Abstract
Background: Chemotherapy for advanced cholangiocarcinoma (CCA) is largely ineffective; thus innovative combinations of chemotherapeutic agents and natural compounds represent a promising strategy. This study aimed to investigate the synergistic effects of forbesione combined with 5-fluorouracil (5-FU) in hamster cholangiocarcinoma (Ham-1) cells both in vitro and in vivo. The anti-tumor effects of 5-FU combined with forbesione in vitro were determined using the Sulforhodamine B (SRB) assay and the effects in vivowere assessed in transplanted Ham-1 allograph models. Using ethidium bromide/acridine orange (EB/AO) staining, the morphological changes of apoptotic cells was investigated. The expressions of apoptosis-related molecules after combined treatment with forbesione and 5-FU were determined using real-time RT-PCR and western blot analysis. Forbesione or 5-FU alone inhibited proliferation of Ham-1 cells in a dose-dependent manner and their combination showed a synergistic proliferation inhibitory effect in vitro. In vivostudies, forbesione in combination with 5-FU exhibited greater inhibition of the tumor in the hamster model compared with treatment using either drug alone. Forbesione combined with 5-FU exerted stronger apoptotic induction in Ham-1 cells than did single drug treatment. The combination of drugs strongly suppressed the expression of B-cell lymphoma 2 (Bcl-2) and procaspase-3 while enhancing the expression of p53, Bcl-2-associated X protein (Bax), apoptotic protease activating factor-1 (Apaf-1), caspase-9 and caspase-3, compared with single drug treatments. These results explained the decreased expression of cytokeratin 19 (CK19) positive cells and proliferation cell nuclear antigen (PCNA) positive cells in Ham-1 cell tumor tissues of the treated hamsters. There was no apparent systemic toxicity observed in the treated animals compared with the control groups. Forbesione combined with 5-FU strongly induced apoptosis in Ham-1 cells. The growth inhibitory effect of combined treatment using these two drugs was much greater than treatment with either drug alone, both in vitroand in vivo.
Keywords: Forbesione, 5-fluorouracil, cholangiocarcinoma, synergism, apoptosis
Introduction
Cholangiocarcinoma (CCA) is the second commonest primary hepatic tumor (Huether et al., 2007). CCA can be located deep in the liver and be anatomically concealed, rendering treatment and early diagnosis extremely difficult (Tang et al., 2007). Rates of survival can vary depending on the anatomic location of the carcinoma and the extent of metastasis (Koprowski et al., 2015). Five-year survival rates for patients with a diagnosis of intrahepatic, distal extrahepatic, and hilar CCA receiving surgical intervention are 22%-44%, 27%-37%, and 11%-41%, respectively (Hasegawa et al., 2007). The treatment options for CCA patients are limited. Moreover, CCAs have a poor response to currently available chemotherapeutic agents and no standard chemotherapy has been established (Iwahashi et al., 2011). Various drugs have been used for the treatment of CCA patients, such as 5-fluorouracil (5-FU), gemcitabine (GEM), cisplatin (CIS), and doxorubicin (DOX) (Khan et al., 2012; Ramirez-Merino et al., 2013). Thus, novel therapeutic approaches, including combination chemotherapies, are urgently needed. 5-FU is widely used for treatment of digestive system cancer, but response rates in the biliary tract are very low (Iwahashi et al., 2011). This drug has historically been ineffective for therapy of cholangiocarcinoma (Iwahashi et al., 2011). Clinically effective antineoplastic treatment with 5-FU is generally limited by dose-related toxicity (Pederson et al., 1997). However, 5-FU is cheap and its combination with other anti-cancer agent has led to improved response rates up to 40-50% (Douillard et al., 2000; de Kort et al., 2007). In clinical practice, the effect of 5-FU combined with cisplatin, BAF (bleomycetin + adriamycin + 5-FU) and FAM (5-FU + adriamycin + mitomycin) is not sufficient. To minimize the toxicity of chemotherapeutic treatment, natural compounds have been developed (Glimelius et al., 1997) and there has been increased interest in use of traditional medicines for prevention or treatment of cancer (Zhao et al., 2010). Synergistic anticancer effects of combinations of drugs, including natural compounds, is well known and use of combinations is becoming popular because of reduction in individual drug-related toxicity and suppression of multi-drug resistance through different mechanisms of action (Hahnvajanawong et al., 2014). Thus combined treatment of 5-FU and natural compounds has been proposed to enhance the efficacy of treatment, increase cost-effectiveness and reduce toxic effects.
Previous reports have suggested that 5-FU elevates the expression of apoptosis-related protein by down-regulating the Bcl-2 family of genes and induction of the caspase family (Yim et al., 2004). The anti-hepatocellular carcinoma (HepG2 cells) effects of 5-FU were increased in combination with the natural flavonoid oroxylin A in vitroand in vivoby modulating the metabolic enzymes of 5-FU and apoptosis-related proteins. Oroxylin A enhanced the sensitivity of HepG2 cells to 5-FU by modulating the expression of apoptosis-inducing protein p53, cleavage of Poly (ADP-ribose) polymerase (PARP) and decreasing the expression of anti-apoptosis proteins cyclooxygenase-2 (COX-2), Bcl-2, and procaspase 3 (Zhao et al., 2010). Similarly, the combination of 5-FU with gambogic acid exhibited synergistic inhibitory effects on human gastric carcinoma (BGC-823) cells by elevating the activity of caspase-3, enhancing PARP cleavage and decreasing the expression of Bcl-2/Bax (Wang et al., 2009).
Plant-derived compounds are gaining increasing attention as potential cancer treatment agents, particularly for treatment-refractory cancers such as cholangiocarcinoma. Forbesione is extracted from resin and fruits of Garcinia hanburyiHook. f. (Family Guttiferae). It is used in folk medicine as a purgative and externally for infected wounds in Thai traditional medicine (Saralamp et al., 1996). We have previously shown that forbesione has potent antitumor activities (Mitra et al., 2007) and cytotoxic effects in several cancer cell lines (Wu et al., 2004; Han et al., 2006; Mitra et al., 2007). Forbesione inhibits the proliferation of human CCA cell lines KKU-100 and KKU-M156 by inducing apoptosis through the mitochondrial pathway (Hahnvajanawong et al., 2010), and inducing G0/G1-phase cell cycle arrest through p53 and the nuclear factor-kappa B (NF-κB)-signaling pathway (Hahnvajanawong et al., 2012). Moreover, the combinations of isomorellin/doxorubicin and forbesione/doxorubicin showed significant synergy for inhibition of cell growth and induction of apoptosis in KKU-M156 and KKU-100 cells, respectively, through suppression of the MRP1 protein, NF-κB activation, enhanced expression of Bax/Bcl-2 and activation of caspase-9 and caspase-3, while suppressing the expression of survivin, procaspase-9 and procaspase-3 (Hahnvajanawong et al., 2014). However, the effects of combining forbesione with 5-FU are completely unknown. Thus, our aim was to systematically determine the combination effect of forbesione and 5-FU for the treatment of cholangiocarcinoma in vitroand in vivo, and to investigate the possible mechanisms.
Materials and Methods
Cell culture
The cell line Ham-1 (Puthdee et al., 2013), derived from the CCA tissue of a Syrian golden hamster infected with Opisthorchis viverriniand treated with N-nitrosodimethylamine, was cultured in DMEM (Gibco, USA) medium supplemented with 10% heat-inactivated fetal bovine serum, and 100 U/ml penicillin, 100 µg/ml streptomycin (Gibco BRL) and maintained at 37°C in 5% CO2 in a humidified incubator (90-95%).
Preparation of forbesione and 5-FU
Forbesione was extracted and provided by Professor Dr. Vichai Reutrakul, Department of Chemistry, Faculty of Science, Mahidol University (Reutrakul et al., 2007). Forbesione was dissolved in dimethylsulfoxide (DMSO, Sigma, USA) to a concentration of 16,000 µM. 5-FU purchased from Boryung Pharmaceutic Co., Ltd. (Korea) was aliquoted and kept at 4 °C. Both drugs were prepared to appropriate final concentration in 1% fetal bovine serum (FBS) in DMEM medium, immediately before each experiment.
Sulforhodamine B (SRB) assay
To evaluate the viability of Ham-1 cells under different concentrations of forbesione and 5-FU, separately or in combination, the SRB assay was used as previously described (Pinmai et al., 2008). Cells (5 x 103 cells/well) were seeded in a 96-well microplate (Costar, USA) which was then incubated for 24 h. Various concentrations of forbesione and 5-FU (MASU, Thailand), separately or in combination, were added to Ham-1 cells in 96-well plates and the plates incubated for a further 72 h. Cell viability was quantified by measuring the absorbance at 540 nm and calculating the IC50 values using concentration-effect curves after linear regression analysis.
Combined effect evaluation
Drug interaction between forbesione and 5-FU was assessed at a fixed concentration ratio of 2:1, using the combination index (CI) method according to Chou and Talalay (1984). Inhibition of cell growth was determined using the SRB assay, as described above. Values for the combination index (CI) less than 1 (CI < 1), equal to 1 (CI = 1), and more than 1 (CI > 1) indicate synergistic, additive, and antagonistic effects, respectively.
Nuclear morphological characterization of cell death
Characteristic apoptotic nuclear morphological changes were determined by confocal fluorescent microscopy using ethidium bromide/acridine orange (EB/AO) staining. Ham-1 cells were treated with varying concentrations of forbesione or 5-FU, or their combination, for 24 h and then stained with 14 μl of 100 μg/ml of the EB/AO mixture (Sigma). The nuclear morphological of cells that were apoptotic (exhibiting condensed or fragmented chromatin) were assessed at 20x magnification.
Western blot analysis
Ham-1 cells were seeded in 10-cm-diameter dishes (Costar, USA) at a density of 1x106 cells per dish and incubated with forbesione or 5-FU alone or in combination for 24 h. Harvested cells were washed twice with cold PBS and lysed with ice-cold RIPA buffer (50 mM Tris-HCL, pH 7.5, 150 mM NaCl, 0.5% Nonidet P-40, 1 mM EDTA, 1 mM dithiothreitol, 0.5% deoxycholate, and 0.1% sodium dodecyl sulfate) containing protease inhibitor cocktail and phosphatase inhibitor cocktail (Pierce Biotechnology, USA). The cell lysates were homogenized and clarified by centrifugation at 15,000 x g for 30 min at 4°C. The protein concentration of total cell lysate was determined by the method of Bradford (Bradford, 1976). Protein concentration was determined using the Coomassie protein assay kit (Pierce Biotechnology, USA). Cell lysates were fractionated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Millipore, USA). Membranes were incubated with primary antibody against Bcl-2, Bax, activated caspase-9, procaspase-3 (Santa Cruz Biotechnology, Inc., USA), activated caspase-3 and β-actin (Sigma, USA) at 4°C overnight and subsequently incubated with the appropriate secondary antibody (anti-mouse, anti-goat or anti-rabbit) (Santa Cruz Biotechnology, USA). The bound secondary antibody-peroxidase (POD) conjugates were visualized using an enhanced chemiluminescence reagent (Pierce Biotechnology, USA), quantified by densitometry (GE Healthcare, USA) and analyzed using the program Scion Image (Scion Corp., USA). Data for expression of each protein were normalized to β-actin.
Establishment and treatment of Ham-1 tumor allograft
The hamster cholangiocarcinoma allograft model (Boueroy et al., 2016) was used to investigate the synergistic growth inhibitory effects of forbesione combined with 5-FU. Tumors were given three days to become established, after which each tumor had reached a volume of about 5-25 mm3. Hamsters with tumors that were too large or too small were not used. Hamsters were then randomly divided into 5 groups (each with n = 8): (1) control group (normal saline (NSS), ip, every other day), (2) control group (DMSO, oral, daily), (3) 5-FU 10 mg/kg, ip, every other day, (4) forbesione 25 mg/kg, oral, daily and (5) the combination (5-FU 10 mg/kg + forbesione 25 mg/kg). These treatment regimens were continued for 3 weeks. The size of each tumor was assessed once every other day. Tumor volume was determined using the equation: tumor volume (mm3) = (a/b2)/2 (a and b refer to the longest and shortest dimensions in mm, respectively). The body weight, food and water intake of hamsters were measured daily. The physical activity and mortality of the animals was monitored daily during the experimental period to assess toxicity of the treatments. All animals were euthanized at 3 weeks. Tumor weight is measured at the end of experiment, so the weight of tumor is depend on the initial size of the tumor. Whereas, the fold increase tumor volume is calculated from the final tumor volume divided by the initial tumor volume. Therefore, these values should reflex the real effect of the treatment. This may explain the difference results of tumor weight and the fold increase tumor volume. All the protocols were approved by the animal ethics committee, Khon Kaen University (AEKKU 2/2556).
RNA extraction and cDNA synthesis
Total RNA was extracted from Syrian hamster tumor samples using TRIzol® solution (Invitrogen, USA) (Boonjaraspinyo et al., 2011). Tumor samples were homogenized with a glass homogenizer in 1 ml of Trizol reagent, then incubated at room temperature for 10 min and centrifuged at 12,000 rpm at 4°C for 10 min. After that, 200 μl of chloroform was added and mixed for 30 min and then the tube incubated at room temperature for 3-5 min. After centrifugation at 12,000 rpm at 8°C for 10 min, the upper phase of the mixture was transferred to a new tube, and 500 μl of isopropanol was added to precipitate RNA and then the tube was incubated at room temperature for 10-15 min. The samples were centrifuged at 12,000 rpm at 4°C for 10 min and the supernatant was removed. RNA pellets were washed with 75% ethanol and centrifuged at 12,000 rpm at 4°C for 5 min, and supernatants were discarded. The pellet was then dissolved in 105 μl RNase-free water.
cDNA was synthesized according to the method of Boonjaraspinyo et al., (2011). Briefly, total RNA (3 μg) was prepared in a new tube with the addition of 1 μl of oligo (dT) 18 primer and 12 μl DEPC-treated water, and incubated at 70°C for 5 min in a water bath. Then the reaction buffer containing 5× reaction buffer (4 μl), ribonuclease inhibitor (0.5 μl), and 10 mM deoxyribonucleotide triphosphate (dNTP) mix (2 μl) and 1 μl of MMLV reverse transcriptase (Fermentas, Canada). The reaction mixture was incubated at 42°C for 60 min. After that, the reaction was terminated by incubation at 70°C for 10 min and the resultant cDNA was stored at -80°C until use.
Real-time reverse transcription PCR
qRT-PCR was performed using the SYBR® Green method on an CFX96 Touch™ Real-time PCR detector system (BioRad, USA). The relative abundance of individual mRNA was analyzed relative to that of glyceraldehyde-3-phosphate dehydrogenase (G3PDH) as an endogenous control. The reaction mixture contained 3 μl of 1:10 diluted-single strand cDNA, 2 μl of 10x HotStar Taq buffer, 1 µl of each 5 mM dNTP, 2.4 μl of 25 mM MgCl2, 1 μl of 5 μM primer pairs, 0.2 μl of HotStar Taq DNA polymerase (Pierce Biotechnology) and 7.4 μl of distilled water to give a final volume of 20 μl. The PCR primers for G3PDH, CK19, Apaf-1, Bax, and p53 (Integrated DNA Technologies, Coralville, IA) are listed in Table 1, along with cycling conditions. Relative expression of CK19, Apaf-1, Bax and p53 mRNA was calculated using the comparative ΔΔCt method as previously described (Lee et al., 2005). All the values were reported as fold change over background levels detected in the untreated controls.
Table 1.
Primer Sequences Used for Real-Time PCR
| Gene | Sequences | Cycles | Anealing temperatures (Ta) (°C) | References/GenBank accession number |
|---|---|---|---|---|
| G3PDH | Forward: 5’-GGCATTGTGGAAGGGCTCAT-3’ | 35 | 60 | 25 |
| Reverse: 5’-GACACATTGGGGGTAGGAACAC-3’ | ||||
| CK19 | Forward: 5’-GCGGGACAAGATTCTTGGTG-3’ | 40 | 62 | NM_008471.2 |
| Reverse: 5’-CTTCAGGCCTTCGATCTGCAT-3 | ||||
| Bax | Forward: 5’-AGCTGCAGAGGATGATTGCT-3’ | 40 | 62 | 25 |
| Reverse: 5’-CTCTCGGAGGAAGTCCAGTG-3’ | ||||
| Apaf1 | Forward: 5’-ATCCTGGTGCTTTGCCTCTA-3’ | 50 | 62 | 26 |
| Reverse: 5’-TACACCCCCTGAAAAGCAAC-3’ | ||||
| P53 | Forward: 5’- AAGGCGATAGTTTGGCTCCT-3’ | 40 | 60 | Y08900 |
| Reverse: 5’- CTGGGGTCTTCCAGTGTGAT-3’ |
Immunohistochemical (IHC) staining
Immunohistochemical staining was performed to determine the localization of biliary CK19, a marker of bile duct epithelium, PCNA and apoptosis-related proteins (Bcl-2, Bax, caspase-9 and caspase-3) in tumor sections from Syrian hamsters (Wu et al., 2012). Briefly, deparaffinized sections were autoclaved at 120°C for 5 min in 50 mM citrate buffer (pH 6) for epitope antigen retrieval, and treated for 30 min in methanol containing 3% H2O2 to block endogenous peroxidase activity. After blocking with 5% skim milk in phosphate-buffered saline (PBS), the sections were incubated with the primary monoclonal antibodies to CK19, PCNA, Bcl-2, Bax, caspase-9 and caspase-3 at 37°C for 90 min. After rinsing, anti-rabbit horseradish peroxidase-labeled secondary antibody (diluted 1:100; Zymed Laboratories, USA) was used to detect primary antibody. Sections were then washed twice with PBS. The peroxidase reaction was developed with 9-ethylcarbazol-3-amine (AEC) (Sigma) as a chromogen. The sections were counterstained with hematoxylin. The IHC results were evaluated by two observers by semiquantifying the percentage of cells that were immunopositive in ten fields per slide and scored as 0% = negative, < 25% = 1+, 25–50% = 2+, > 50–75% = 3+, and > 75% = 4+.
Results
The effect of forbesione or 5-FU single-drug treatment, or their combination, on the proliferation of Ham-1 cells in vitro
The inhibitory effects of forbesione or 5-FU as single-agent therapies on cell viability in Ham-1 cells were tested using the SRB assay. Results showed that forbesione and 5-FU showed inhibitory effects in a concentration-dependent manner (Figure 1A -1B), the IC50 values for forbesione and 5-FU being 3.34 ± 0.31 and 1.22 ± 0.04 µM, respectively. In combination treatment of forbesione and 5-FU, the inhibitory effects were significantly stronger as compare to single drug treatment (Figure 1C). Forbesione and 5-FU showed a synergistic effect with CI < 1 (Figure 1D).
Figure 1.
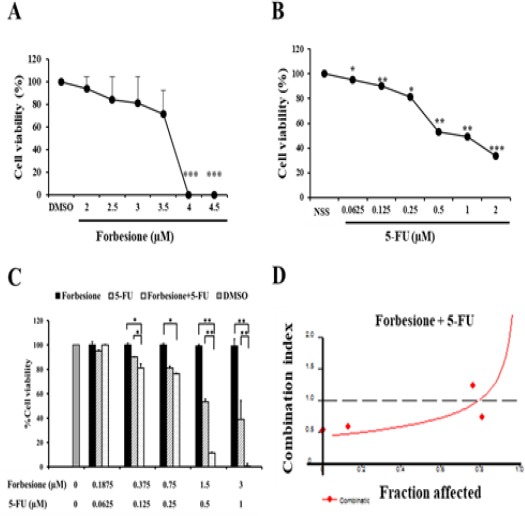
Inhibitory Effects of Two Drugs, Separately or in Combination, on Ham-1 Cells. Ham-1 cells were treated with various concentrations of (A) forbesione, (B) 5-FU alone for 72 h. Cells were treated with the combination of forbesione and 5-FU with a ration 1:2, (C) either single drug alone or the combinations, and (D) the CI values were determined. Combination index vs. fraction affected (fa) plots were analyzed by the median-effect analysis program (CalcuSyn, Biosoft, Cambridge, UK). Cells (5x103 cells/well) were seeded on a 96-well plate. After 24 h of incubation, the cells were treated with various concentrations of two drugs, singly or in combination, and incubated for 72 h. Viability of the cells was determined using the SRB assay. Each value represents the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001, vs. the NSS, DMSO group.
Effects of 5-FU and forbesione, singly or in combination, on nuclear morphology of Ham-1 cells
To investigate the effects of 5-FU and forbesione, singly or in combination, on Ham-1 cell nuclear morphology, the cells were treated with 5-FU (0.125, 0.25 and 0.5 µM) or forbesione (0.375, 0.75 and 1.5 µM) and their combinations for 24 h and were then subjected to EB/AO staining. The results indicated that 5-FU and forbesione induced nuclear morphological changes indicating apoptotic cell death, chromosomal condensation and nuclear fragmentation (indicated by bright green bodies) (Figure 2). The combination of drugs markedly induced cell death compared with single drugs or controls. The result indicated that 5-FU combined with forbesione markedly induced apoptotic cell death, characterized by nuclear morphological changes, in Ham-1 cells (Figure 2).
Figure 2.
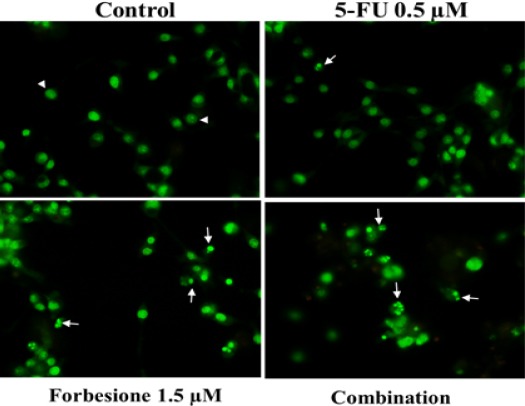
Apoptotic Effects on Ham-1 Cells after Treatment with 5-FU or Forbesione or Their Combination. Nuclear morphological features of Ham-1 cells treated with different concentrations of 5-FU (0.125, 0.25 and 0.5 µM) or forbesione (0.375, 0.75 and 1.5 µM), or the combination of the two drugs, for 24 h followed by EB/AO staining and analysis by confocal microscopy. Acridine orange (AO) is a vital dye that will stain nucleus of both viable and nonviable cells (eg. an early apoptotic cells) as shown in green color, whereas ethidium bromide (EB) will stain only those cells that have lost their membrane integrity or nonviable cells (eg. a late apoptotic or necrotic cells) represent with red color. Normal cells were uniformly stained green (arrowheads), while early apoptotic cells shown condensed chromatin (arrow).
Effect of forbesione and 5-FU, singly or in combination, on the expression of apoptosis-related proteins in Ham-1 cells
To further elucidate the growth inhibitory mechanisms of forbesione and 5-FU, singly or in combination, in Ham-1 cells, the expression levels of some apoptosis-related proteins were determined by western blot analysis. As shown in Figure 3, forbesione combined with 5-FU significantly decreased the expression level of Bcl-2 and procaspase-3, whereas the expression of Bax, activated caspase-9 and activated caspase-3 markedly increased compared with forbesione or 5-FU single treatment.
Figure 3.
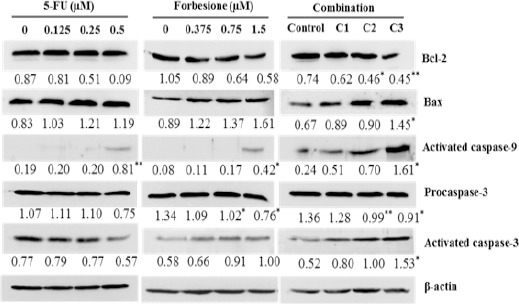
Effects of 5-FU or Forbesione, or Their Combination, on the Expression of Apoptosis- Related Proteins in Ham-1 Cells. Representative western blots of lysates of cells treated with different concentrations of forbesione and 5-FU, either alone or in combination, for 24 h. Ham-1 cells were treated with various concentrations of two drugs, singly or in combination, for 24 h. The total cell lysates were separated on SDS-PAGE and transferred to nitrocellulose membranes. The membranes were probed with a primary antibody specific to Bcl-2, Bax, activated caspase-9, pro-caspase-3 or activated caspase-3. Staining intensities of apoptosis regulatory proteins were quantified by densitometry and normalized to β-actin. Data are expressed as mean ± SD of three independent experiments. Statistical analyses were performed using Student’s t-test, *p < 0.05, **p < 0.01 indicate a significant difference.
Effects of forbesione and 5-FU, single or in combination, on Ham-1 cell tumor growth in vivo
We examined the effects of forbesione and 5-FU, singly or in combination, on the growth of Ham-1 cells as allograft tumors in the hamster model. As shown in Figure 4A, forbesione alone or combined with 5-FU had inhibitory effects on the growth of Ham-1 cells as allograft tumors in hamsters. Tumor weights in the combination treatment group (mean 128.68 mg) were smaller than in the forbesione group (mean 178.41 mg), 5-FU group (232.51 mg), the DMSO treated group (182.64 mg) or the normal saline group (280.89 mg) (Figure 4B). In the normal saline and DMSO control groups, tumors grew continuously and the fold increase of tumor volume was 3.72 and 3.65, respectively at the end of the experiment (Figure 4C). When hamsters were treated with forbesione combined with 5-FU, the fold increase of tumor volume was only 1.26, whereas in hamsters treated with 5-FU (10 mg/kg) and forbesione (25 mg/kg), fold increases were 1.58 and 2.73, respectively (Fig. 4C). These results indicated that the inhibitory effect of forbesione combined with 5-FU was superior to that of either drug on its own.
Figure 4.
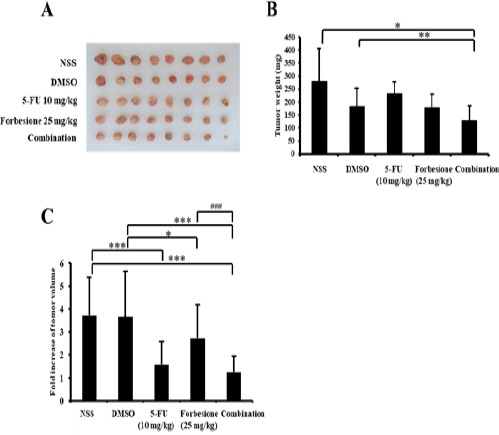
Inhibitory Effect of 5-FU or Forbesione, or Their Combination, on the Growth of Ham-1 Transplantation Tumors. Hamsters bearing tumors were administered forbesione 25 mg/kg orally once every day and 5-FU 10 mg/kg intraperitoneally once every other day, alone or in combination. After 3 weeks of treatment, hamsters were euthanized and (A) tumors were collected from individual hamsters and (B) weighed. (C) The tumors volume were measured once every other day and calculated the mean fold increase of tumor volume for 3 weeks of treatment. Data are represented as mean ± SD, n = 8; *p < 0.05 compared with control; **p < 0.01 compared with control; ***p < 0.001 compared with control, indicates a significant difference; ###p < 0.001 combination compared with the monotherapy.
Changes in mRNA expression of CK19, p53, Bax and Apaf-1 in Ham-1 tumor tissues after treatment with forbesione and 5-FU, single or in combination
To further elucidate the synergistic tumor growth suppression of forbesione and 5-FU, the relative mRNA expression levels of CK19, p53, Bax, and Apaf-1 in tumor tissues derived from each group were determined by qRT-PCR. The combination of forbesione with 5-FU resulted in slightly increased in mRNA levels of Bax and Apaf-1 at 1.29- and 1.23-fold (Fig. 5C and 5D), respectively, while the mRNA expression of CK19 was slightly decreased to 0.80-fold compared to the control treatment (Figure 5A). Interestingly, a combination of forbesione with 5-FU resulted in significantly increase in p53 mRNA levels at 1.61-fold compared to drug alone or control group.
Figure 5.
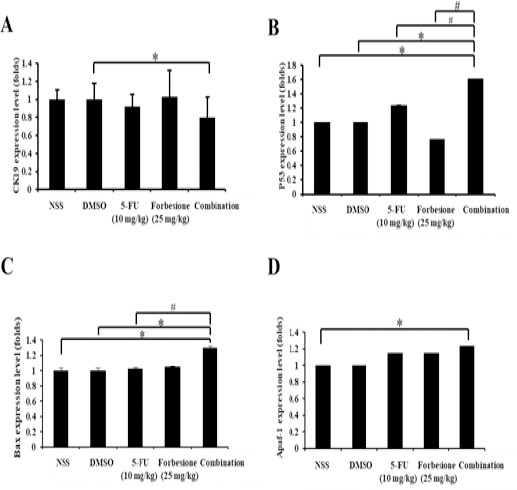
Changes of mRNA Levels of CK19, p53, BAX and Apaf-1 in Ham-1 Transplantation Tumors. The RNA was collected from the tumor tissues, converted to cDNA and relative gene expressions were determined. Relative expression of (A) CK19, (B) p53, (C) BAX and (D) Apaf-1 mRNA are shown. All values are reported as fold change over background levels detected in the untreated control. Data are expressed as mean ± SD of three independent experiments. Statistical analyses were performed using Student’s t-test, *p < 0.05 indicate a significant difference, #p < 0.05 combination compared with the monotherapy
Changes in the protein expression levels of CK19, PCNA, Bcl-2, Bax, caspase-9 and caspase-3 in tumor tissues from hamsters after treatment with forbesione and 5-FU, singly or in combination
To further illuminate the mechanism of tumor growth suppression, the tumor tissues derived from four groups of hamsters, including the controls, the single drug treatments (2 groups), and the combination treatment group, were stained for CK19, PCNA (a proliferation index) and apoptosis-related proteins (Bcl-2, Bax, caspase-9 and caspase-3). Semi-quantitative analyses using the IHC scores were performed. Forbesione combined with 5-FU significantly decreased the protein levels of CK19, PCNA and Bcl-2 (Figure 6A-6D) whereas the protein level of Bax, caspase-9 and caspase-3 (Figure 6A, 6E, 6F and 6G) were significantly increased compared with controls and single drug treatments.
Figure 6.
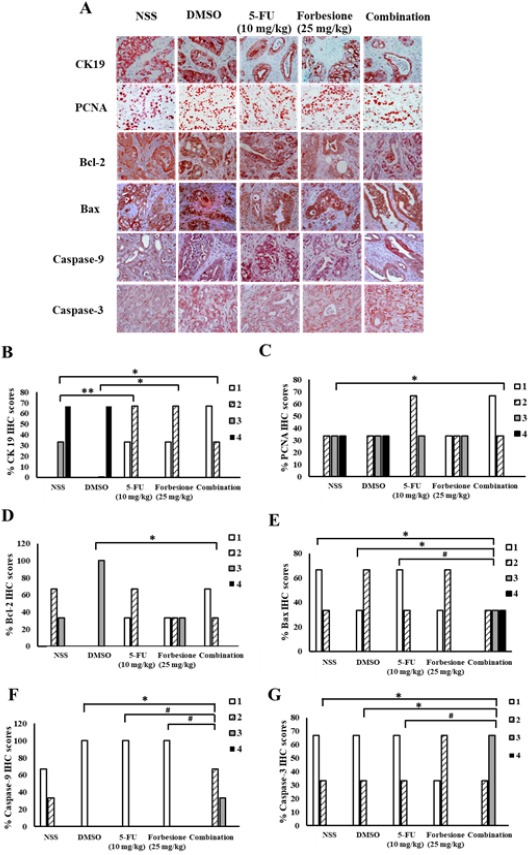
Expression Changes of Proteins Associated with Epithelial Bile Duct Marker (CK19), Proliferation Marker (PCNA) and Apoptosis-Related Proteins (Bcl-2, Bax, caspase-9, caspase-3). The tumor sections in each group were incubated with primary antibodies including CK19, PCNA, Bcl-2, Bax, caspase-9, caspase-3 and with secondary antibodies. The peroxidase reaction was developed with AEC as a chromogen and counterstained with hematoxylin. (A) Representative immunohistochemical staining of Ham 1 allograft tissue (magnification, x40). Effect of forbesione and 5-FU or their combinations on % IHC scores for the expression of (B) CK19, (C) PCNA, (D) Bcl-2, (E) Bax, (F) Caspase-9 and (G) Caspase-3 in allograft tissues.*p < 0.05 compared with control; **p < 0.01 compared with control; #p < 0.05 combination treatment compared with the monotherapy
Toxicity effects in hamsters of forbesione and 5-FU, singly or in combination
To test whether forbesione or 5-FU alone, or their combination, had side effects on the treated hamsters, general parameters including physical activity, body weight (Figure 7A), food intake (Figure 7B) and water intake (Figure 7C) were determined. The hamsters in the forbesione-treated group and the combination-treated group were healthy with no changes in normal physical activities and eating habits as compared to the DMSO-treated and NSS-treated hamsters. The hamsters in the 5-FU treatment alone were healthy with no changes in normal physical activities as compared to the NSS-treated group, but food and water intake was significantly decreased as compared to the NSS-treated group. None of the treated animals in these experiments showed changes of liver and kidney serum markers suggestive of pathology due to drug toxicity (data not shown).
Figure 7.
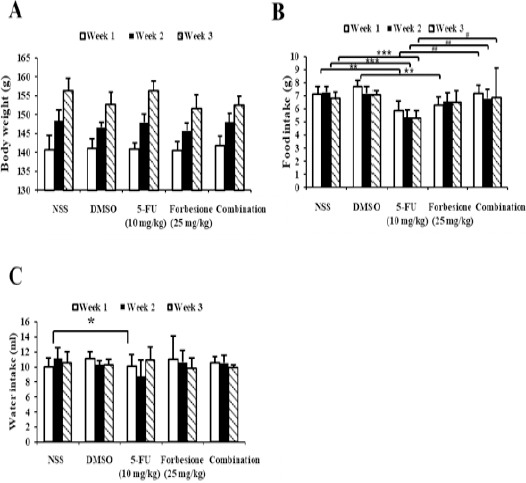
Effect of Forbesione Administration on the General Parameters in the Hamster Model. Hamsters bearing tumors were administered forbesione 25 mg/kg orally once every day and 5-FU 10 mg/kg intraperitoneally once every other day, alone or in combination. (A) The body weight, (B) food and (C) water intake of hamsters were measured once every other day. Data are represented as mean ± SD, n = 8; *p < 0.05 compared with control; **p < 0.01 compared with control. ##p < 0.01 combination compared with the monotherapy.
Discussion
Treatment responses of CCA to radiation therapy and currently available chemotherapeutic regimens are unsatisfactory, and consequently the prognosis is poor. Thus, novel combination drug approaches are much needed. In addition to improved treatment response rates in CCA, combination therapies should also reduce problems associated with the dose-limiting toxicities of individual agents. 5-FU has been widely used in patients with CCA, but has not improved median survival time or response rates, which are only 2–12 months and 0-40%, respectively. The co-administration of doxorubicin and mitomycin C with methyl CCNU or streptozotocin with 5-FU has not increased the overall response rate or survival time (Choi et al., 2000; Thongprasert, 2005). 5-FU in combination with cisplatin has been reported to yield an overall response rate of 24% (Wu et al., 2004). The combination of interferon-α (IFN-α) and 5-FU resulted in a partial response rate of 34% (Douillard et al., 2000). However, the addition of other drugs (cisplatin, doxorubicin) to the 5-FU/IFN-α combination has been shown to increase toxicity without increasing the response rate (Thongprasert, 2005; Patt et al., 2001). Preliminary in vivostudies have shown that 5-FU, alone at concentrations of 30 mg/kg (and above) or in combination with histone deacetylase inhibitor (PXD101), shows evidence of toxicity and the combined treatment did not show a significant effect on tumor growth suppression over PXD101 treatment alone (Tumber et al., 2007). In addition, 5-FU (20 mg/kg) combined with 20 mg/kg tanshinone IIA (a natural product extraction from Salvia miltiorrhiza root) exhibited an antitumor effect in SCID mice bearing Colo205 cell xenografts (Su, 2012). Therefore, the combination of chemotherpeutic agent and naturally occurring dietary supplement can reduce the systemic toxicity of chemotherapy (Taylor-Robinson et al., 2001; Khan et al., 2002; Hahnvajanawong et al., 2014)
In recent years, the interest in exploiting traditional medicines for prevention or treatment of tumors has increased. Caged xanthones have shown significant inhibitory effects against the growth of various human cancer cell lines (Guo et al., 2004; Qi et al., 2008b) as well as anti-cancer and antitumor activities (Guo et al., 2006; Hahnvajanawong et al., 2014). Our recent studies show that four caged xanthones, isomorellin, isomorellinol, forbesione and gambogic acid have inhibitory effect against human CCA cell lines by inducing cell-cycle arrest and apoptotic cell death (Hahnvajanawong et al., 2012). In this study, forbesione and 5-FU significantly inhibited hamster CCA cells (Ham-1 cells) in a dose-dependent manner. Our previous study showed that isomorellin and forbesione had selective inhibitory effect against human CCA cell lines compared with normal human peripheral blood mononuclear cells and Chang cells (Guo et al., 2004; Hahnvajanawong et al., 2010; Hahnvajanawong et al., 2014). These results suggest that forbesione has antiproliferative and apoptotic effects in both human and hamster CCA cell lines. Moreover, results from our previous studies in vivodemonstrated remarkable tumor suppression effects of forbesione (50 mg/kg) on Ham-1 transplantable tumors with low toxicity to hamsters (Boueroy et al., 2016).
The combination of chemotherapeutic agent and natural compound with different molecular mechanisms is promising therapeutic strategy with good clinical efficacy and patient survival (Huang et al., 2011). This is the first report to show that forbesione enhances the effects of 5-FU on Ham-1 cells and Ham-1 allograft tumors. We have shown that forbesione or 5-FU alone significantly inhibited the proliferation of Ham-1 cells in a dose-dependent manner. The combination of forbesione and 5-FU showed synergistic effects in Ham-1 cells, with the CI < 1 (Figure 1). Furthermore, the anti-tumor activity in Ham-1 allograft tumors in the combination treatment group showed a greater effect than did treatment with either drug alone (Figure 4). These results were confirmed by the reduction of CK19- and PCNA-positive cells in tumor allograft tissues (Figure 6). We further explore the anti-tumor mechanism of forbesione, 5-FU and their combination on Ham-1 cells in vitroand in vivo. We found that the synergistic anti-proliferative effect of forbesione and 5-FU on Ham-1 cells was through apoptosis induction mediated by increased expression of p53, Bax, caspase-9, caspase-3 and Apaf-1 and decreased expression of Bcl-2 (Figures 3, 5 and 6). The hamsters remained healthy with normal physical activities and eating habits in the forbesione-treated and in both control groups, whereas hamsters in the 5-FU-treated group show slight effects on their eating habits as compared to the control group. Histopathological features of liver, kidney and stomach of the 5-FU-, forbesione- and the combination-treated hamsters were not significantly different from the control groups (data not show). Levels of markers indicating liver function (ALT and ALP) and kidney function (BUN and creatinine) of the hamsters treated with forbesione (25 mg/kg), 5-FU (10 mg/kg) and the combination (10 mg/kg 5-FU + 25 mg/kg forbesione) were not significantly different from those in the control groups (data not show). These results suggest a lack of toxic effects on liver and kidney at the dosage levels used. Recently, we showed the remarkable tumor repression effect in forbesione (50 mg/kg) treated Ham-1 transplantable tumors (Boueroy et al., 2016). Gambogic acid, derived from resin of G. hanburyi, at a high dose (120 mg/kg) in a long-term follow-up study in rats showed toxic effects to the liver and kidney. Moreover, rats treated at dosage of 60 mg/kg of gambogic acid for a long time exhibited a non-significant increase in levels of blood biochemical markers (Wu et al., 2004; Qi et al., 2008b). Taken together these results suggested that Ham-1 cells in vitroand in vivodisplay higher sensitivity to 5-FU when combined with forbesione.
In summary, Ham-1 cells in vitroand in vivoshowed higher sensitivity to 5-FU when combined with forbesione. This combination could regulate the expression of p53, Bax, Bcl-2, Apaf-1, caspase-9 and caspase-3, the key molecules in apoptosis, in Ham-1 cells more strongly than could treatment with either drug alone. In addition, we found that the hamsters were healthy with normal physical activities and eating habits when the two drugs were used in combination. All the results suggested the combination of forbesione and 5-FU showed synergistic effects in Ham-1 cells and might be a potential candidate in clinical treatment of cholangiocarcinoma in the future.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
The present study was supported in part by grants from the Center of Excellence for Innovation in Chemistry, Commission on Higher Education (Bangkok, Thailand; grant no. 48 03 3 00 144), Khon Kaen University Research Fund (Khon Kaen, Thailand; grant no. 573301582701). We would like to acknowledge Prof. Dr. David Blair, for editing the MS via Publication Clinic KKU, Thailand.
References
- Boonjaraspinyo S, Boonmars T, Aromdee C, Puapairoj A, Wu Z. Indirect effect of a turmeric diet:enhanced bile duct proliferation in Syrian hamsters with a combination of partial obstruction by Opisthorchis viverriniinfection and inflammation by N-nitrosodimethylamine administration. Parasitol Res. 2011;108:7–14. doi: 10.1007/s00436-010-2031-7. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Boueroy P, Hahnvajanawong C, Boonmars T, et al. Antitumor effect of forbesione isolated from Garcinia hanburyion cholangiocarcinoma in vitroand in vivo. Oncol Lett. 2016;12:4685–98. doi: 10.3892/ol.2016.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CW, Choi IK, Seo JH, et al. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J Clin Oncol. 2000;23:425–8. doi: 10.1097/00000421-200008000-00023. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships:the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- de Kort SJ, Kenny N, van Dijk P, et al. Cost issues in new disease-modifying treatments for advanced cancer:In-depth interviews with physicians. Eur J Cancer. 2007;43:1983–9. doi: 10.1016/j.ejca.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Douillard J, Cunningham D, Roth A, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer:a multicentre randomised trial. Lancet. 2000;355:1041–7. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–8. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- Guo QL, You QD, Wu ZQ, Yuan ST, Zhao L. General gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitroand in nude mice. Acta Pharmacol Sin. 2004;25:769–74. [PubMed] [Google Scholar]
- Guo Q, Qi Q, You Q, et al. Toxicological studies of gambogic acid and its potential targets in experimental animals. Basic Clin Pharmacol Toxicol. 2006;99:178–84. doi: 10.1111/j.1742-7843.2006.pto_485.x. [DOI] [PubMed] [Google Scholar]
- Hahnvajanawong C, Boonyanugomol W, Nasomyon T, et al. Apoptotic activity of caged xanthones from Garcinia hanburyiin cholangiocarcinoma cell lines. World J Gastroenterol. 2010;16:2235–43. doi: 10.3748/wjg.v16.i18.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnvajanawong C, Ketnimit S, Pattanapanyasat K, et al. Involvement of p53 and nuclear factor-κB signaling pathway for the induction of G1-phase cell cycle arrest of cholangiocarcinoma cell lines by isomorellin. Biol Pharm Bull. 2012;35:1914–25. doi: 10.1248/bpb.b12-00118. [DOI] [PubMed] [Google Scholar]
- Hahnvajanawong C, Wattanawongdon W, Chomvarin C, et al. Synergistic effects of isomorellin and forbesione with doxorubicin on apoptosis induction in human cholangiocarcinoma cell lines. Cancer Cell Int. 2014;14:68–82. doi: 10.1186/1475-2867-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han QB, Wang YL, Yang L, et al. Cytotoxic polyprenylated xanthones from the resin of Garcinia hanburyi. Chem Pharm Bull. 2006;54:265–7. doi: 10.1248/cpb.54.265. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Ikai I, Fujii H, Hatano E, Shimahara Y. Surgical resection of hilar cholangiocarcinoma:analysis of survival and postoperative complications. World J Gastrointest Surg. 2007;31:1258–65. doi: 10.1007/s00268-007-9001-y. [DOI] [PubMed] [Google Scholar]
- Huang H, Chen D, Li S, et al. Gambogic acid enhances proteasome inhibitor-induced anticancer activity. Cancer Lett. 2011;301:221–8. doi: 10.1016/j.canlet.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huether A, Hopfner M, Baradari V, Schuppan D, Scherubl H. Sorafenib alone or as combination therapy for growth control of cholangiocarcinoma. Biochem Pharmacol. 2007;73:1308–17. doi: 10.1016/j.bcp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Ha J, Park OJ. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem Biophys Res Commun. 2005;332:433–40. doi: 10.1016/j.bbrc.2005.04.143. [DOI] [PubMed] [Google Scholar]
- Iwahashi S, Ishibashi H, Utsunomiya T, et al. Effect of histone deacetylase inhibitor in combination with 5-fluorouracil on pancreas cancer and cholangiocarcinoma cell lines. J Med Invest. 2011;58:106–9. doi: 10.2152/jmi.58.106. [DOI] [PubMed] [Google Scholar]
- Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma:an update. Gut. 2012;61:1657–69. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- Khan SA, Taylor-Robinson SD, Toledano MB, et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–13. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- Koprowski S, Sokolowski K, Kunnimalaiyaan S, Gamblin TC, Kunnimalaiyaan M. Curcumin-mediated regulation of Notch1/hairy and enhancer of split-1/survivin:molecular targeting in cholangiocarcinoma. J Surg Res. 2015;198:434–40. doi: 10.1016/j.jss.2015.03.029. [DOI] [PubMed] [Google Scholar]
- Lee SO, Nadiminty N, Wu XX, et al. Selenium disrupts estrogen signaling by altering estrogen receptor expression and ligand binding in human breast cancer cells. Cancer Res. 2005;65:3487–92. doi: 10.1158/0008-5472.CAN-04-3267. [DOI] [PubMed] [Google Scholar]
- Mitra R, Agricola S, Mitchell B, et al. Medicinal Plants of Thailand. Asia-Pacific Biotech News. 2007;11:508–18. [Google Scholar]
- Pederson LC, Buchsbaum DJ, Vickers SM, et al. Molecular chemotherapy combined with radiation therapy enhances killing of cholangiocarcinoma cells in vitroand in vivo. Cancer Res. 1997;57:4325–32. [PubMed] [Google Scholar]
- Patt YZ, Hassan MM, Lozano RD, et al. Phase II trial of cisplatin, interferon alpha-2b, doxorubicin, and 5-fluorouracil for biliary tract cancer. Clin Cancer Res. 2001;7:3375–80. [PubMed] [Google Scholar]
- Pinmai K, Chunlaratthanabhorn S, Ngamkitidechakul C, Soonthornchareon N, Hahnvajanawong C. Synergistic growth inhibitory effects of Phyllanthus emblicaand Terminalia bellericaextracts with conventional cytotoxic agents:doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J Gastroenterol. 2008;14:1491. doi: 10.3748/wjg.14.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthdee N, Vaeteewoottacharn K, Seubwai W, et al. Establishment of an allo transplantable hamster cholangiocarcinoma cell line and its application for in vivoscreening of anti cancer drugs. Korean J Parasitol. 2013;51:711–17. doi: 10.3347/kjp.2013.51.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Gu H, Yang Y, et al. Involvement of matrix metalloproteinase 2 and 9 in gambogic acid induced suppression of MDA-MB-435 human breast carcinoma cell lung metastasis. Int J Mol Med. 2008a;86:1367–77. doi: 10.1007/s00109-008-0398-z. [DOI] [PubMed] [Google Scholar]
- Qi Q, You Q, Gu H, et al. Studies on the toxicity of gambogic acid in rats. J Ethnopharmacol. 2008b;117:433–8. doi: 10.1016/j.jep.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Ramirez-Merino N, Aix SP, Cortes-Funes H. Chemotherapy for cholangiocarcinoma:an update. World J Gastrointest Oncol. 2013;5:171–6. doi: 10.4251/wjgo.v5.i7.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul V, Anantachoke N, Pohmakotr M, et al. Cytotoxic and anti-HIV-1 caged xanthones from the resin and fruits of Garcinia hanburyi. Planta Med. 2007;73:33–40. doi: 10.1055/s-2006-951748. [DOI] [PubMed] [Google Scholar]
- Saralamp P, Chuakul W, Temsiririrkkul R, Clayton T. Medicinal plants in Thailand. Vol. 1. Bangkok: Amarin printing and publishing public Co., Ltd; 1996. pp. 209–11. [Google Scholar]
- Su CC. Tanshinone IIA potentiates the efficacy of 5-FU in Colo205 colon cancer cells in vivothrough downregulation of P-gp and LC3-II. Exp Ther Med. 2012;3:555–9. doi: 10.3892/etm.2011.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T, Zheng JW, Chen B, et al. Effects of targeting magnetic drug nanoparticles on human cholangiocarcinoma xenografts in nude mice. Hepatobiliary Pancreat Dis Int. 2007;6:303–7. [PubMed] [Google Scholar]
- Taylor-Robinson S, Toledano M, Arora S, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001;48:816–20. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Ann Oncol. 2005;16:93–6. doi: 10.1093/annonc/mdi712. [DOI] [PubMed] [Google Scholar]
- Tumber A, Collins LS, Petersen KD, et al. The histone deacetylase inhibitor PXD101 synergises with 5-fluorouracil to inhibit colon cancer cell growth in vitroand in vivo. Cancer Chemother Pharmacol. 2007;60:275–83. doi: 10.1007/s00280-006-0374-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu W, Zhao Q, et al. Synergistic effect of 5-fluorouracil with gambogic acid on BGC-823 human gastric carcinoma. Toxicology. 2009;256:135–40. doi: 10.1016/j.tox.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Wonkchalee O, Boonmars T, Aromdee C, et al. Anti-inflammatory, antioxidant and hepatoprotective effects of Thunbergia laurifoliaLinn. on experimental opisthorchiasis. Parasitol Res. 2012;111:353–9. doi: 10.1007/s00436-012-2846-5. [DOI] [PubMed] [Google Scholar]
- Wu ZQ, Guo QL, You QD, Zhao L, Gu HY. Gambogic acid inhibits proliferation of human lung carcinoma SPC-A1 cells in vivoand in vitroand represses telomerase activity and telomerase reverse transcriptase mRNA expression in the cells. Biol Pharm Bull. 2004;27:1769–74. doi: 10.1248/bpb.27.1769. [DOI] [PubMed] [Google Scholar]
- Wu Z, Boonmars T, Nagano I, et al. Alteration of galectin-1 during tumorigenesis of Opisthorchis viverriniinfection-induced cholangiocarcinoma and its correlation with clinicopathology. Tumor Biol. 2012;33:1169–78. doi: 10.1007/s13277-012-0360-0. [DOI] [PubMed] [Google Scholar]
- Yim EK, Lee KH, Bae JS, et al. Proteomic analysis of antiproliferative effects by treatment of 5-fluorouracil in cervical cancer cells. DNA Cell Biol. 2004;23:769–76. doi: 10.1089/dna.2004.23.769. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen Z, Wang J, et al. Synergistic effect of 5-fluorouracil and the flavanoid oroxylin A on HepG2 human hepatocellular carcinoma and on H22 transplanted mice. Cancer Chemother Pharmacol. 2010;65:481–9. doi: 10.1007/s00280-009-1053-2. [DOI] [PubMed] [Google Scholar]


