Abstract
Cancer is one of the most common causes of death in the developed world, with one-third of people diagnosed with cancer during their lifetime. Oral cancer commonly occurs involving the buccal mucosa (cheeks), tongue, floor of the mouth and lip. It is one of the most devastating and disfiguring of malignancies. Morinda citrifolia L., commonly known as ‘noni’, belongs to the Rubiaceae family. It is native to the Pacific islands, Hawaii, Caribbean, Asia and Australia. The plant displays broad curative effects in pharmacological studies. Damnacanthal (DAM) and Nordamnacanthal (NDAM), anthraquinone compounds isolated from the roots of Morinda citrifolia L., has been used for the treatment of several chronic diseases including cancer. The objectives of this study were to evaluate cytotoxicity, morphological changes, cell death mode (apoptosis/necrosis), and cell migration induced by DAM and NDAM on the most common type of oral cancer, oral squamous cell carcinoma (OSCC)cells. Anti-proliferative effects of these compounds against OSCC cell lines were determined by MTT assay. The mode of cell death was analysed by phase contrast and fluorescent microscopy as well as flow cytometry. In addition, cell migration was assessed. The results showed that DAM and NDAM exerted cytotoxicity against OSCC cells with IC50 values of 1.9 to >30 μg/ml after 72 h treatment. Maximum growth inhibition among the tested cell lines for both compounds was observed in H400 cells, and thus it was selected for further study. The study demonstrated inhibition of H400 OSCC cell proliferation, marked apoptotic morphological changes, induction of early apoptosis, and inhibition of cell migration by DAM and NDAM. Therefore, this information suggests that these compounds from noni have potential for used as anti tumor agents for oral cancer therapy.
Keywords: Damnacanthal and nordamnacanthal, Human oral squamous cell carcinoma (OSCC), Cytotoxicity
Introduction
Cancer is one of the most common causes of death in the developed world. One-third of people will be diagnosed with cancer during their lifetime. Oral cancer, a subtype of head and neck cancer, is an abnormal growth found in the mouth region. Malignant tumours of the oral cavity account for approximately 30% of all head and neck cancers, and 80% of these tumours are oral squamous cell carcinoma (OSCC) (Krishnan et al., 2014). Oral carcinomas are the world’s eleventh most common form of human neoplasm and account for 3% of all newly diagnosed cancer cases (Khan et al., 2015). Oral cancer is a significant disease worldwide with up to 400,000 new cases every year and almost 130,000 deaths annually (Al-Maweri et al., 2014). Oral cancers may originate in any of the tissues of the mouth, and may be of varied histologic types: teratoma, adenocarcinoma derived from a serious or minor salivary gland, lymphoma from tonsillar or other lymphoid tissue, or melanoma from the pigment-producing cells of the oral mucosa. There are several types of oral cancers, but around 90% are squamous cell carcinomas, originating in the tissues that line the mouth and lips. Oral or mouth cancer most commonly involves the tongue. It may also occur on the floor of the mouth, cheek lining, gingiva (gums), lips, or palate (roof of the mouth). The most prevalent oral cancer is oral squamous cell carcinoma (OSCC), which comprises 90% of all oral cancers (Neville and Day, 2002). In Malaysia, the incidence of oral cancer differs by gender and ethnic group. Indian and indigenous people of Malaysia have the highest prevalence of this cancer (Ghani et al., 2011; Al-Dubail et al., 2012). Oral squamous cell carcinoma has high mortality and also morbidity rates around the world since it is commonly seen in advanced stages before treatment (Johnson et al., 2011). Heavy or regular use of tobacco (Castellsagué et al., 2004), excessive alcohol consumption (Johnson and Warnakulasuriya, 1993), fruit and vegetable deficiency in diet (Pavia et al., 2006), chewing paan and betel nut in addition to poor oral hygiene (Balaram et al., 2002) are the major risk factors regarding oral cancer, with smoking and alcohol having synergistic effects (Blot et al., 1988). Generally, oral cancer risk is linked with age and gender. The number of people affected by oral cancer is increased with advancing the age and also oral cancer occurrence is especially high in males (Ariyoshi et al., 2008).
The typical strategies of OSCC treatment rely on surgery, radiotherapy, chemotherapy and targeted therapy (Scully and Bagan, 2009). Oral cancer therapy basically aims to recognize chemopreventive as well as treatment agents that selectively target OSCC cells without having cytotoxic impacts on normal cells (Sato et al., 2013). Regardless of recent progress in oral squamous cell carcinoma therapy, the prognosis of these patients has not ameliorated significantly over the past 20 years (Sato et al., 2013). Development of resistance and unbearable side effects of current chemotherapy drugs are considerable problems that need to be settled (Haghiac and Walle, 2005). Roughly 74% of therapeutic agents that were accepted for cancer therapy have been derived from natural origins (Gordaliza, 2007). Therefore, one of the reasonable and effective strategies regarding cancer chemoprevention is the research for new anti-tumour agents from plant sources (George et al., 2012).
Damnacanthal and nordamnacanthal comprise a general class of athraquinone derivatives. Damnacanthal or 3-hydroxy-1- me thoxyanthr aquinone -2- carboxaldehyde (C16H10O5), occurs as pale yellow crystals with a melting point of 210-211°C whereas nordamnacanthal or 2-formyl-1,3-dihydroxyanthraquinone (C15H8O5), are orange yellow crystals with melting point of 214- 218°C (Alitheen et al., 2010). Damnacanthal and nordamnacanthal have some unique chemical and biological properties. Both these naturally occurring quinone are present widely in the Morinda species. Morinda citrifolia L., commonly known as ‘noni’, belongs to the Rubiaceae family. It is native to the Pacific islands, Hawaii, Caribbean, Asia and Australia (Brown, 2012; Chan-Blanco et al., 2006). Both DAM and NDAM incorporate some exclusive chemical and biological characteristics (Alitheen et al., 2010). Ali et al., (2000) reported that damnacanthal and nordamnacanthal isolated from M. ellipticawere cytotoxic towards the MCF-7 (breast carcinoma) and CEM-SS (T-lymphoblastic leukemia) cell lines (Kanokmedhakul et al., 2005). DAM displayed cytotoxic activity against breast cancer cell lines along with small cell lung cancer cell lines (Kanokmedhakul et al., 2005). In addition, it was documented that DAM isolated from the root of noni acted as an inhibitor associated with ras function, which is considered to be linked to the signal transduction in various human cancers including colon, lungs and leukaemia (Hiramatsu et al., 1993). Among the reported anthraquinones, DAM has distinctive features regarding the function for its anti-cancer and anti-HIV activity (Hiramatsu et al., 1993; Kamata et al., 2006). Furthermore, DAM demonstrated a potent tyrosine kinase and also topoisomerase II inhibitory activity (Faltynek et al., 1995; Tosa et al., 1998). Additionally, DAM showed antifungal activity against Candida albicans along with antituberculosis activity against Mycobacterium tuberculosis (Kanokmedhakul et al., 2005). NDAM also features many biological properties, which includes antioxidant activities and anti-cancer effects on human B-lymphoblastoid cell lines (Jasril et al., 2003). Moreover, NDAM has been reported to demonstrate antiviral, antimicrobial in addition to cytotoxic properties (Ali et al., 2000).
Apoptotic induction is an advantageous feature in cancer therapy, due to its highly requested nature as well as absence of inflammatory response (Hsieh et al., 2006, Alabsi et al., 2013) therefore; it is worthwhile to screen apoptotic inducers coming from natural product. There are two main pathways involve in apoptosis and have been defined as extrinsic (death receptor) pathway and intrinsic (mitochondrial) pathway (Lee et al., 2015; Antonsson, 2004). Mitochondria perform crucial roles in apoptotic cell death (Kumar et al., 2009), and it is becoming one of the key targets in screening treatment agents against cancer (Galluzzi et al., 2006). The objectives of this study were to evaluate cytotoxicity, anti-proliferative, morphological changes, cell death mode (apoptosis/necrosis), and cell migration induced by DAM and NDAM on the most common type of oral cancer, oral squamous cell carcinoma cells (OSCC).
Materials and Methods
Extract preparation
Isolated Damnacanthal and Nordamnacanthal from the roots of Morinda citrifolia were kindly supplied by Prof. Dr. Nor Hadiani Ismail from Universiti Teknologi MARA (Ismail et al. 1997). The powdered-form compounds were dissolved in dimethylsulphoxide (DMSO) (Vivantis, USA) to get a stock solution of 10 mg/mL. The stock solution was then stored at -20 ºC in aliquots for future use.
Cell lines and culture conditions
The human oral squamous cell carcinoma cell lines used in this study, H103, H400, H413, H357, H376 and H314, were kindly provided by Professor. Dr. Ian Charles Paterson (University of Malaya). In addition, 3T3 (normal mouse fibroblast) cells were used as normal cells. OSCC cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 medium (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (J R Scientific, Inc., USA), 100 Units/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich) at 37ºC in a humidified atmosphere of 5% CO2. Growth and morphology of the cells were regularly monitored and the medium renewal was done every 2 to 3 days until the cells were confluent.
MTT assay
Cell growth and viability were determined using 3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. In brief, the cells were seeded into 96-well plates (Nunclon™, Denmark) at a density of 1 × 105 cells/mL in total volume of 100 μL in each well of 96-well plate. Following a culture at 37 ºC for 24 h, the cells were treated with various concentrations of DAM and NDAM (0.46, 0.93, 1.87, 3.75, 7.5, 15 and 30 μg/mL) by serial dilution in DMEM media to give a volume of 100 μL in each well of the plate. The assay for each concentration of compounds was performed in triplicate. Then, cells were incubated further at 37 ºC for 72 h. Finally, 20 μL of MTT (5 mg/mL in phosphate buffer saline [PBS]) was added directly to each well, and the plate was incubated for 4 h at 37ºC and 5% CO2 (Mosmann, 1983). After incubation, MTT were reduced to insoluble purple formazan crystals by metabolically active cells in the wells. Subsequently the media were removed and MTT formazan crystals were dissolved in 150 μL of DMSO. The optical density (OD) was measured at 575nm with reference at 650nm wavelength using Tecan Infinite 200 Pro ELISA plate reader (Tecan, Männedorf, Switzerland). The inhibitory concentration of compounds that inhibit 50% (IC50) of cell growth was determined from absorbance versus concentration curve.
Cell proliferation assay
The effect of DAM and NDAM on the proliferation of OSCC cell lines was determined by the 3- (4, 5-dimethythiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Briefly, H400 OSCC cells were plated in 96-well plate (Nunclon™, Denmark) at a concentration of 1 × 105 cells/mL in medium. The cells were then treated with three different concentrations of compounds. The selected concentrations were IC25, IC50 and IC75. The assay for each concentration of compounds was performed in triplicate, and control samples include cells without DAM and NDAM. Then the plate was incubated at 37ºC in a 5% CO2 environment for 24, 48 and 72 h. After the designated time period, The MTT assay was performed. Finally, the proliferation graph was determined by plotting the optical density values versus time.
Morphological analysis of cells by phase contrast inverted microscope
H400 OSCC cell lines were seeded in 6-well plates at a concentration of 1×105 cells/mL overnight and were then treated with DAM and NDAM at IC50 of compounds for 24, 48 and 72 hours. At the end of the incubation period, the media was removed and the cells were washed once with PBS and the morphological changes were assessed and photographed using a phase-contrast inverted microscope (Leica DMI3000B, Wetzlar, Germany) at 200× magnifications.
Morphological assessment of cells by fluorescent microscope
H400 OSCC cell lines were treated with DAM and NDAM at IC50 value of compounds for 24, 48 and 72 hours. Cells without treatment were used as a control and the assay was performed in triplicate. Following the incubation, the cells were harvested and the pellets were washed with PBS. Then the pellets were suspended in 1 μL of YO-PRO -1 (100 μM solution in DMSO) and 1 μL of propidium iodide (1.0 mg/mL solution in water) (Invitrogen, USA) for 20 min and then 10.0 μL of stained cells were pipetted onto glass slide and covered with a cover slip. Finally, the viable, apoptotic and necrotic cells were observed using fluorescent microscope (Leica DMI3000B, Wetzlar, Germany) at 200 × magnifications.
Annexin V-FITC/PI double staining
Apoptotic cell death was quantitatively determined by FITC Annexin V/propidium iodide (PI) assay kit (BD Bioscience-Pharmingen, USA) using flow cytometry. In brief, 5×105 H400 OSCC cells were washed with cold PBS and resuspended in 100 μL binding buffer, as well as stained with 5 μL of FITC-conjugated Annexin V and 5 μL of PI. Following the 15 min incubation at room temperature in the dark, 400 μL of binding buffer was added. Finally the cells were analyzed using CyAn ADP flow cytometer (Beckman Coulter, USA). Early and late apoptosis was examined on fluorescence 2 (FL2 for propidium iodide) versus fluorescence 1 (FL1 for Annexin) plots. For each sample, 10,000 events were collected. The results were analysed using Summit v4.3 software.
Cell migration assay
H400 OSCC cell lines were seeded in μ-Dish within an IBIDI culture insert (IBIDI GmbH) consists of two reservoirs separated by a 500 µm thick wall. An equal number of untreated and treated cells (70 µl; 5×105 cells/ml) were added into the two reservoirs of the same insert and incubated at 37°C and 5% CO2 for overnight. Media from the wells were removed and replaced with 70μl of media containing Mitomycin-C (10μg/ml) (Sigma Aldrich) and incubated in 37°C for 2 hours. The insert was gently removed creating a gap of ~500 µm. Finally, the μ-Dish was filled with complete media and wound closure or cell migration was observed for every 3 hours using phase-contrast inverted microscope (Leica DMI3000B, Wetzlar, Germany) at 4× magnifications. Phase-contrast images were taken from 0 to 12 h incubation for the same scratch area. The pictures were analyzed using the WimScratch software (Wimasis image analysis platform). The results were expressed as percentages of scratched and cell-covered areas.
Statistical analysis
The data were presented as mean ± SEM. The statistical differences between the treated and control groups were determined using independent-sample t test. One-way analysis of variance (ANOVA) was also used for multiple comparisons, where p<0.05 was considered to be statistically significant. Asterisks indicate significant difference (*p<0.05, **p<0.01) as compared to control.
Results
Cell viability assay
In order to determine the anticancer capability of DAM and NDAM in vitro, these compounds were examined for cell growth inhibition in OSCC cell lines (Table 2) at various concentrations after 72 hours. The compounds did not show any significant inhibitory effect against 3T3 cells. As shown in Table 1, the inhibitory effect against oral cancer cell lines was assessed with the half-maximal inhibition concentration (IC50) of DAM and NDAM. For all cell lines, maximum growth inhibition for both compounds was observed in H400 OSCC cells (1.9 ± 0.01 and 6.8 ± 0.03 μg/mL for DAM and NDAM respectively), and thus H400 was selected for further study.
Table 2.
Human OSCC Cell Lines and the Sites which the Cell Lines have been Derived.
| Cell line | H103 | H400 | H413 | H357 | H376 | H314 |
|---|---|---|---|---|---|---|
| Site | Tongue | Alveolar process | Buccal mucosa | Tongue | Floor of mouth | Floor of mouth |
Table 1.
MTT Cell Viability Assay
| Cell line | Damnacanthal | Nordamnacanthal |
|---|---|---|
| H103 | 3 ± 0.040 | 8.0 ± 0.045 |
| H400 | 1.9 ± 0.014 | 6.8 ± 0.019 |
| H413 | 2.9 ± 0.005 | 12.2 ± 0.030 |
| H357 | 2 ± 0.005 | 13 ± 0.026 |
| H376 | 2.6 ± 0.005 | 10 ± 0.062 |
| H314 | > 30 | > 30 |
| 3T3 | >30 | >30 |
Table 1. MTT cell viability assay. The effects of Damnacanthal and Nordamnacanthal on OSCC cell lines and normal cells at their respective IC50 value (μg/mL). Results are represented as mean ± SEM. The assay for each cell lines was performed in triplicate and three independent experiments.
Cell proliferation assay
The effect of DAM and NDAM on H400 OSCC cells proliferation was examined in vitro using MTT proliferation assay. This assay was performed in three different concentrations of DAM (IC25: 1.4, IC50: 1.9 and IC75: 3.4 μg/mL) and NDAM, (IC25: 3.6, IC50: 6.8 and IC75: 22 μg/mL) for 24, 48 and 72h. Untreated cells were used as control and the optical density is in proportion to the quantity of viable cells. As shown in Figure 1, untreated H400 cells exhibited an increase in OD from 24 to 72h after incubation. However, H400 cells treated with DAM and NDAM after 24, 48 and 72h, showed decrease in OD from IC25 to IC75. In other words, DAM and NDAM treatment on H400 cells showed that the optical density was lower than control in all tested concentration of compounds. Besides, Figure 2 shows the percentage of viable and non-viable cells after 24, 48 and 72h treatment with IC25, IC50 and IC75. According to bar charts the percentage of non-viable cells increased from IC25 to IC75 in all time tested.
Figure 1.
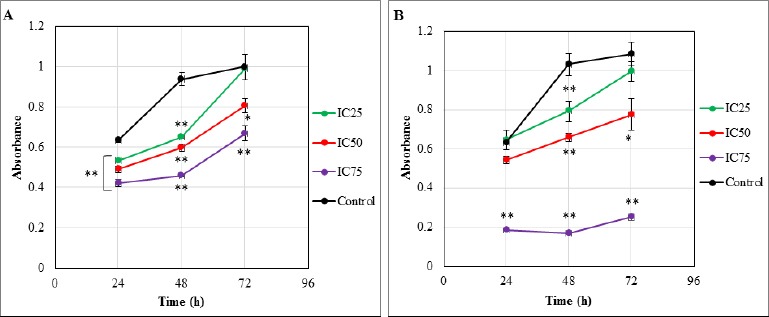
MTT Cell Proliferation Assay. The effects of DAM and NDAM on the proliferation of H400 OSCC cells after 24, 48 and 72h. (A) DAM-treated H400 cell lines and (B) NDAM-treated H400 cell lines. Untreated control H400 cells exhibited an increase in OD from 24 to 72h after incubation. In contrast, treated cells after 24, 48 and 72h, showed decrease in OD from IC25 to IC75. Besides, the optical density of treated cells was lower than control in all tested concentration of compounds. Data are presented as mean ± SEM from three independent experiments (n=4). Significant differences were compared between control and treated cells (* p < 0.05 and **p < 0.01).
Figure 2.
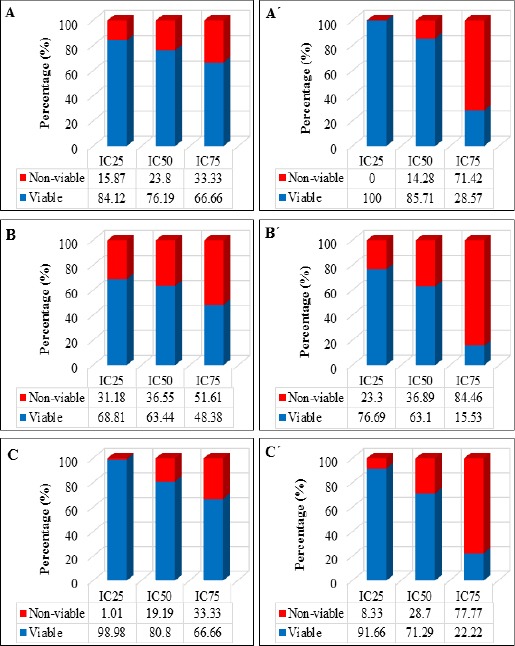
The Percentage of Viable and Non-Viable H400 OSCC Cells after 24, 48 and 72h Treated with DAM and NDAM at Three Different Concentrations (IC25, IC50 and IC75). (A), DAM-treated cells for 24h; (B), DAM-treated cells for 48h; (C), DAM-treated cells for 72h; (A´), NDAM-treated cells for 24h; (B´), NDAM-treated cells for 48h; (C´), NDAM-treated cells for 72h.
Morphological assessment of cells by phase contrast inverted microscope
To evaluate the effect of the DAM and NDAM on cell-morphological changes of H400 cells, the treatment was performed with IC50 of compounds for 24, 48 and 72 h. Morphological observation of untreated cells exhibited that the cells preserved their original morphology and also were adherent to the plates. On the other hand, the treated cells with DAM and NDAM displayed apoptotic features such as shrinkage of cells, cell blebbing, nuclear chromatin condensation and formation of apoptotic bodies. Apoptotic cells also ended in other types of morphological alteration such as losing contact with neighbouring cells and some delicate cells even detached from the plates (Figure 3).
Figure 3.
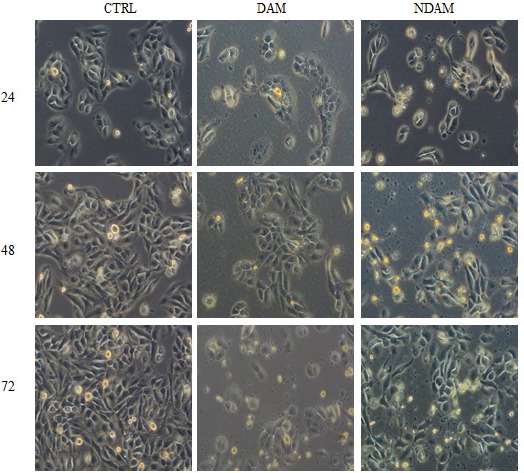
Photo- Micrographs Showing Phase Contrast Microscope Examination of H400 OSCC Cells Treated with IC50 value of DAM and NDAM for 24, 48 and 72 Hours (Magnification 200×). Untreated (control) H400 cells preserved their original morphology and were adherent to the plates. In contrast, treated H400 OSCC cells displayed obvious apoptotic features. Images are representative of two independent experiments.
Morphological analysis of cells by fluorescent microscope
The morphological changes of H400 OSCC cells treated with DAM and NDAM after 24, 48 and 72 hours were observed by YO-PRO-1 and propidium iodide staining and photographed by fluorescent microscope. The cells were categorized as viable, apoptotic and necrotic. Viable cells with undamaged DNA and nucleus showed a round and green nuclei whilst apoptotic cells with condensed chromatin displayed several green coloured nuclei and also apoptotic blebs. In addition, the DNA of the necrotic cells was stained orange to red. As shown in figure 4, H400 cells treated with DAM and NDAM demonstrated characteristic alteration and different signs of programed cell death. Furthermore, the numbers of necrotic cells were generally very low in the treated population which revealed the cell death mode was apoptosis.
Figure 4.
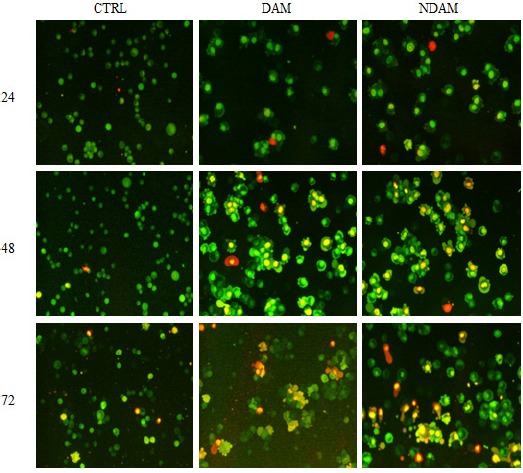
Photo- Micrographs of Fluorescence Microscopy Examination of H400 OSCC Cells Treated with IC50 value of DAM and NDAM for 24, 48 and 72 Hours (Magnification 200×). Live cells with round and green nuclei and cytoplasm were observed in high population in control cells. Apoptotic cells with condensed chromatin and blebbing feature had a uniformly green fluorescent whereas necrotic cells stained orange. Images are representative of two independent experiments.
Flow cytometric analysis of apoptosis by Annexin V-FITC/PI
Flow cytometric analysis results showed the percentage of viable cells in H400 OSCC cells treated with DAM and NDAM decreased dramatically in comparison to the control cells (Table 3 and Figure 5). Whilst, the percentage of early apoptotic cells increased from 1.22 in control to 64.04 in DAM and 10.86 in NDAM-treated cells after 12h treatment. In addition, no considerable changes were observed in the percentage of early apoptotic cells after 24 and 48h treatment with DAM and NDAM compare to control cells. On the other hand, the percentage of late apoptotic/secondary necrotic cells were increased significantly (from 3.23 in control to 22.74 and 86.04 after 12h treatment, from 6.47 in control to 83.75 and 85.86 after 24h treatment, and finally from 8.50 in control to 84.11 and 88.12 after 48h treatment in DAM and NDAM-treated cells respectively) (Table 3 and Figure 5). Therefore, this study confirms that apoptosis is the major mode of cell death induced by these two compounds in H400 OSCC cells.
Table 3.
Annexin-V FITC/PI Staining Flow Cytometery
| Time (hour) | Treatment | Quadrants | |||
|---|---|---|---|---|---|
| R3 (%) | R4 (%) | R5 (%) | R6 (%) | ||
| 12 | CTRL | 1.47 ± 0.04 | 3.23 ± 0.13 | 94.09 ± 0.75 | 1.22 ± 0.06 |
| DAM | 0.53 ± 0.05 | 22.74 ± 0.67 | 12.69 ± 0.42 | 64.04 ± 0.70 | |
| NDAM | 1.68 ± 0.04 | 86.04 ± 1.00 | 1.42 ± 0.08 | 10.86 ± 0.43 | |
| CTRL | 1.53 ± 0.03 | 6.47 ± 0.34 | 85.53 ± 0.87 | 6.47 ± 0.08 | |
| 24 | DAM | 1.38 ± 0.03 | 83.75 ±1.51 | 6.63 ± 0.35 | 8.24 ± 0.13 |
| NDAM | 4.67 ± 0.09 | 85.86 ± 1.79 | 8.27 ± 0.25 | 1.20 ± 0.06 | |
| CTRL | 0.14 ± 0.01 | 8.50 ± 0.20 | 82.82 ± 0.92 | 8.53 ± 0.21 | |
| 48 | DAM | 2.70 ± 0.13 | 84.11± 1.38 | 6.18 ± 0.32 | 7.01 ±0.23 |
| NDAM | 3.74 ± 0.17 | 88.12 ± 0.69 | 7.44 ± 0.12 | 0.70 ± 0.16 | |
Figure 5.
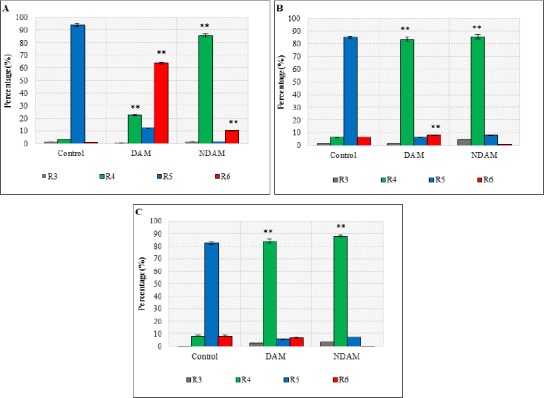
The Effects of DAM and NDAM on H400 OSCC Cells after 12, 24 and 48h Using Annexin V-FITC/PI Staining Flow Cytometery. (A) 12h, (B) 24h, and (C) 48h. Treatment of H400 OSCC cells with both compounds induced apoptosis through the observation of a shift in live cell population from early to late stage of apoptosis /secondary necrosis. Significant difference from the control was *p < 0.05 and **p < 0.01.
Figure 6.
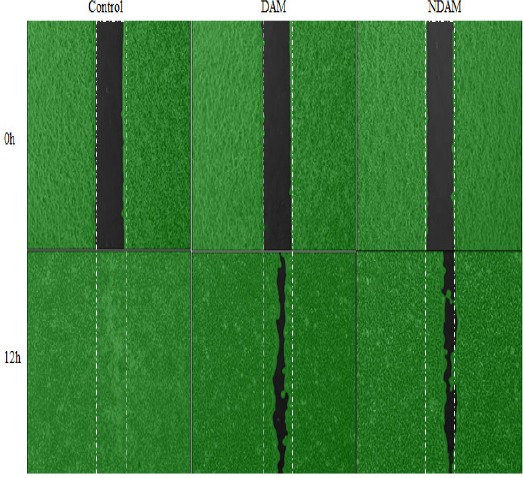
The Effect of DAM and NDAM on H400 OSCC Cells Migration. Cell migration was observed and photographed, for every 3 hours after creation of 500 µm gap, using phase-contrast inverted microscope at 50× magnifications. The images were analyzed using the WimScratch software (Wimasis image analysis platform) and are representative of three independent experiments.
Figure 7.
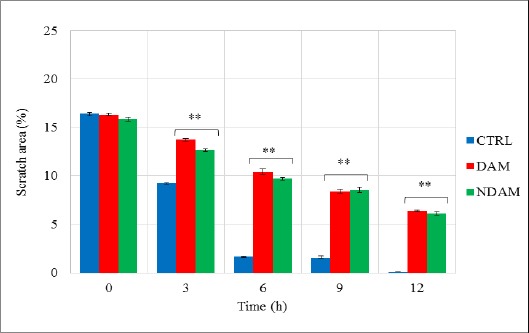
DAM and NDAM Effects on the Cellular Migratory Ability of H400 OSCC Cells. The results were expressed as percentages of scratched area revealing that DAM and NDAM significantly reduced migration of H400 OSCC cells compared to the control. The values are expressed as means ± SEM from three individual experiments. Statistical significant differences between control and treated cells were set at *p < 0.05 and **p < 0.01.
Cell migration analysis
The migratory ability of H400 OSCC cells were analysed in vitro using μ-Dish contains an IBIDI culture insert consists of two reservoirs separated by a 500 µm thick wall. Monolayer scratch migration assay was performed in untreated H400 OSCC cell line (Control) and treated with DAM and NDAM. This assay was performed in the presence of Mitomycin-C (10μg/ml). Mitomycin-C was chosen to block the cell proliferation activity and to prove that wound healing assay (gap closure) entirely attributed to the cell migration. In this study, following the creation of ~500 µm gap, phase-contrast images were taken from the same scratch area and the pictures were analysed using the WimScratch software. According to the results, upon incubation, the untreated H400 cells started to migrate in order to fill the gap area and complete confluency was achieved after 12 hours while the exposure of the H400 cells to DAM and NDAM slowed down the cells’ migration. Therefore, these results suggest that DAM and NDAM inhibited the migration capability of H400 OSCC cell line significantly (**p < 0.01).
Discussion
Damnacanthal and nordamnacanthal are anthraquinones that are generated mostly by plants of the Rubiaceae family (Alitheen et al., 2010). There is little knowledge of the effectiveness of noni and its anthraquinones in cancer therapy, particularly regarding the effect of DAM and NDAM on various cancer cells. First studies regarding the effect of Morinda citrifolia L. (noni) on cancer cells go back to early 1990s (Hiramatsu et al., 1993). Therefore, in the present study, the cytotoxic effect of Damnacanthal and Nordamnacanthal on proliferation, apoptosis and migration of oral squamous cell carcinoma (OSCC) was studied and our results showed that DAM and NDAM led to an anti-growth effect on H400 OSCC cells by both suppression of cell proliferation and activation of apoptosis pathways.
In the current study, cytotoxic potential of DAM and NDAM on oral squamous cell carcinoma cell lines were evaluated by MTT assay. According to the United States National Cancer Institute Plant Screening Program, a crude extract is generally considered to induce active cytotoxic effect if the IC50 value in cancer cells, following incubation between 48 and 72 hours, is 20 μg/mL or less, while it is less than 4 μg/mL for pure compounds (Boik, 2001; Ramasamy et al., 2012). As shown in Table 1, among the treated cell lines with DAM and NDAM, the maximum growth inhibition for both compounds was detected in H400 OSCC cells with IC50 values of 1.9 and 6.8 μg/mL, respectively. In other words, DAM and NDAM demonstrated significant cytotoxic effect against H400 OSCC cell lines with the lowest IC50 value compared to other cell lines. Moreover, DAM and NDAM showed no significant cytotoxicity to normal cell line (3T3). Therefore, based on the US National Cancer Institute Guidelines, cytotoxically active DAM compound was chosen against H400 cell lines for further study. Although the IC50 of NDAM exceeded 4 μg/mL, its apoptotic effects against H400 cell lines was still investigated in this study. This is because the chemical structure of DAM and NDAM is very similar and for this reason both DAM and NDAM have often been compared one another in many studies.
The cytotoxic effect of damnacanthal and nordamnacanthal, in the current study, is in accordance with several previous studies. Konoshima et al., (1989) showed that DAM displayed cytotoxic activity against breast cancer cell lines along with small cell lung cancer cell lines. In addition, Ali et al., (2000) reported that DAM and NDAM isolated from M. ellipticawere cytotoxic towards the MCF-7 (breast carcinoma) and CEM-SS (T-lymphoblastic leukemia) cell lines. Furthermore, Lin et al., (2011) claimed the selective cytotoxic effect of DAM for human liver adenocarcinoma SKHep 1 cells. The selective cytotoxic effect associated with DAM and NDAM is important, because currently available anticancer medicines target both normal and cancer cells, leading to severe side effects. Therefore, these findings invite additional analysis on the apoptotic effects of these compounds and their potency as anticancer drugs for OSCC cells therapy.
The chemical structure between DAM and NDAM is tightly associated. In this study, there was a significant difference on the cytotoxicity effect of compounds against oral cancer cell lines which may be due to the presence of methoxyl group (-OCH3) of DAM at position C-1 whilst NDAM possesses hydroxyl group (-OH) at the same position. The presence of this different group was forecasted to be the contributing factor to the various effects of DAM and NDAM against cancer cell lines (Alitheen et al., 2010). The existence of hydroxyl group at C-1 and C-3 and/or a formyl group at C-2 in the anthraquinone structure may be responsible for their cytotoxicity effects against several cancer cell lines (Ali et al., 2000). The cytotoxicity effects of DAM and NDAM towards different cancer cell lines seemed to be variable because of the exclusive chemical and biological properties of these compounds (Alitheen et al., 2010). Furthermore, the number and position of hydroxyl groups in the structure of DAM and NDAM can be considerable factors regarding the activity of anthraquinones (Alitheen et al., 2010; Kamei et al., 1998; Konoshima et al., 1989).
Apoptosis is one of the most important objectives for cancer therapy including chemotherapy and chemoprevention (Kang et al., 2007). Apoptosis was initially explained by (Kerr et al., 1972) which is characterized by particular morphological as well as biochemical alteration of dying cells, which includes cell shrinkage, nuclear condensation and fragmentation, membrane blebbing, formation of apoptotic bodies in addition to loss of cell attachment to neighbours (Acebedo et al., 2014). Biochemical changes of apoptosis consist of chromosomal DNA cleavage into internucleosomal fragments, externalization of phosphatidylserine and the cleavage of some intracellular substrate by specific proteolysis (Cohen et al., 1994; Ali et al., 2011). In this study, three different types of assays were executed to prove the mode of cell death induced by DAM and NDAM against H400 OSCC cells: phase contrast and fluorescent microscopy analysis of the cells and Annexin V-FITC/PI flow cytomtry. Morphological analysis with applying phase contrast and fluorescence microscope is one of the best methods to determine apoptosis (Doonan and Cotter, 2008). Apoptotic cells were first identified from the morphological alteration, especially some changes in the nucleus. Obvious morphological changes of apoptosis in treated H400 cells comprise loss of cellular adhesion to their neighbours, membrane blebbing and chromatin condensation along with creation of apoptotic bodies. Annexin V-FITC is commonly used in conjugation with propidium iodide to determine early apoptotic cells before the loss of cell membrane integrity (Aubry et al., 1999; Alabsi et al., 2012). Flow cytometric study of apoptosis by Annexin V-FITC/PI demonstrated that DAM and NDAM induce apoptosis in H400 OSCC cells through the observation of shift in live cell population from early to late stage of apoptosis/secondary necrosis from 12 to 48h.
Metastasis performs a crucial function in oral carcinogenesis (Yen et al., 2013). The ability of cancer cells to migrate marks the initial steps of metastasis (van Zijl et al., 2011). On the other hand, there is little knowledge regarding the anti-migration impact of DAM and NDAM on oral squamous cell carcinoma cells. Therefore, the effect of DAM and NDAM against H400 OSCC cell lines was studied in IC50 value compounds using μ-Dish contains an IBIDI culture insert consists of two reservoirs separated by a 500 µm thick wall. The results obtained from this assay revealed that consequent to the creation of a gap, the control cells exhibited a time-dependent migration in order to occupy free spaces and they finally reached a complete confluency, whereas DAM and NDAM-treated cells showed slower migration compared to the control cells. In other words, DAM and NDAM compounds demonstrated a significant suppressive effect on H400 cells’ migration compared to the control cells. This result is in line with the study as reported by Nualsanit et al., (2012), who reported on the anti-migration capability of DAM against human colorectal cancer cells (HCT-116).
In conclusion, in summary, DAM and NDAM exhibited selective cytotoxic effects against H400 oral squamous cell carcinoma cells. In addition, the study demonstrated the inhibition of H400 OSCC cells proliferation, activation of apoptosis pathway, and inhibition of cell migration by DAM and NDAM. These outcomes suggest that DAM and NDAM may function as a promising experimental anti tumor agent for oral squamous cell carcinoma cells therapy.
Acknowledgements
This study was financially supported by UMRG Grant (RP002D-13HTM) from University of Malaya, Malaysia.
References
- Acebedo AR, Amor EC, Jacinto SD. Apoptosis-inducing activity of HPLC fraction from Voacanga globosa (Blanco) Merr on the human colon carcinoma cell. Asian Pac J Cancer Prev. 2014;15:617–22. doi: 10.7314/apjcp.2014.15.2.617. [DOI] [PubMed] [Google Scholar]
- Alabsi AM, Ali R, Ali AM, et al. Apoptosis induction, cell cycle arrest and in vitro anticancer activity of gonothalamin in a cancer cell lines. Asian Pac J Cancer Prev. 2012;13:5131–6. doi: 10.7314/apjcp.2012.13.10.5131. [DOI] [PubMed] [Google Scholar]
- Alabsi AM, Ali R, Ali AM, et al. Induction of caspase-9, biochemical assessment and morphological changes caused by apoptosis in cancer cells treated with goniothalamin extracted from Goniothalamus macrophyllus. Asian Pac J Cancer Prev. 2013;14:6273–80. doi: 10.7314/apjcp.2013.14.11.6273. [DOI] [PubMed] [Google Scholar]
- Ali A, Ismail N, Mackeen M, et al. Antiviral, cyototoxic and antimicrobial activities of anthraquinones isolated from the roots of Morinda elliptica. Pharm Biol. 2000;38:298–301. doi: 10.1076/1388-0209(200009)3841-AFT298. [DOI] [PubMed] [Google Scholar]
- Ali R, Alabsi AM, Ali AM, et al. Cytolytic effects and apoptosis induction of Newcastle disease virus strain AF2240 on anaplastic astrocytoma brain tumour cell line. Neurochem Res. 2011;36:2051–62. doi: 10.1007/s11064-011-0529-8. [DOI] [PubMed] [Google Scholar]
- Alitheen N, Mashitoh A, Yeap S, et al. Cytotoxic effect of damnacanthal, nordamnacanthal, zerumbone and betulinic acid isolated from Malaysian plant sources. Int Food Res J. 2010;17:711–9. [Google Scholar]
- Al-Maweri SA, Addas A, Tarakji B, et al. Public awareness and knowledge of oral cancer in Yemen. Asian Pac J Cancer Prev. 2014;15:10861–5. doi: 10.7314/apjcp.2014.15.24.10861. [DOI] [PubMed] [Google Scholar]
- Antonsson B. Mitochondria and the Bcl-2 family proteins in apoptosis signalling pathways. Mol Cell Biochem. 2004;256:141–55. doi: 10.1023/b:mcbi.0000009865.70898.36. [DOI] [PubMed] [Google Scholar]
- Ariyoshi Y, Shimahara M, Omura K, et al. Epidemiological study of malignant tumours in the oral and maxillofacial region:survey of member institutions of the Japanese Society of Oral and Maxillofacial Surgeons, 2002. Int J Clin Oncol. 2008;13:220–8. doi: 10.1007/s10147-007-0756-9. [DOI] [PubMed] [Google Scholar]
- Aubry JP, Blaecke A, Lecoanet-Henchoz S, et al. Annexin V used for measuring apoptosis in the early events of cellular cytotoxicity. Cytometry. 1999;37:197–204. doi: 10.1002/(sici)1097-0320(19991101)37:3<197::aid-cyto6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Balaram P, Sridhar H, Rajkumar T, et al. Oral cancer in southern India:The influence of smoking, drinking, paan-chewing and oral hygiene. Int J Cancer. 2002;98:440–5. doi: 10.1002/ijc.10200. [DOI] [PubMed] [Google Scholar]
- Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–7. [PubMed] [Google Scholar]
- Boik J. Natural compounds in cancer therapy. Minnesota, USA: Oregon Medical Press; 2001. [Google Scholar]
- Brown AC. Anticancer activity of Morinda citrifolia (Noni) fruit:a review. Phytother Res. 2012;26:1427–40. doi: 10.1002/ptr.4595. [DOI] [PubMed] [Google Scholar]
- CastellsaguéX Quintana MJ, Martínez MC, et al. The role of type of tobacco and type of alcoholic beverage in oral carcinogenesis. Int J Cancer. 2004;108:741–9. doi: 10.1002/ijc.11627. [DOI] [PubMed] [Google Scholar]
- Chan-Blanco Y, Vaillant F, Perez AM, et al. The noni fruit (Morinda citrifolia L.). A review of agricultural research, nutritional and therapeutic properties. J Food Comp Anal. 2006;19:645–54. [Google Scholar]
- Cohen GM, Sun XM, Fearnhead H, et al. Formation of large molecular weight fragments of DNA is a key committed step of apoptosis in thymocytes. J Immunol. 1994;153:507–16. [PubMed] [Google Scholar]
- Doonan F, Cotter TG. Morphological assessment of apoptosis. Methods. 2008;44:200–4. doi: 10.1016/j.ymeth.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Dubai SARA, Ganasegeran K, Alabsi AM, Alshagga MA, Ali RS. Awareness and knowledge of oral cancer among university students in Malaysia. Asian Pac J Cancer Prev. 2012;13:165–8. doi: 10.7314/apjcp.2012.13.1.165. [DOI] [PubMed] [Google Scholar]
- Faltynek CR, Schroeder J, Mauvais P, et al. Damnacanthal is a highly potent, selective inhibitor of p56lck tyrosine kinase activity. Biochemistry. 1995;34:12404–10. doi: 10.1021/bi00038a038. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Larochette N, Zamzami N, Kroemer G. Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene. 2006;25:4812–30. doi: 10.1038/sj.onc.1209598. [DOI] [PubMed] [Google Scholar]
- George VC, Kumar D, Suresh P, Kumar RA. Apoptosis-induced cell death due to oleanolic acid in HaCaT keratinocyte cells-a proof-of-principle approach for chemopreventive drug development. Asian Pac J Cancer Prev. 2012;13:2015–20. doi: 10.7314/apjcp.2012.13.5.2015. [DOI] [PubMed] [Google Scholar]
- Ghani WM, Razak IA, Yang YH, et al. Factors affecting commencement and cessation of betel quid chewing behaviour in Malaysian adults. BMC Public Health. 2011;11:82–8. doi: 10.1186/1471-2458-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–76. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- Haghiac M, Walle T. Quercetin induces necrosis and apoptosis in SCC-9 oral cancer cells. Nutr Cancer. 2005;53:220–31. doi: 10.1207/s15327914nc5302_11. [DOI] [PubMed] [Google Scholar]
- Hiramatsu T, Imoto M, Koyano T, Umezawa K. Induction of normal phenotypes in ras-transformed cells by damnacanthal from Morinda citrifolia. Cancer Lett. 1993;73:161–6. doi: 10.1016/0304-3835(93)90259-c. [DOI] [PubMed] [Google Scholar]
- Hsieh WT, Huang KY, Lin HY, Chung JG. Physalis angulata induced G2/M phase arrest in human breast cancer cells. Food Chem Toxicol. 2006;44:974–83. doi: 10.1016/j.fct.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Ismail NH, Ali AM, Aimi N, et al. Anthraquinones from Morinda elliptica. Phytochemistry. 1997;45:1723–5. [Google Scholar]
- Jasril LN, Mooi LY, Abdullah MA, Sukari MA, Ali AM. Antitumor promoting and antioxidant activities of anthraquinones isolated from the cell suspension culture of Morinda elliptica. Asia Pac J Mol Biol Biotechnol. 2003;11:3–7. [Google Scholar]
- Johnson N, Warnakulasuriya K. Epidemiology and aetiology of oral cancer in the United Kingdom. Community Dent Health. 1993;10:13–29. [PubMed] [Google Scholar]
- Johnson NW, Warnakulasuriya S, Gupta P, et al. Global oral health inequalities in incidence and outcomes for oral cancer:causes and solutions. Adv Dent Res. 2011;23:237–46. doi: 10.1177/0022034511402082. [DOI] [PubMed] [Google Scholar]
- Kamata M, Wu RP, An DS, et al. Cell-based chemical genetic screen identifies damnacanthal as an inhibitor of HIV-1 Vpr induced cell death. Biochem Biophys Res Commun. 2006;348:1101–6. doi: 10.1016/j.bbrc.2006.07.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei H, Koide T, Kojima T, Hashimoto Y, Hasegawa M. Inhibition of cell growth in culture by quinones. Cancer Biother Radiopharm. 1998;13:185–8. doi: 10.1089/cbr.1998.13.185. [DOI] [PubMed] [Google Scholar]
- Kang K, Lee HJ, Kim CY, et al. The chemopreventive effects of Saussurea salicifolia through induction of apoptosis and phase II detoxification enzyme. Biol Pharm Bull. 2007;30:2352–9. doi: 10.1248/bpb.30.2352. [DOI] [PubMed] [Google Scholar]
- Kanokmedhakul K, Kanokmedhakul S, Phatchana R. Biological activity of Anthraquinones and Triterpenoids from Prismatomeris fragrans. J Ethnopharmacol. 2005;100:284–8. doi: 10.1016/j.jep.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis:a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan FK, Ali S, Javed N, et al. Genetic susceptibility to oral cancer due to combined effects of GSTT1, GSTM1 and CYP1A1 gene variants in tobacco addicted patients of pashtun ethnicity of Khyber Pakhtunkhwa province of Pakistan. Asian Pac J Cancer Prev. 2015;16:1145–50. doi: 10.7314/apjcp.2015.16.3.1145. [DOI] [PubMed] [Google Scholar]
- Konoshima T, Kozuka M, Koyama J, Tagahara TO, Tokuda H. Studies on inhibitors of skin tumour promotion, VI. Inhibitory effects of quinones on Epsterin-Barr virus activation. J Nat Prod. 1989;52:987–95. doi: 10.1021/np50065a012. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Thayalan DK, Padmanaban R, et al. Association of serum and salivary tumour necrosis factor-αwith histological grading in oral cancer and its role in differentiating premalignant and malignant oral disease. Asian Pac J Cancer Prev. 2014;15:7141–8. doi: 10.7314/apjcp.2014.15.17.7141. [DOI] [PubMed] [Google Scholar]
- Kumar B, Kumar A, Pandey B, Mishra K, Hazra B. Role of mitochondrial oxidative stress in the apoptosis induced by diospyrin diethylether in human breast carcinoma (MCF-7) cells. Mol Cell Biochem. 2009;320:185–95. doi: 10.1007/s11010-008-9920-4. [DOI] [PubMed] [Google Scholar]
- Lee WS, Yun JW, Nagappan A, et al. Flavonoids from Orostachys japonicus A. Berger induces caspase-dependent apoptosis at least partly through activation of p38 MAPK pathway in U937 human leukemic cells. Asian Pac J Cancer Prev. 2015;16:465–9. doi: 10.7314/apjcp.2015.16.2.465. [DOI] [PubMed] [Google Scholar]
- Lin FL, Hsu JL, Chou CH, et al. Activation of p38 MAPK by damnacanthal mediates apoptosis in SKHep 1 cells through the DR5/TRAIL and TNFR1/TNF-αand p53 pathways. Eur J Pharmacol. 2011;650:120–9. doi: 10.1016/j.ejphar.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival:application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- Nualsanit T, Rojanapanthu P, Gritsanapan W, et al. Damnacanthal, a noni component, exhibits antitumorigenic activity in human colorectal cancer cells. J Nutr Biochem. 2012;23:915–23. doi: 10.1016/j.jnutbio.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer:a meta-analysis of observational studies. Am J Clin Nutr. 2006;83:1126–34. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- Ramasamy S, Wahab NA, Abidin NZ, Manickam S, Zakaria Z. Growth inhibition of human gynecologic and colon cancer cells by Phyllanthus watsoniithrough apoptosis induction. PLoS One. 2012;7:e34793. doi: 10.1371/journal.pone.0034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D, Kondo S, Yazawa K, et al. The potential anticancer activity of extracts derived from the roots of Scutellaria baicalensis on human oral squamous cell carcinoma cells. Mol Clin Oncol. 2013;1:105–11. doi: 10.3892/mco.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully C, Bagan J. Oral squamous cell carcinoma:overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009;15:388–99. doi: 10.1111/j.1601-0825.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- Tosa H, Iinuma M, Asai F, et al. Anthraquinones from Neonauclea calycina and their inhibitory activity against DNA topoisomerase II. Biol Pharm Bull. 1998;21:641–2. doi: 10.1248/bpb.21.641. [DOI] [PubMed] [Google Scholar]
- Van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis:cell invasion and endothelial transmigration. Mutat Res Rev Mutat Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CY, Liang SS, Han LY, et al. Cardiotoxin III inhibits proliferation and migration of oral cancer cells through MAPK and MMP signalling. Sci World J. 2013;2013 doi: 10.1155/2013/650946. http://dx.doi.org/10.1155/2013/650946. [DOI] [PMC free article] [PubMed] [Google Scholar]


