Abstract
Background:
High-grade gliomas, with glioblastomas as the most frequently observed histologic subtype, are the most common primary brain tumours in adults. It is considered that inflammatory responses play a major role in malignancies, including tumour progression. This study aimed to determine the prognostic significance of the neutrophil to lymphocyte ratio (NLR) and the thrombocyte to lymphocyte ratio (PLR) as indicators of systemic inflammatory response (SIR) in glioblastoma patients.
Methods:
A total of 90 patients treated for glioblastoma were retrospectively evaluated. Absolute counts were used to generate NLR and PLR. A SIR was considered to be present with an NLR ≥5 and/or PLR ≥150.
Results:
Median follow-up time was 11.3 months (range: 1-70 months). The 1-year and 2-year overall survival rates were 55.2% and 19.5%, respectively. Univariate analysis showed that there was no correlation between overall survival and gender (p=0.184), comorbid disease (p = 0.30), clinical presentation (p = 0.884), or tumour lateralization (p = 0.159). Multivariate analysis showed that overall survival was significantly correlated with SIR based on NLR (HR: 2.41), and ECOG performance status (HR: 1.53). The prognostic factors that affected survival, other than SIR, were Eastern Cooperative Oncology Group (ECOG) performance status (p = 0.003), and tumour localization (p = 0.006).
Conclusion:
The present findings confirm that NLR based on peripheral blood counts prior to treatment can be used as a prognostic factor in patients with glioblastoma. Since tumour aggression increases and survival decreases as the NLR value rises, choice of treatment modality is facilitated for glioblastoma patients.
Keywords: Glioblastoma, prognosis, neutrophil to lymphocyte ratio, thrombocyte to lymphocyte ratio
Introduction
High-grade gliomas, among which glioblastomas are the most frequently observed histologic subtype, are the most common primary brain tumours in adults. The incidence of glioblastoma in adults is 3-4/100,000 patients, versus 1/1,000,000 in children (Van Gool, 2015). The standard treatment for glioblastoma consists of maximal safe resection, followed by concomitant chemoradiotherapy using temozolomide and adjuvant temozolomide therapy, which is known as the Stupp protocol (Stupp et al., 2005). Despite advances in treatment, median survival in patients with glio-blastoma is 12-15 months. Due to the high rate of mortality in patients with glioblastoma, researchers have been searching for prognostic factors other than age, Karnofsky Performance Status (KPS), etc.
In addition to such benign disorders as infectious and autoimmune diseases, malignancies can also cause chronic inflammation (Turkdogan et al., 2016; Hussein and Ahmed, 2005). It was reported that the inflammatory response plays a major role in malignancy, including tumour progression (Grivennikov, 2010). Neutrophils and lymphocytes are immune cells, and are a part of the tumour microenvironment that plays a regulatory role in carcinogenesis. Even though the precise mechanism of systemic inflammatory response (SIR) in cancer patients is not known, hypoxia due to tumour necrosis, changes in neuroendocrine metabolism, synthesis of interleukin, and acute phase proteins are considered possible causes of SIR. SIR is reported to be associated with poor prognosis in patients with a variety of solid tumours (Forrest et al., 2003).
Recently, numerous studies reported that the neutrophil to lymphocyte ratio (NLR), based on the neutrophil and lymphocyte counts in peripheral blood, is a good indicator of SIR (Gunaldi et al., 2015; Atzpodien et al., 2003; Fogar et al., 2006). The neutrophil and lymphocyte counts in peripheral blood are also correlated with immune cell counts in tumour stroma (Pollard, 2004). As such, it was concluded that NLR is a simple and inexpensive method for detecting SIR. In addition to NLR, the thrombocyte to lymphocyte ratio (PLR) has also been investigated as an indicator of SIR (McMillan, 2009). The present study aimed to determine the prognostic role of the SIR indica-tors, NLR and PLR, in patients with glioblastoma.
Materials and Methods
Patients and Methods
Patients diagnosed as glioblastoma that were treated between 2011 and 2015 at Süleyman Demirel University, Department of Radiation Oncology, Isparta, Turkey, and Medicalpark Gazian-tep Hospital, Gaziantep, Turkey, were retrospectively evaluated. Patients with a high-grade glioma other than glioblastoma, patients that had undergone blood transfusion ≤3 months prior to diagnosis, and those with active bleeding, bleeding diathesis, hyper or hypothyroidism, infection, disseminated intravascular coagulation, heparin treatment, or connective tissue diseases were excluded from the study. Patients that underwent a complete blood count and biochemical analysis, including liver and renal function tests, prior to receiving any treatment including steroids was analysed. NLR was calculated as the ratio of the absolute neutrophil count to the absolute lymphocyte count, and PLR was calculated as the ratio of the absolute thrombocyte count to the absolute lymphocyte count. SIR was considered to be present in patients with an NLR ≥5 and/or PLR ≥150. Statistical analysis was performed using SPSS for Windows v.15.0 (SPSS, Inc., Chicago, IL). The logrank test was used for univariate analysis of survival. Prognostic factors for survival were analysed via Cox regression analysis (multivariate analysis). Survival rates were calculated using KaplanMeier survival analysis. Results were considered statistically significant when the type 1 error was<0.05.
Results
Patient and tumour characteristics Among the 90 patients analysed, 39 (43%) were female and 51 (57%) were male. Median age of the patients was 58.5 years (range: 16-93 years) (Table 1). Eastern Cooperative Oncology Group (ECOG) performance status was as follows: 0 (n = 34 (37.8%)); 1 (n = 16 (17.8%)); 2 (n = 25 (27.8%)); 3 (n = 15 (16.7%)). Comorbid disease was noted in 25 patients, and type II diabetes mellitus (n = 6) and essential hypertension (n = 6) were the most common. In total, 6 patients had both type II diabetes mellitus and essential hypertension.
Table 1.
Laboratory Values of Patients
| Mean. Standard Deviation | Median | |
|---|---|---|
| Age | 55.7±16.3 | 58.5 |
| AST (U/L) | 22.2±10.8 | 20 |
| ALT (U/L) | 34.8±24.4 | 30 |
| BUN (mg/dl) | 17.3±8.3 | 15 |
| Cre (mg/dl) | 0.89±0.0.7 | 0.78 |
| WBC (103/mm3) | 8.34±3.31 | 7.6 |
| HGB (g/dl) | 12.9±1.6 | 13 |
| PLT(103/mm3) | 249.8±74.9 | 245 |
| Neutrophils | 7.89±5.0 | 6.1 |
| Lymphocytes | 1.84±1.06 | 1.7 |
Headache was the most common symptom at presentation (n = 35 (38.9%)). Neurological deficit, epileptic seizures, and syncope were observed in 30 (38.9%), 17 (18.9%), and 5 (8.9%) patients, respectively. In total, 87 (96.7%) patients were diagnosed as primary glioblastoma and 3 (3.3%) as secondary glioblastoma. Lesion lateralisation was the left hemisphere in 44 (48.9%) pa-tients and the right hemisphere in 40 (44.4%). Tumour localisation was the frontal, temporal, and occipital lobes in 30%, 30%, and 6.7% of the patients, respectively. Tumour localisation could not be determined in 7 patients. Gross total excision was performed in 38 (53.3%) patients, whereas 34 (37.8 %) patients underwent subtotal excision. In 6 patients biopsy only was performed. In 18 patients diagnostic procedures information was failed to obtain. Postoperatively, 11 patients received radiotherapy only, and 75 (83.3%) received chemoradiotherapy. Adjuvant temozolomide was given to 65% (n = 59) of the patients, and the median number of adjuvant treatment cycles was 6 (range: 1-12). Adjuvant treatment data were missing for 3 of the patients.
NLR, PLR, and SIR
Based on NLR values, SIR was present in 32 (35.6%) patients and absent in 58 (64.4%). Based on PLR values, SIR was present in 48 (53 %) patients and absent in 42 (46.7%). Median duration of follow up was 11.3 months (range: 1-70 months). The 1-year overall survival (OS) rate was 55.2%, versus 2-year OS of 19.5% (Figure 1). OS was significantly correlated with SIR based on NLR (P = 0.048) (Figure 2). In SIR-negative patients (according to NLR) median survival was 15.7 ± 2.5 months (95% CI: 10.6-20.7), versus 11.8 ± 4.7 months (95% CI: 10.4-18.9) in SIR positive patients (according to NLR). SIR based on PLR was not significantly correlated with OS (P = 0.854, Figure 3): however, OS in patients with SIR based on PLR tended to be shorter than in patients without SIR (13.9 ± 1.5 months (95%CI: 10.4-17.3) and 15.7 ± 3.8 months (95% CI: 8.1-23.3), respectively). When the presence of SIR was based on NLR and PLR values above the limits mentioned above SIR was a prognostic factor for poor survival (P = 0.026) (Figure 4). Median survival was 16 ± 2.2 months (95% CI: 11.7-20.4) in patients without SIR, versus 11.8 ± 4.7 months (95% CI:2.6-21) in those with SIR. Univariate analysis showed that there wasn’t a correlation between OS and gender (p = 0.184), comorbid disease (p = 0.30), clinical presentation (p = 0.884), or tumour lateralisation (p = 0.159). The only prognostic factors other than SIR that affected survival were ECOG performance status (p = 0.003) and tumour localisation (p= 0.006). Multivariate analysis showed that OS was significantly correlated with the presence of SIR (based on NLR) (HR: 2.41; 95% CI: 1.26-4.58) and ECOG performance status (HR: 1.53; 95% CI: 1.15-2.05).
Figure 1.
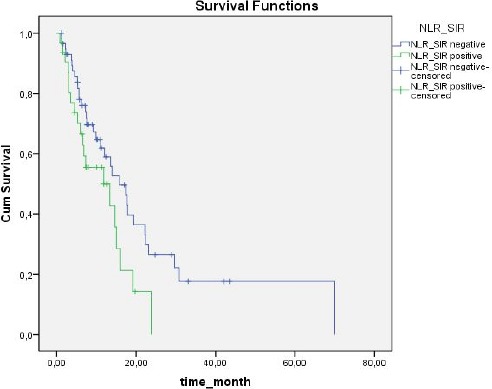
All Patients Survival
Figure 2.
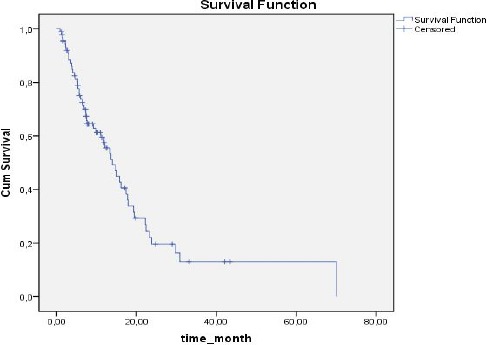
Survival According to SIR Based on NLR
Figure 3.
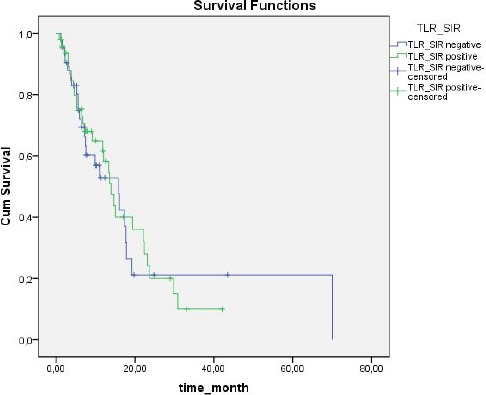
Survival According to SIR Based on PLR
Figure 4.
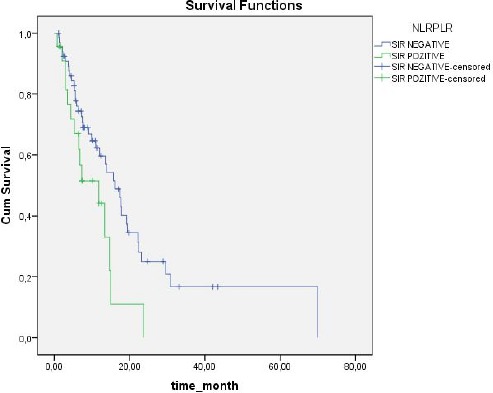
Survival According to SIR was Based on NLR and PLR Values Above the Limits Mentioned Above SIR
Discussion
The present retrospective study analysed the correlation between SIR and survival in pa-tients with glioblastoma. The presence of SIR was a poor prognostic factor and OS was significantly shorter in patients with SIR. This correlation was stronger when SIR was based on the NLR value. PLR- another marker of SIR-was not significantly correlated with survival, but there was a trend toward shorter survival in the patients with SIR based on PLR. Recent studies reported the prognostic role of NLR in patients with a variety of solid tumours, including lung, gastric, extrahepatic cholangiocarcinoma, ovarian, and colorectal cancer, and in patients with liver metastasis of colorectal cancer (Halazun, et al., 2008; Sharma et al., 2008, Kitano et al., 2017). Inflammatory cells play a major role in tumour progression and an elevated neutrophil count in tumour stroma is a poor prognostic factor (Turkdogan et al., 2016). Cytokines and chemokine secreted by neutrophils increase tumour angiogenesis, which induces tumour progression and metastasis. NLR obtained via the peripheral blood count is a good indicator of inflammation in tumour stroma, and as an elevated neutrophil count in tumour biopsy specimens is prognostically important, inflammation based on NLR in peripheral blood is also of prognostic importance (Balkwill and Mantovani, 2001).
Inflammatory cells direct the interaction between tumour cells and the tumour microenvironment (Grivennikov et al., 2010; Del Prete et al., 2011). Hanahan and Weinberg (2011) showed that uncontrolled inflammation is a critical factor for tumour proliferation. Even though the underlying mechanisms are not fully known, the neutrophil count does increase in patients with cancer. Some hypothesised that inflammatory cytokines or reactive oxygen species secreted by tumour cells cause the neutrophil count to increase, both in tumour stroma and in peripheral blood. This increase in the neutrophil count causes a decrease in lymphocytes and lymphocyte apoptosis. In patients with locally advanced lung cancer a decreased lymphocyte count has been shown to be a poor prognostic factor and it was postulated that an elevated NLR could be a sensitive marker of tumour recurrence (Gregory and Houghton, 2011; McMillan, 2008).
The role of host immunity in glioblastoma biology was first suggested in an epidemiological study in which it was observed that the incidence of glioblastoma was lower in patients with a history of allergy (Schlehofer et al., 1999). Another study that evaluated pathological specimens of patients with glioblastoma reported that effector T-cell infiltration in tumour stroma was a good prognostic factor (Lohr et al., 2011). The underlying mechanisms of the role of an elevated NLR in cancer prognosis are not fully understood. One possible mechanism is the driving force of systemic inflammation on secretion of angiogenic factors, growth factors, and other proneoplastic signals, as proposed by Hanahan and Weinberg (2011). An increase in the neutrophil count causes a decrease in the lymphocyte count, and as a result cellular immunity in other words, immunity against tumour cells is depressed (Zadora et al., 2015). Bambury et al., (2013) studied the effect of SIR on prognosis in patients with glioblastoma, based on retrospective evaluation of 84 patients. They considered SIR to be present in patients with an NLR >4. They also reported that based on multivariate analysis the presence of SIR was an independent poor prognostic factor. Furthermore, they showed that when NLR was added to nomograms that included age, ECOG performance status, and extension of surgical resection the index’s concordance increased. Another retrospective study by Han et al., (2015) that in-cluded 154 patients with glioblastoma reported that NLR was an independent prognostic factor for survival. They reported that there was a significant correlation between NLR and PLR values, but that based on multivariate analysis PLR was not a significant prognostic factor for survival. They also studied the concordance between NLR, and the neutrophil count and CD3+ T-lymphocyte infiltration in tumour tissue, reporting that patients with an NLR >4 had an elevated neutrophil count and decreased CD3+ T-lymphocyte infiltration in tumour tissue. McNamara et al., (2014) reported the importance of NLR prior to reapportion for glioblastoma. They reported that patients with an NLR ≤4 had significantly longer survival (9.7 months) than those with an NLR >4 (5.9 months) (P = 0.02). Zadora et al., (2015) retrospectively studied 424 patients with brain tumours, and observed a significant correlation between tumour grade and NLR. The NLR cut-off value was highest in patients with glioblastoma and was 2.579 in patients with high-grade lesions.
The prognostic importance of NLR and PLR varies according to tumour type. Whereas PLR is a better prognostic factor for oesophageal cancer, NLR is a significantly better prognostic factor for endometrial cancer (Feng et al., 2014; Haruma et al., 2015). It has been shown that NLR is an important prognostic factor for glioblastoma (Han et al., 2015). In accordance with the literature, the present findings show that NLR is a better prognostic factor than PLR in patients with glioblastoma. The difference between the cut-off value noted in the present study and that in earlier studies might be due to differences in the populations studied.
The NLR cut-off value also varies according to tumour type; 5 for lung cancer, versus 2.56 for gastric cancer (Gunaldi et al., 2015; Kaya et al., 2013). Our study was done with patients from two separate centers. Blood count devices were different but, we think that our study does not affect the results of using different blood count devices because it contains the ratio for neutrophil lymphocytes and platelets. Furthermore, the present findings confirm that NLR based on the peripheral blood count prior to treatment can be used as a prognostic factor in patients with glioblastoma. As tumour aggression increases and survival decreases as the NLR value increases, aggressive treatment modalities (such as new targeted therapies or immunotherapy can be used in this selected patient population.
Conflict of interest
No conflict of interest.
References
- Atzpodien J, Royston P, Wandert T, Reitz M. DGCIN-German cooperative renal carcinoma chemo-immunotherapy trials group metastatic renal carcinoma comprehensive prognostic system. Br J Cancer. 2003;88:348–53. doi: 10.1038/sj.bjc.6600768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer:back to virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Bambury RM, Teo MY, Power DG, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114:149–54. doi: 10.1007/s11060-013-1164-9. [DOI] [PubMed] [Google Scholar]
- Del Prete A, Allavena P, Santoro G, et al. Molecular path-ways in cancer-related inflammation. Biochem Med. 2011;21:264–75. doi: 10.11613/bm.2011.036. [DOI] [PubMed] [Google Scholar]
- Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. doi: 10.1186/1477-7819-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer:an index of adverse outcome. Pancreas. 2006;32:22–8. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]
- Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumula-tive prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–30. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AD, Houghton AM. Tumor-associated neutrophils:new targets for cancer therapy. Cancer Res. 2011;71:2411–16. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–9. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaldi M, Goksu S, Erdem D, et al. Prognostic impact of platelet/lymphocyte and neutro-phil/lymphocyte ratios in patients with gastric cancer:a multicenter study. Int J Clin Exp Med. 2015;8:5937–42. [PMC free article] [PubMed] [Google Scholar]
- Halazun KJ, Aldoori A, Malik HZ, et al. Elevated preoperative neutrophil to lymphocyte ra-tio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Han S, Liu Y, Li Q, et al. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15:617. doi: 10.1186/s12885-015-1629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer:the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Haruma T, Nakamura K, Nishida T, et al. Pre-treatment neutrophil to lymphocyte ratio is a predictor of prognosis in endometrial cancer. Anticancer Res. 2015;35:337–43. [PubMed] [Google Scholar]
- Hussein MR, Ahmed RA. Analysis of the mononuclear inflammatory cell infiltrate in the non-tumorigenic, pre-tumorigenic and tumorigenic keratinocytic hyperproliferative lesions of the skin. Cancer Biol Ther. 2005;4:819–21. doi: 10.4161/cbt.4.8.1864. [DOI] [PubMed] [Google Scholar]
- Kaya V, Yildirim M, Demirpence O, Yildiz M, Yalcin AY. Prognostic significance of basic laboratory methods in non- small-cell-lung cancer. Asian Pac J Cancer Prev. 2013;14:5473–6. doi: 10.7314/apjcp.2013.14.9.5473. [DOI] [PubMed] [Google Scholar]
- Kitano Y, Yamashita YI, Yamamura K, et al. Effects of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios on survival in patients with extrahepatic cholan-giocarcinoma. Anticancer Res. 2017;37:3229–37. doi: 10.21873/anticanres.11685. [DOI] [PubMed] [Google Scholar]
- Lohr J, Ratliff T, Huppertz A, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17:4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc U S A. 2008;67:257–62. doi: 10.1017/S0029665108007131. [DOI] [PubMed] [Google Scholar]
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–6. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- McNamara MG, Lwin Z, Jiang H, et al. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutro-phil/lymphocyte ratio and time to first progression. J Neurooncol. 2014;117:147–52. doi: 10.1007/s11060-014-1366-9. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Schlehofer B, Blettner M, Preston-Martin S, et al. Role of medical history in brain tumour development. Results from the international adult brain tumour study. Int J Cancer. 1999;19:155–60. doi: 10.1002/(sici)1097-0215(19990719)82:2<155::aid-ijc1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Sharma R, Hook J, Kumar M, Gabra H. Evaluation of an inflammation-based prognostic score in patients with advanced ovarian cancer. Eur J Cancer. 2008;44:251–6. doi: 10.1016/j.ejca.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Turkdogan KA, Eren SH, Coskun A, et al. Ratio of neutrophil to lymphocyte counts in crimean congo hemorrhagic fever kırım. J Clin Anal Med. 2016;7:10–13. [Google Scholar]
- Van Gool SW. Brain Tumor Immunotherapy:What have We Learned so Far? Front Oncol. 2015;17:98. doi: 10.3389/fonc.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadora P, Dabrowski W, Czarko K, et al. Preoperative neutrophil-lymphocyte count ratio helps predict the grade of glial tumor - a pilot study. Neurol Neurochir Pol. 2015;49:41–4. doi: 10.1016/j.pjnns.2014.12.006. [DOI] [PubMed] [Google Scholar]


