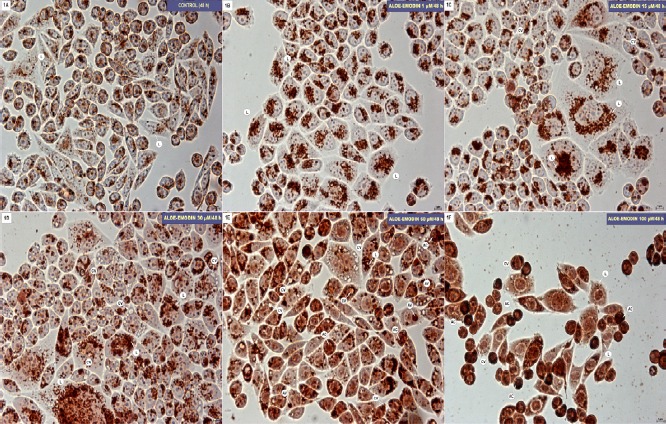
A-F: Hela cell line after 48-hour incubation in basal medium (control, A) and treated with aloe-emodin in concentrations of: 1 µM (B), 15 µM (C), 30 µM (D), 60 µM (E) and 100 µM (F). Cells were stained with neutral red: lysosomes (L), cytoplasmic vacuoles (CV), autophagic vacuole (VA), apoptotic cells (AC). Magnification × 400.
Aloe-emodin induces increased lysosomal membrane permeability in HeLa cells-acridine orange staining
Figure 2A shows an image of HeLa control cells in which few red-stained lysosomes are visible. The cells treated with 1 µM and 15 µM aloe-emodin are characterized by increases in the number of lysosomes and autophagic vacuoles (Figure 2B and C). The intensification in the red color of the fluorescence, indicating a significant increase in activity of the lysosomal system, was shown at a concentration of 30 µM (Figure 2D). The exposure of cells to 60 µM aloe-emodin resulted in visible saturation of fluorescent dye at the location of the autophagic vacuoles, and the presence of numerous cells with the gradual extinction of the fluorescent color in lysosomes (Figure 2E). The highest concentration (100 µM) of aloe-emodin used caused a significant gradual extinction of fluorescence in the tagged single lysosomes (Figure 2F).
Figure 2.
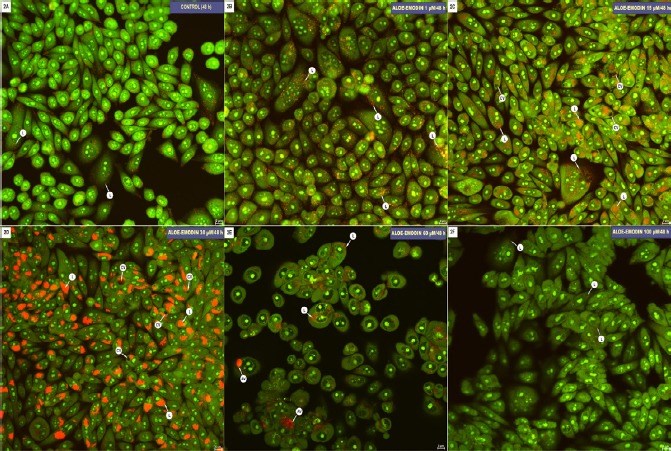
A-C. Hela cells after 48-hour incubation in basal medium (control, A) and treated with aloe-emodin at concentraction of: 1 µM (B), 15 µM (C), 30 µM (D), 60 µM (E) and 100 µM (F). Cells were stained with acridine orange: labeled on red lysosomes (L) (red emission), cytoplasmic vacuoles (CV), autophagic vacuoles (AV). Magnification × 400.
Aloe-emodin induces an increase activity of cathepsin in the extralysosomal fraction of Hela cells
As a consequence of aloe-emodin in a concentration of 1 µM was found to produce a highly statistically significant increase in the activity of cathepsin D and L to 181.21% in the lysosomal fraction (Figure 3), and to 104.44% in the extralysosomal fraction. Aloe-emodin at a concentration of 15 µM resulted in a highly statistically significant increase in the activity of cathepsin D and L (of 226.90%) in the lysosomal fraction and 139.48% in the case of extralysosomal fractions. The effect of exposure to 60 µM aloe-emodin was a highly significant decrease in the activity of cathepsin D and L in the lysosomal fraction to 41.87%, and a highly statistically significant increase in the activity of the proteolytic enzyme to 162.95% in the extralysosomal fraction, which may indicate an increase in lysosomal membrane permeability. The effect of 100 µM aloe-emodin on the HeLa cell line was a highly significant decrease in the activity of cathepsin to 13.40% in the lysosomal fraction and a highly statistically significant increase in the activity of the extralysosomal fraction (224.69%).
Figure 3.
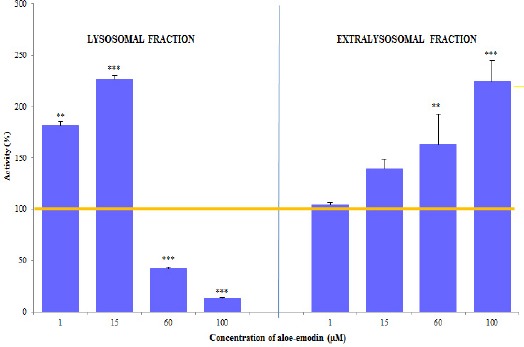
Activity (mean±SD) of Cathepsin D and L in the Lysosomal and Extralysosomal Fraction of HeLa Cells after 48 h of Exposure to Differences Concentrations of Aloe-Emodin. Statistically significant differences with: ** p <0.01, *** p<0.001.
Aloe-emodin exhibits cytotoxic activity against HeLa cells
Even at a concentration of 1 µM, significant reduction in cell viability to 83.9%, relative to the control (100%), were observed. 20 µM of aloe-emodin caused a decrease in cell viability to 76.7% (Figure 4). In contrast, a 50% reduction in viability (IC50) was demonstrated for a concentration of 39.20 µM. Further reductions in cell viability to 27.89% have been shown to result from a concentration of 60 µM. A 90% reduction in viability (IC90) was seen with a concentration of 78.71 µM. As a consequence of the 48-hour incubation with aloe-emodin at concentrations of 80 µM and 100 µM, a further reduction in the viability of the cells was seen: 9.98% and 6.03%, respectively.
Figure 4.
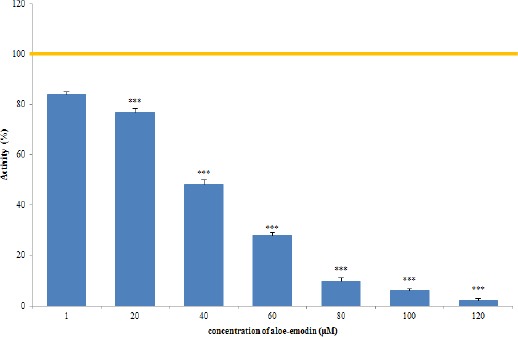
HeLa Cell Viability Determined by Neutral Red (NR) Uptake assay after 48-h Exposure to Different Concentrations of Aloe-Emodin. Differences statistically significant to: *** p <0.001. IC50/IC90: 39,20µM/78,71µM.
Aloe-emodin induces apoptosis of HeLa cells
Exposition of HeLa cells to the aloe-emodin in a concentration of 100 µM for 48 hours clearly induces apoptosis. Apoptotic cells were visualized by staining with DAPI. Nucleus stain increased the number of nuclei showing symptoms characteristic of apoptosis, such as shown in Figure 5A chromatin condensation and fragmentation of the nucleus.
Fig. 5.
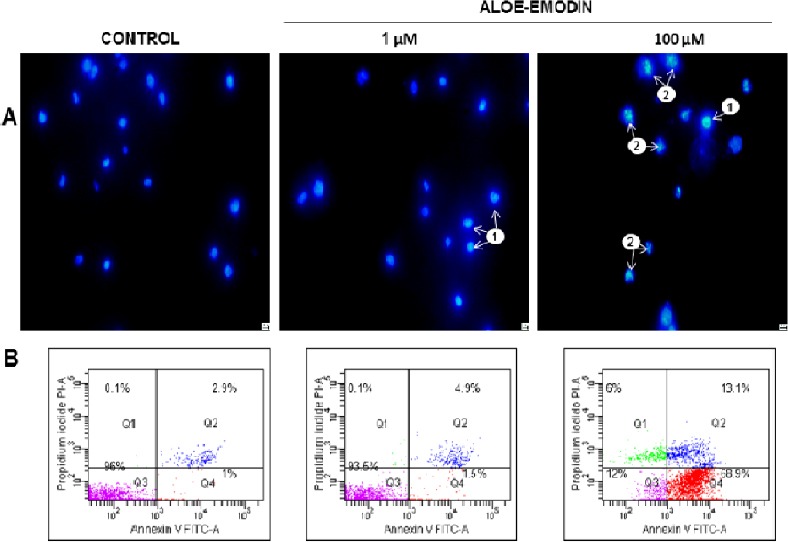
Apoptosis observed in HeLa cells using 4’,6-Diamidine-2-phenylindole staining (A). Control cells (not exposed to the test agent) showed normal morphology of the nucleus. After 48 hours of aloe-emodin action at the concentration of 1 µM, mainly cells with chromatin condensation were observed (1), whereas among cells exposed to aloe-emodin at the concentration of 100 μM numerous cells with both condensation (1) and nucleus fragmentation (2) were observed. Magnification × 400. Representative cytograms showing apoptosis analysis in a flow cytometer (B). Cells were treated for 48 hours with 1 μM and 100 μM concentration of aloe-emodin and apoptosis level was assessed by annexin V-FITC/PI staining. Control cells (not in apoptosis)-without Annexin V-FITC and PI staining. After the action of aloe-emodin, three populations of cells were demonstrated: living cells (Annexin V-FITC-/PI-), cells in the early (Annexin V-FITC +/PI-) and late stage of apoptosis (Annexin V-FITC+/PI+) and dead cells (Annexin V-FITC-/PI+). Data are representative of three parallel experiments.
In addition, apoptosis was measured by flow cytometry. After 48 hours of treatment with aloe-emodin at 1 μM and 100 μM, the percentages of apoptotic cells were respectively 6.4% and 82%, compared with the control cells (3.9%) (Figure 5B). These results indicate the concentration-dependent induction of apoptosis.
Discussion
Cells correctly perform most life functions because of their specific internal structure
A significant role in adaptation of the cells, both normal and cancer, to the changed environment, is attributed to the lysosomal system. The adaptive functions of the lysosomal compartment were highlighted by following, inter alia, their variability with respect to certain anti-cancer compounds of plant origin, such as taxol, vinblastine and vincristine (Król at al., 1994; 1996; 1998). Studies of the effects of selected plant alkaloids (vinblastine, vincristine) showed that their introduction into a cell can significantly modulate the activity of lysosomal enzymes, and to modify the structure of lysosomes and to induce various accompanying changes (Król at al., 1994; 1996). For many years, it was believed that in the process of apoptosis, caspases play a major role, and the role of lysosomes in apoptosis is limited only to digesting the contents of the apoptotic bodies. It is now accepted, that the destabilization of lysosomal membranes is critical not only for the organelles, but also for the functioning of the cell (Boya, 2012; Bursch, 2001; Česen at al., 2012; Johansson at al., 201; Kågedal at al., 2001). The gradual release of enzymes from lysosomes can lead to apoptosis, while rupture of lysosomal membrane can lead to necrosis (Boya, 2012; Bursch, 2001; Česen at al., 2012; Johansson at al., 201; Kågedal at al., 2001; Linder and Shoshan, 2005; Pivtoraiko at al., 2009; Yi at al., 2006; Appelqvist at al., 2013).
Rapidly dividing cancer cells are highly dependent on the effective functioning of the lysosomes and are characterized by large changes in the lysosomal compartment, i.e. modification of their volume, number, as well as an increase in the expression, secretion and/ or activation of lysosomal enzymes, including cathepsin. These changes can affect the invasive tumor growth, angiogenesis and the formation of drug resistance (Glunde at al., 2003; Jäättelä, 2004; Fehrenbacher and Jäättelä, 2005; Kallunki at al., 2013).
As is clear from many reports cancer cells may have the ability to reduce the activity of both apoptotic and lysosomal pathway (Kirkegaard and Jäättelä, 2009).
In contrast, increasing the area of lysosomes, as well as pH change in tumor cells may contribute to stronger sensitivity of their membranes and increases in permeability (Glunde at al., 2003; Fehrenbacher at al., 2004; Kirkegaard and Jäättelä, 2009; Boya, 2012; Kallunki at al., 2013).
Thus, for new cancer therapies it is important to activate in cancer cells pathways other than apoptosis, leading to their death, including the lysosomal cell death involving specific lysosomal enzymes-cathepsins (Johansson at al., 2010; Kirkegaard and Jäättelä, 2009; Erdal at al., 2005; Morselli at al., 2008; Piao and Amaravadi, 2016; Tardy at al., 2016).
An example of the compounds inducing alternative to apoptosis kind of death in cancer cells, that is, death as mediated by the lysosomal system, is aloe-emodin used in our study.
Lysosomes are organelles that react most quickly to all changes in the cell, responding with increase in their number and size. A morphological and functional remodeling takes place, associated with activation of lysosomal hydrolases and increased permeability of the lysosomal membrane (Boya and Kroemer, 2008; Xu and Ren, 2015).
The studies conducted on Hela cell line show that with increasing concentrations of aloe-emodin used, increased of lysosomal membrane permeability took place, which manifested i.a. in reduction of cathepsin D and L concentration in the lysosomal fraction, in favor of the increase of its concentration in the extralysosomal fraction (Figure 3).
This may indicate a high level of aloe-emodin accumulation in the lysosomes and a concentration-dependent (1 μM and 100 μM) induction of apoptotic processes in the studied tumor cells, as shown in Figures 5A and 5B.
Cathepsin are lysosomal aspartyl endopeptidases involved in many physiological and pathophysiological processes in the cell. In the last decade an increased number of studies demonstrated that the enzymatic functions of cathepsins (in particular D and L) are not limited to protein digestion, but also to the regulation of apoptosis, in particular with regard to the lysosomal cell death (Benes at al., 2008; Leist and Jäättelä, 2001).
Lysosomal membrane damage in HeLa cells is also demonstrated using acridine orange lysosomal marker. The exposure of the tested cells at the increasing concentration of aloe-emodin resulted in the gradual extinction of red fluorescent color in labeled lysosomes. The highest degree of extinction was found with tested cells exposed to the aloe-emodin in a concentration of 100 µM (Figure 2F). The cells were then characterized by a significant extinction of red fluorescence in labeled lysosomes, and the emission of green fluorescence from accumulation of acridine orange in the cytosol (Figure 2F).
As is clear from the literature acridine orange staining is a very good method for visualizing changes in the lysosomal system. The staining used is the only lipophilic fluorochrome having the ability to accumulate in the lysosomes, dye molecules after entering the cell membrane are protonated and then pumped together with the hydrogen ions to the inside of the lysosomes (then there is marking) (Choi at al., 2002; Terman at al., 2006; Kurz at al., 2008).
The fluorescence emission of acridine orange is variable and depends on its concentration in the lysosomes and pH changes, varying from red at a high concentration in the lysosomes to green at a low concentration in the cytosol (Moriyama at al., 1982; Rundquist at al., 1984; Nicolini at al., 1979; Denamur at al., 2011). The fluorochrome is thus widely used as a marker in studies of lysosomal membrane permeability (Zdolsek at al., 1990).
The sensitivity of lysosomes to the action of aloe-emodin was also confirmed by the neutral red uptake by lysosomes assay with the optical microscopy technique where, similarly to fluorescence microscopy, showed a reduced uptake of dye in cells exposed to its concentration (Figure 1E and F).
Using spectrophotometric method (NR cytotoxicity test) IC50 and IC90 values were determined for HeLa cells, 39,20 µM and 8,71 µM respectively for concentrations and were a response to relatively low concentrations anthraquinone used (Figure 4). This further confirms the cytotoxic effect of aloe-emodin on lysosomal compartment in the studied cancer cells.
The observed lysosomal membrane damage can also be associated with the ability of aloe-emodin to generate reactive oxygen species formed during the damage of mitochondrion function, as was shown in studies Chiu et al., (2009); Lee et al., (2006); Lin et al., (2009); Chen et al., (2010).
The biochemical and morphological results obtained for the lysosomal compartment indicate that aloe-emodin has high cytotoxic activity with respect to the lysosomal system of the cancer HeLa cell line. The test anthraquinone activates the lysosomal system in tumor cells, and when used in high concentrations destabilizes the lysosomal membranes, resulting in release of lysosomal enzymes into the cytoplasm, which can induce apoptosis dependent on activity of cathepsins.
In conclusion, studies of the effects of anthraquinone on the lysosomal compartment require further extension and continuation, especially in the context of new cancer treatments.
Acknowledgements
This work was supported by Jan Kochanowski University, grant: 066/R/11.
References
- Agarwal SK, Singh SS, Verma S, Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol. 2000;72:43–7. doi: 10.1016/s0378-8741(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Appelqvist H, Wäster P, Kågedal K, Öllinger K. The lysosome:from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5:214–26. doi: 10.1093/jmcb/mjt022. [DOI] [PubMed] [Google Scholar]
- Arosio B, Gagliano N, Fusaro LM, et al. Aloe-emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacol Toxicol. 2000;87:229–33. doi: 10.1034/j.1600-0773.2000.d01-79.x. [DOI] [PubMed] [Google Scholar]
- Benes P, Vetvicka V, Fusek M. Cathepsin D-many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68:12–28. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24:119–24. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Boya P. Lysosomal function and dysfunction:mechanism and disease. Antioxid Redox Signal. 2012;17:766–74. doi: 10.1089/ars.2011.4405. [DOI] [PubMed] [Google Scholar]
- Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–51. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8:569–81. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- Česen MH, Pegan K, Spes A, Turk B. Lysosomal pathways to cell death and their therapeutic applications. Exp Cell Res. 2012;318:1245–51. doi: 10.1016/j.yexcr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Chen S-H, Lin K-Y, Chang C-C, et al. Aloe-emodin-induced apoptosis in human gastric carcinoma cells. Food Chem Toxicol. 2007;45:2296–303. doi: 10.1016/j.fct.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Chen YY, Chiang SY, Lin JG, et al. Emodin, aloe-emodin and rhein induced DNA damage and inhibited DNA repair gene expression in SCC-4 human tongue cancer cells. Anticancer Res. 2010;30:945–52. [PubMed] [Google Scholar]
- Chiu T-H, Lai W-W, Hsia T-Ch, et al. Aloe-emodin induces cell death through S-phase arrest and caspase-dependent pathways in human tongue squamous cancer SCC-4 Cells. Anticancer Res. 2009;29:4503–12. [PubMed] [Google Scholar]
- Choi S, Chung M-H. A review on the relationship between aloe vera components and their biologic effects. Semin Integr Med. 2003;1:53–62. [Google Scholar]
- Choi SH, Choi DH, Lee JJ, et al. Imidazoline drugs stabilize lysosomes and inhibit oxidative cytotoxicity in astrocytes. Free Radic Biol Med. 2002;32:394–405. doi: 10.1016/s0891-5849(01)00819-x. [DOI] [PubMed] [Google Scholar]
- Coopoosamy RM, Magwa ML. Antibacterial activity of aloe emodin and aloin A isolated from Aloe excels. Afr J Biotech. 2006;5:1092–4. [Google Scholar]
- Denamur S, Tyteca D, Marchand-Brynaert J, et al. Role of oxidative stress in lysosomal membrane permeabilization and apoptosis induced by gentamicin, an aminoglycoside antibiotic. Free Radic Biol Med. 2011;51:1656–65. doi: 10.1016/j.freeradbiomed.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Erdal H, Berndtsson M, Castro J, et al. Induction of lysosomal membrane permeabilization by compounds that activate p53-independent apoptosis. Proc Natl Acad Sci U S A. 2005;102:192–7. doi: 10.1073/pnas.0408592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher N, Gyrd-Hansen M, Poulsen B, et al. Sensitization to the lysosomal cell death pathway upon immortalization and transformation. Cancer Res. 2004;64:5301–10. doi: 10.1158/0008-5472.CAN-04-1427. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher N, Jäättelä M. Lysosomes as targets for cancer therapy. Cancer Res. 2005;65:2993–95. doi: 10.1158/0008-5472.CAN-05-0476. [DOI] [PubMed] [Google Scholar]
- Glunde G, Gugginoy SE, Solaiyappan M, et al. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia. 2003;5:533–45. doi: 10.1016/s1476-5586(03)80037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J-M, Xiao B-X, Liu Q, et al. Anticancer effect of aloe-emodin on cervical cancer cells involves G2/M arrest and induction of differentiation. Acta Pharmacol Sin. 2007;28:1991–5. doi: 10.1111/j.1745-7254.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- Harhaji L, Isakovic A, Raicevic N, et al. Multiple mechanisms underlying the anticancer action of nanocrystalline fullerene. Eur J Pharmacol. 2007;568:89–98. doi: 10.1016/j.ejphar.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Hsu S-Ch, Chung J-G. Anticancer potential of emodin. Bio Med. 2012;2:108–16. doi: 10.1016/j.biomed.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäättelä M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene. 2004;23:2746–56. doi: 10.1038/sj.onc.1207513. [DOI] [PubMed] [Google Scholar]
- Johansson ACh, Appelqvist H, Nilsson C, et al. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis. 2010;15:527–40. doi: 10.1007/s10495-009-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kågedal K, Johansson U, Öllinger K. The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 2001;15:1592–4. doi: 10.1096/fj.00-0708fje. [DOI] [PubMed] [Google Scholar]
- Kallunki T, Olsen OD, Jäättelä M. Cancer-associated lysosomal changes:friends or foes? Oncogene. 2013;18:1995–2004. doi: 10.1038/onc.2012.292. [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, Jäättelä M. Lysosomal involvement in cell death and cancer. Biochim Biophys Acta. 2009;1793:746–54. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Kirschke H, Wiederanders B. Methoden zur aktivitätsbestimmung von proteinasen. Martin-luther-universität halle-wittenberg wissenschaftliche beitrage halle/salle. 1984:11–17. [Google Scholar]
- Kovacevic N, Subotic A, Budimir S, Grubišic D. Comparative study of anthraquinones from embryogenic callus tissue and zygotic embryos of Frangula alnus and Rhamnus aatharticus. Pharm Biol. 2000;38:321–5. [Google Scholar]
- Król T. Vincristine-induced autophagy in mouse liver. Acta Biol Cracov series Zool. 1996;38:5–8. [Google Scholar]
- Król T. Activity of lysosomal system in mouse liver after taxol administration. Gen Pharmac. 1998;30:239–43. doi: 10.1016/s0306-3623(97)00091-8. [DOI] [PubMed] [Google Scholar]
- Król T, Schmidt M, Kołątaj A, Witek B. Vinblastine-induced autophagy in mouse liver. Comp Biochem Physiol Pharmacol Toxicol Endocrinol. 1994;107:165–69. [Google Scholar]
- Kuo P-L, Lin T-C, Lin Ch-Ch. The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sci. 2002;71:1879–92. doi: 10.1016/s0024-3205(02)01900-8. [DOI] [PubMed] [Google Scholar]
- Kurz T, Terman A, Gustafsson B, Brunk UT. Lysosomes in iron metabolism, ageing and apoptosis. Histochem Cell Biol. 2008;129:389–406. doi: 10.1007/s00418-008-0394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner J, Wakil A, Zimmermann M, et al. Aktivitätsbestimmung proteolytischer Enzyme mit Azokasein als Substrat. Acta Biol Med Ger. 1973;31:1–18. [PubMed] [Google Scholar]
- Lee H-Z. Protein kinase C involvement in aloe-emodin and emodin-induced apoptosis in lung carcinoma cell. Br J Pharmacol. 2001;134:1093–103. doi: 10.1038/sj.bjp.0704342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HZ, Lin ChJ, Yang WH, et al. Aloe-emodin induced DNA damage through generation of reactive oxygen species in human lung carcinoma cells. Cancer Lett. 2006;239:55–63. doi: 10.1016/j.canlet.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Leist M, Jäättelä M. Triggering of apoptosis by cathepsins. Cell Death Differ. 2001;8:324–6. doi: 10.1038/sj.cdd.4400859. [DOI] [PubMed] [Google Scholar]
- Lin Ch-W, Wu Ch-F, Hsiao N-W, et al. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. Int J Antimicrob Agents. 2008;32:355–9. doi: 10.1016/j.ijantimicag.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J-G, Chen G-W, Li T-M, et al. Aloe-emodin induces apoptosis in T24 Human Bladder Cancer Cells through the p53 dependent apoptotic pathway. J Urol. 2006;175:343–7. doi: 10.1016/S0022-5347(05)00005-4. [DOI] [PubMed] [Google Scholar]
- Lin SY, Lai WW, Ho ChCh, et al. Emodin induces apoptosis of human tongue squamous cancer SCC-4 cells through reactive oxygen species and mitochondria-dependent pathways. Anticancer Res. 2009;29:327–35. [PubMed] [Google Scholar]
- Linder S, Shoshan MC. Lysosomes and endoplasmic reticulum:Targets for improved, selective anticancer therapy. Drug Resist Updat. 2005;8:199–204. doi: 10.1016/j.drup.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Marzella L, Glaumann H. Increased degradation in rat liver induced by vinblastine. I. Biochemical characterization. Lab Invest. 1980;42:8–17. [PubMed] [Google Scholar]
- Michalik M, Pierzchalska M, Pabiańczyk-Kulka A, Korohoda W. Procaine-induced enhancement of fluid-phase endocytosis and inhibition of exocytosis in human skin fibroblasts. Eur J Pharmacol. 2003;475:1–10. doi: 10.1016/s0014-2999(03)02000-4. [DOI] [PubMed] [Google Scholar]
- Mijatovic S, Maksimovic-Ivanic D, Radovic J, et al. Anti-glioma action of aloe emodin:the role of ERK inhibition. Cell Mol Life Sci. 2005;62:589–98. doi: 10.1007/s00018-005-4425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic S, Maksimovic-Ivanica D, Radovica J, et al. Aloe-emodin prevents cytokine-induced tumor cell death:the inhibition of auto-toxic nitric oxide release as a potential mechanism. Cell Mol Life Sci. 2004;61:1805–15. doi: 10.1007/s00018-004-4089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y, Takano T, Ohkuma S. Acridine orange as a fluorescent probe for lysosomal proton pump. J Biochem. 1982;92:1333–6. doi: 10.1093/oxfordjournals.jbchem.a134053. [DOI] [PubMed] [Google Scholar]
- Morselli E, Tasdemir E, Maiuri MCh, et al. Mutant p53 protein localized in the cytoplasm inhibits autophagy. Cell Cycle. 2008;7:3056–61. doi: 10.4161/cc.7.19.6751. [DOI] [PubMed] [Google Scholar]
- Nicolini C, Belmont A, Parodi S, et al. Mass action and acridine orange staining:static and flow cytofluorometry. J Histochem Cytochem. 1979;27:102–13. doi: 10.1177/27.1.86559. [DOI] [PubMed] [Google Scholar]
- Pecere T, Gazzola MV, Mucignat C, et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2002;60:2800–4. [PubMed] [Google Scholar]
- Pecere T, Sarinella F, Salata C, et al. Involvement of p53 in specific anti-neuroectodermal tumor activity of aloe-emodin. Int J Cancer. 2003;106:836–47. doi: 10.1002/ijc.11312. [DOI] [PubMed] [Google Scholar]
- Piao S, Amaravadi RK. Targeting the lysosome in cancer. Ann N Y Acad Sci. 2016;1371:45–54. doi: 10.1111/nyas.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivtoraiko VN, Stone SL, Roth KA, Shacka JJ. Oxidative Stress and Autophagy in the Regulation of Lysosome-Dependent Neuron Death. Antioxid Redox Signal. 2009;11:481–96. doi: 10.1089/ars.2008.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundquist I, Olsson M, Brunk U. Cytofluorometric quantitation of acridine orange uptake by cultured cells. Acta Pathol Microbiol Immunol Scand A. 1984;92:303–9. doi: 10.1111/j.1699-0463.1984.tb04408.x. [DOI] [PubMed] [Google Scholar]
- Sydiskis RJ, Owen DG, Lohr JL, et al. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob Agents Chemother. 1991;35:2463–6. doi: 10.1128/aac.35.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardy C, Codogno P, Autefage H, et al. Lysosomes and lysosomal proteins in cancer cell death (new players of an old struggle) Biochim Biophys Acta. 2016;1765:101–25. doi: 10.1016/j.bbcan.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Terman A, Kurz T, Gustafsson B, Brunk UT. Critical Review Lysosomal Labilization. Life. 2006;58:531–9. doi: 10.1080/15216540600904885. [DOI] [PubMed] [Google Scholar]
- Tian B, Hua Y-J, Ma X-Q, Wang G-L. Relationship between antibacterial activity of aloe and its antraquinone compounds. Zhongguo Zhong Yao Za Zhi. 2003;28:1034–7. [PubMed] [Google Scholar]
- Wang X, Feng Y, Wang N, et al. Chinese Medicines Induce Cell Death:The Molecular and Cellular Mechanisms for Cancer Therapy. Biomed Res Int. 2014;2014:1–14. doi: 10.1155/2014/530342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ren D. Lysosomal Physiology. Annu Rev Physiol. 2015;77:57–80. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Zhang T, Tian G. Preparative isolation and purification of hydroxyanthraquinones from Rheum officinale Baill by high-speed counter-current chromatography using pH-modulated stepwise elution. J Chromatogr A. 1999;858:103–7. doi: 10.1016/s0021-9673(99)00827-4. [DOI] [PubMed] [Google Scholar]
- Yen G-C, Duh P-D, Chuang D-Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000;70:437–41. [Google Scholar]
- Yi Y-P, Wang X, Zhang G, et al. Phosphatidic Acid Osmotically Destabilizes Lysosomes through Increased Permeability to K+and H+ Gen Physiol Biophys. 2006;25:149–60. [PubMed] [Google Scholar]
- Zdolsek JM, Olsson GM, Brunk UT. Photooxidative damage to lysosomes of cultured macrophages by acridine orange. Photochem Photobiol. 1990;51:67–76. doi: 10.1111/j.1751-1097.1990.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Zhang L-M, Xie W-G, Wen T-T, Zhao X. Thermal behavior of five free anthraquinones from rhubarb. J Therm Anal Calorim. 2010;100:215–18. [Google Scholar]
- Zhong J, Huang Y, Ding W, et al. Chemical constituents of Aloe barbadensis Miller and their inhibitory effects on phosphodiesterase-4D. Fitoterapia. 2013;91:159–65. doi: 10.1016/j.fitote.2013.08.027. [DOI] [PubMed] [Google Scholar]


