Abstract
Purpose:
Liquid biopsy has entered the arena of cancer diagnostics in the past decade and detection of circulating tumor cells (CTC) is one diagnostic component. CTCs in gallbladder cancer (GBC) have hitherto not been comprehensively analysed.
Methods and Results:
The current study focused on the diagnostic role of CTCs in 27 cases of treatment-naive GBC and 6 normal controls as well as 6 cases of cholecystitis. An EasySep kit featuring negative immunomagnetic bead separation and flow cytometric detection of EpCAM positive and CD45 negative cells revealed CTCs in 25 of the 27 cases. At a cut-off point of ≥1, the CTC count discriminated GBC from controls with a sensitivity, specificity and diagnostic accuracy of 92.6%, 91.7% and 92.3%, respectively. CTC levels in turn correlated significantly with clinico-pathological parameters of cases in terms of known prognostic indicators, with significant diagnostic potential at a cut-off point of >4, to discriminate disease stage I and II vs. III and IV GBC. With a cut-off of >3, the CTC count discriminated tumor stages I and II vs. III and IV and at >6 CTCs could discriminate metastatic vs. non metastatic GBCs with a sensitivity, specificity and diagnostic accuracy of 55. 6%, 100.0% and 85.2, respectively. A review of CTC in pancreatico-biliary malignancies is included.
Conclusion:
Detection and quantification of CTCs may serve as a non-invasive biomarker for GBC diagnosis in correlation with radiological studies.
Keywords: Circulating tumor cells, gall bladder cancer, liquid biopsy, flowcytometry, EpCAM
Introduction
Gallbladder cancer (GBC) is a frequent malignancy in Asian countries including North India, Pakistan, Japan, and South Korea and South American Countries like Chile, Bolivia and Ecuador (Diehl, 1990). The prognosis and post-treatment outcome of cases with GBC is dismal. The disease is diagnosed in late stages and no effective treatment options are available. Advanced genetic diagnosis and profiling of tumors has brought forward cancer biomarkers which have been applied for early diagnosis, prediction of treatment response and prognosticating tumor behaviour in various tumor categories, cancer stage and metastatic phenotype (Cohen et al., 2008; Krebs et al., 2011).
Liquid biopsy, a generic term for detection of tumor derivatives in circulating blood or other body fluids, is a concept which has entered the arena of cancer diagnostics in the past decade. Liquid biopsy comprehensively includes circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), circulating microRNAs, circulating proteins, extracellular vesicles. CTCs and ctDNA are crucial components in the area of liquid biopsy. Apart from detection of malignancy CTCs have been used in various studies (1) achieving early diagnosis; (2) dynamic monitoring of treatment response (3) evaluating relapse and metastatic risk; (4) studying genetic evolution of the tumor and its heterogeneity (Gao et al., 2016; Nurwidya et al., 2016). In relation to the Gastro Intestinal Tract (GIT), CTCs have been studied in detail with reference to the above applications in colonic cancers, gastric carcinoma, esophageal cancer, pancreatic carcinomas, hepatocellular carcinoma (Weiss et al., 2013; Zhu et al., 2016; Iwatsuki et al., 2015). In an extensive review of literature we could find a single study of CTC in tumors of the biliary tract including mostly cholangiocarcinoma with only 3 cases of gall bladder carcinoma (Al Ustwani et al., 2012). The current study was designed to evaluate the diagnostic role of CTC in GBC as well as correlate CTC in relation to the Clinico-pathological parameters of cases in terms of known prognostic indicators affecting managements of gall bladder cancer.
Materials and Methods
Patients Selection: Study sample comprised of 27 patients diagnosed with GBC and 12 controls including 6 cholecystitis and 6 normal controls. The mean ± SD age of the normal control, cholecystitis and GBC patients were 35.33 ± 4.08, 37.00 ± 6.54 and 52.63 ± 9.95 respectively. Male to female (M/F) ratio was 5:22 in cancer group, 2/4 in cholecystitis and 3/3 in normal controls. Of the 27 patients with GBC 22 (81.5%) were female, 13 (48.1%) had stage IV disease, with T stage of T3 in 15 (55.6%) and with metastasis in 9 (33.3%). Ethical approval was obtained from Institutional Ethics Committee before recruiting patients. Inclusion criteria were radiologically or cyto-histologically diagnosed cases of GBC having not undergone prior chemotherapy or radiation. Cases were recruited from Surgical Oncology and Gastro Surgery services of our referral hospitals. All participants signed an informed consent. Exclusion criteria included cases who had undergone previous chemotherapy/radiotherapy as well as those with evidence of a significant clinical disorder or laboratory finding (which, in the opinion of the any other investigator would make it undesirable for the patient to participate in the consent to enter the study).
CTCs enrichment from Peripheral blood: Peripheral blood samples (4.0 ml) were collected into sodium heparinized tubes (BD Vacutainer, Cat# 36788, USA) in treatment naive cases after discarding the first 5.0 ml of blood, in order to avoid potential contamination with skin epithelial cells (Wang et al.,2009; Hristozova et al., 2012). Samples were stored at room temperature and processed within 1-2 hour of the collection.
Tumor cells were enriched directly from whole blood by depletion of hematopoietic cells and platelets with antibodies recognizing CD2, CD14, CD16, CD19, CD45, CD61, CD66b and Glycophorin A surface markers, using a Direct Human CTC Enrichment kit based on magnetic bead separation technique (EasySep®, Stem Cells Technologies, Inc., Vancouver, BC, Canada Cat# 19657) according to manufactures’ instructions. Briefly, 100μl of enrichment cocktail was added to 2ml of blood, mixed and incubate at room temperature (RT) for 5min followed by addition of 100μl ofRapidSpheres™ and 500μl of Phosphate buffer saline (PBS) containing 2% fetal bovine serum and 1mM EDTA). After gentle mixing, tubes were placed in aEasySep® magnet and incubated at RT for 10 minute. Cell suspension was transferred to a fresh FACS tube and 100μlRapidSpheres™ was added followed by 10 minute incubation in magnet at RT. Suspension containing enriched sample was poured off in new FACS tube. Same procedure was followed to process another 2 ml of blood.
For staining, fractions containing enriched cell were pooled in one tube and centrifuged at 1400rpm for 10 min at RT. Upper supernatant was discarded, leaving 200µl in the tube and was split into two equal fractions. One tube was stained with 10µl of CD45 (clone TU116, FITC-Labelled, B.D.Biosciences, USA) as auto fluorescence tube and other tube was stained with 10 µl of epithelial cell adhesion molecule (EpCAM, clone EBA-1, PE-Labelled, BD Biosciences, USA), 10µl CD45 and 15µl of 7-AAD (B.D Biosciences, USA). Both tubes were incubated in dark for 15 min at RT. All antibody batches were titrated before use to determine their optimal concentration on cell suspension prepared on fresh GBC tissue. After staining, cells were washed with Phosphate Buffer Saline (PBS, pH 7.4) and re-suspended in 500µl of PBS and acquired within 1 hour on a flowcytometer (FACSCalibur: Becton, Dickinson and Co.; San Jose, CA) equipped with a 488-nm blue laser. Acquisition and analysis were performed on Cell Quest Pro software (B.D. Biosciences, USA).Setup and automatic compensation were performed by using commercially available B.D. CalibriteTM 3-color kit beads (Cat # 340486, B.D. Biosciences, USA). The absolute numbers of tumor cells in 4.0 ml of blood were estimated by acquiring the total number of events in the analyzed sample. All the events were acquired on low flow rate. Cell debris and aggregates were excluded through sequential Boolean gating strategy on forward scatter vs. Side scatter, 7AAD vs. forward scatter and CD45 FITC vs. EpCAM PE. CTCs were defined as EpCAM+CD45− events. A blood sample was considered CTC+ when at least one EpCAM+CD45− event detected (Figure 1) and no event was detected in the auto fluorescence tube. The reproducibility of the method was tested in 6 (22.22%) cases by performing the experiment in duplicate from the same patient sample. Coefficient of variation (SD and mean) were acceptable giving test reproducibility of 100%.
Figure 1.
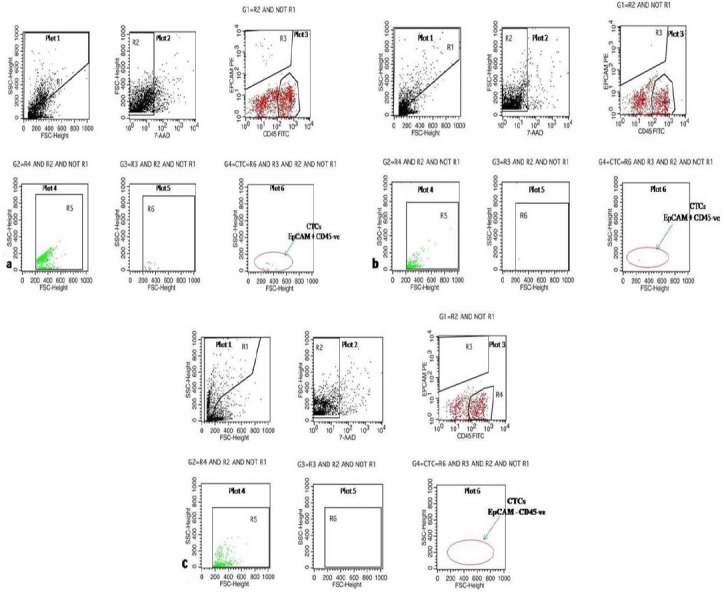
Plot 1, R1 drawn on Forward scatter vs. Side scatter plot to exclude debris (exclusion gate/NOT gate); Plot 2, R2 drawn on Forward scatter vs. 7-AAD to include only viable events which are 7-AAD positive; Plot 3, shows cells in G1 gate = R2 AND NOT R1 on CD45 FITC vs. EpCAM PE, R4 is drawn on bright CD45 positive cells (lymphocyte CD45+, EpCAM –ve) and R3 is drawn around EpCAM + CD45 –ve event (CTCs); Plot 4, is gated on G2=R4 AND R2 AND NOT R1. This plot gives lowest cut-off based on size for lymphocyte on which a further region R5 was made; Plot 5, is gated on G3=R3 AND R2 AND NOT R1. This plot shows CTCs on Forward scatter vs. Side scatter on which further region R6 is created which is exactly same as R5 on X-axis, considering that CTCS would not be smaller than lymphocytes; Plot 6, is gated on G4= R6 AND R3 AND R2 AND NOT R1. This plot shows CTC count after strictly excluding debris, dead cells and events which are smaller in size than lymphocytes. a)shows 18 CTC in Stage IV GBC b) shows 1 CTC in Stage I c) shows No CTC in normal healthy control.
Statistical Analysis
Statistical analysis was performed using the SPSS (Statistical Package for the Social Sciences) software, version 16.0.The CTC counts were reported as mean, median and interquartile range and other categorical variables were reported as percentages. Association between CTC and clinical and histological parameters were analysed by Kruskal-Wallis one way ANOVA and Mann-Whitney U-test. Association between categorical variables were assessed by chi-square or Fisher’s exact test. The Receiver Operating Characteristics (ROC) curve analysis was performed to test the diagnostic potential of CTC count to discriminate GBC cases from control. A cut off value was defined to calculate sensitivity and specificity values defining the curve and the area under the curve (AUC). A p value of less than 0.05 was considered significant.
Results
CTCs were detected in 25/27 (92.59%) of cases with a median of 3.0 CTCs per 4.0 ml bloods (range 0-20). CTC was also detected in 1 (8.33%) controls. CTC count was significantly higher in cases as compared to controls (p=<0.001). The median CTC count was significantly higher and associated with tumor stage, metastasis and disease stage p= 0.024, 0.022 and 0.013 respectively. However median CTC count of node negative vs. node positive GBC was not significantly different (p=0.093). Histopathological grading was available in 14 operated cases of which 7 (50.0%) cases were well differentiated and 7 (50.0%) were poorly differentiated. Cases of PD and WD showed 100% positivity for CTCs, with median count was higher in PD as compared to cases with well differentiated histology, though it was not statistically different (p=0.128) (Table 1).
Table 1.
Case Characteristics
| Patients (N=27) |
Number of CTC positive patients (%) |
Number of CTC Count Mean (Range) |
Number of CTC Count Median (Q1-Q3) |
p value* | |
|---|---|---|---|---|---|
| T stage | |||||
| T1 | 2 | 2 (100.00) | 1.5 (1-2) | 1.5 (1.25- 1.75) | |
| T2 | 4 | 3 (75.00) | 2.00 (0-2) | 2.00 (.75- 2.25) | |
| T3 | 15 | 14 (93.33) | 4.93 (0-18) | 4.00 (2.00-5.00) | 0.024 |
| T4 | 6 | 6 (100.00) | 7.00 (3-20) | 4.50 (3.00-10.25) | |
| N Stage | |||||
| N0 | 15 | 13 (86.66) | 4.27 (0-20) | 2.00 (1.00-4.00) | |
| N1 | 4 | 4 (100.00) | 4.00 (2-5) | 5.00 (3.50-5.00) | 0.093 |
| N2 | 8 | 8 (100.00) | 6.00 (2-18) | 4.00 (2.75- 5.50) | |
| M Stage | |||||
| M0 | 18 | 16 (88.88) | 2.67 (0-5) | 2.50 (1.00-4.25) | 0.022 |
| M1 | 9 | 9 (100.00) | 8.56 (2-20) | 7.00 (2.50-16.50) | |
| Disease stage | |||||
| I | 1 | 1 (100.00) | 1 | 1 | |
| II | 4 | 3 (75.00) | 2.00 (0-3) | 2.00 (0.75-2.25) | 0.013 |
| III | 9 | 8 (88.88) | 2.78 (0-5) | 3.00 (1.00-4.50) | |
| IV | 13 | 13 (100.00) | 7.15 (2-20) | 5.00 (2.50-11.00) | |
| Histological Grade | |||||
| WD | 7 | 7 (100.00) | 2.00 (1-5) | 2.00 (1.50-3.00) | 0.128 |
| PD | 7 | 7 (100.00) | 7.00 (1-18) | 4.00 (3.00-10.00) | |
Diagnostic of CTCs
Cut off values was calculated from ROC curves. The cases and controls were adequately separated at a cut-off of ≥1 CTC/4ml blood. Cut-off values were estimated to distinguish prognostic parameters within GBC cases including the tumor stage, lymphnode metastasis, metastasis, stage and histological grade of the carcinoma. Table 2 depict CTC cut-offs which discriminated stage T1andT2 vs. T3andT4, CTC lymphnode metastasis absent vs. present, systemic metastasis absent vs. present, stage IandII vs. IIIandIV and histological grade WD vs. PD. At a cut-off point of ≥1, CTC count significantly discriminates GBC cases from controls with sensitivity, specificity and diagnostic accuracy of 92.59%, 91.67% and 92.31% respectively. At a cut-off point of ≥3, CTCs count showed significant diagnostic discrimination of the tumor stage T1andT2 vs. T3andT4 with sensitivity, specificity and diagnostic accuracy of 71.43%, 83.33% and 74.04% respectively with an AUC of 0.833. At a cut-off point of ≥2, CTCs count showed significant diagnostic discrimination of no lymphnode metastasis vs. metastasis with sensitivity, specificity and diagnostic accuracy of 100%, 40.00% and 66.66% respectively with an AUC of 0.692 (Table 2).
Table 2.
Diagnostics of CTC in Gall Bladder Carcinoma
| Comparison Groups | Cut-off value | Positive/Total cases | p value | AUC | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Diagnostic Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| Control vs. | ≥1 | 1/12 | <0.001 | 0.954 | 92.59 | 91.67 | 96.15 | 84.62 | 92.31 |
| Cancer | 25/27 | (75.71- 99.09) | (61.52- 99.79) | (79.24- 99.39) | (58.91- 95.47) | ||||
| T-Stage: | ≥3 | 0.027 | 0.833 | 74.04 | |||||
| T1&T2 vs. | 2/5 | 71.43 | 83.33 | 93.75 | 45.45 | ||||
| T3&T4 | 19/22 | (47.82- 88.72) | (35.88- 99.58) | (71.06 -98.92) | (27.94 -64.17) | ||||
| N-Stage: | ≥2 | 0.017 | 0.692 | 66.66 | |||||
| Present vs. | 12/12 | 100 | 40 | 57.14 | 100 | ||||
| Absent | 9/15 | (73.54-100.00) | (16.34- 67.71) | (46.87- 66.84) | |||||
| Metastasis: | ≥6 | 0.002 | 0.772 | 85.18 | |||||
| Present vs. | 05/9 | 55.56 | 100 | 100 | 81.82 | ||||
| Absent | 0/18 | (21.20- 86.30) | (81.47- 100.0) | (68.43-99.33) | |||||
| Disease Stage: | ≥4 | 0.037 | 0.836 | 62.96 | |||||
| I&II vs. | 0/5 | 54.55 | 100 | 100 | 33.33 | ||||
| III&IV | 12/22 | (32.21- 75.61) | (47.82-100.00) | (24.03- 44.14) | |||||
| Histological Grade: | ≥4 | 0.051 | 0.745 | 78.57 | |||||
| WD vs. | 1/7 | 71.43 | 85.71 | 83.33 | 75 | ||||
| PD | 5/7 | (29.04- 96.33) | (42.13- 99.64) | (43.42-97.02) | (47.22-90.96) |
At cut-off point of ≥6 CTCs, CTC count showed significant diagnostic discrimination of non metastatic GBC from metastatic disease with a sensitivity, specificity and diagnostic accuracy of 55.56%, 100.0% and 85.18% respectively and an AUC of 0.772. At cut-off point of ≥4, CTC count significantly discriminates stage IandII vs. III andIV cases with a sensitivity, specificity and diagnostic accuracy of 86.36%, 60.0% and 81.48% respectively and an AUC of 0.836. At cut-off point of ≥4 CTCs, CTC count showed some diagnostic discrimination of well differentiated histology vs. poorly differentiated carcinoma with sensitivity, specificity and diagnostic accuracy of 71.43%, 85.71% and 78.57% respectively with an AUC of 0.745 although p value was borderline at p=0.051 (Table 2).
Discussion
The current paper describes a novel study detecting CTC in a large cases series of GBC for the first time using immunomagnetic bead separation for enrichment from peripheral blood and flowcytometric detection. Till date we could come across only three cases of gall bladder carcinoma reported in literature in a series of other biliary cancers mostly cholangiocarcinoma (Al Ustwani et al., 2012).
CTCs are extremely rare and occur in peripheral blood in a ratio of about 1 CTC per 106-108 leukocytes. Enrichment and their subsequent detection is hence a challenging issue. Since CTC can, in a non invasive way, provide clinically relevant information in cancer follow-up there has been a concerted effort to develop sensitive platforms to isolate, enrich, and analyse CTCs. The process involves two steps: CTC enrichment followed by CTC detection. CTC enrichment has been performed by different techniques: Antibody-based enrichment uses antibodies directed against cell surface markers. Positive immunoselection relies on antibodies directed against cancer cell surfaces markers. The most frequently used antibody is Epithelial Cell Adhesion Molecule (EpCAM) for positive immunoselection. Negative selections deplete leukocytes from the blood sample and usually use CD45-binding antibodies as also done in our cases. Physical/biological assays isolate CTCs on the basis of cell size or bioelectric features. Most CTCs exhibit a larger size and different density, electromagnetic charge and motility than normal blood cells. This allows their separation by dedicated devices (filters, dielectrophoresis), sometimes integrated in microfluidic chips (Autebert et al., 2015; Bobek et al., 2014). A novel label-free and reusable electrochemical cytosensor for direct detection and enrichment of CTCs was successfully developed based on effective surface recognition between EpCAM and EpCAMaptamer with a low detection limit of 10 cells/ml (Shen et al., 2016).
CTC can be detected through proteomic or genomic or on the basis of physical properties. Immunocytochemistry is considered the gold standard for detection and utilizes detection of proteins of epithelial origin to identify CTC. Combined with a morphological examination of the stained cells, the detection rate is highest among prevalent techniques. The CellSearch technique combines an immunomagnetic separation with EpCAM-based enrichment followed by immunocytofluorescence detection utilizing EpCAM and other epithelial markers. CTCs are enumerated on the basis of expression of epithelial cytokeratins, presence of a nucleus and negative CD45 staining. The United State Food and Drug Administration cleared this technique for prognostication of metastatic breast, colorectal and prostate cancers (Lalmahomed et al., 2014). The potential drawback of this positive selection of CTCs, allows only cells with adequate expression of EpCAM to be separated. Therefore, CTCs with down-regulation of EpCAM resulting in low or absent expression will be excluded. In the current study we have used Direct Human CTC Enrichment kit (EasySep®, Stem Cells Technologies, Inc., Vancouver, BC, Canada) based on magnetic bead separation technique which included markers for hematopoietic cells and platelets with antibodies recognizing CD2, CD14, CD16, CD19, CD45, CD61, CD66b and Glycophorin A surface markers which allowed depletion of leucocytes without removing the CTC. Subsequent phenotypic characterization was done using EpCAM and CD45 staining of the CTC concentrate for flowcytometric detection. This method has resulted in a high sensitivity of CTC detection in GBC with only two cases not showing any CTC. Detection rate of CTCs was 92.59% (≥1CTC/4 ml) with a median of 3.0 CTCs per 4.0 ml of blood. We found CTC ≥2 in 21 (77.8%), CTC ≥3 in 16 (59.3%), CTC ≥ 4 in 12 (44.4%) and CTC≥5 9 (33.3%) patients with gall bladder cancers per 4 ml of blood. CTCs were not found in healthy patients, however one of the patient with chronic cholecystitis showed a single CTC in 4 ml blood. A single cases series of biliary cancers has reported three cases of CTC detection in GBC so far with positive CTC in only one of three cases. However, pancreatico-biliary malignancies, liver malignancies and colonic malignancies have been extensively studied for presence of CTC. Table 3 reviews CTC detection and Enrichment Techniques used in major studies in gastrointestinal malignancies emphasizing differences in methods of CTC analysis in tumors of the biliary tract, pancreas and colorectal lesions. Highest CTC detection rate of 96.1 % was reported in pancreas by Gao et al. using immunobeads for enrichment and immunocytometry for detection (Gao et al., 2016). Overall immunocytometry based methods show a higher detection rate in pancreatic malignancies with a mean detection rate of 79% in studies reviewed, while the CellSearch system showed a mean positive CTC detection in 25% biliary cancers, 30% pancreatic and 39.05% colorectal cancers. Overall the flowcytometric detection carries a higher detection rate of 74.63% and we have got a very good detection rate using the immunomagnetic bead separation and EpCAM based flowcytometric detection (Table 3).
Table 3.
Review of CTC Detection and Enrichment Techniques in Gastrointestinal Malignancies
| CTC Count | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Technique | Enrichment | Site | Number of Cases | Positive Rate | Mean(±SD) | Median | Range | Mean % CTC detection | Author & Year |
| Colorectal liver metastases | 75/151 | 43.00% | NR | NR | 0-49 | Lalmahomed ZS et al.,2015 | |||
| Colorectal Cancer | 12/25 | 48.00% | NR/7.5ml | NR | 0-162 | Welinder C et al.,2015 | |||
| Colorectal Cancer | 20/69 | 29.00% | NR | 0/7.5ml | 0–147 | 39.05 | Deneve E et al., 2013 | ||
| CellSearch® system | Integrated EpCAM based enrichment | Colorectal Cancer | 34/94 | 36.20% | 3.4/7.5 ml | NR | 0-61 | Sastre J et al., 2008 | |
| Pancreatic Cancer | 9/79 | 11.00% | 2.7±4.6/7.5 ml | 1 | 0-15 | Bidard FC et al., 2013 | |||
| Pancreatic Cancer | 21/53 | 39.60% | 6/7.5 ml | 0 | 0–15 | Khoja Let al., 2012 | |||
| Pancreatic Cancer | 11/26 | 42.30% | 16.9 ± 31.0/7.5 ml | NR | 0-105 | 30.96 | Kurihara T et al., 2008 | ||
| Biliary origin cancer | 4/16 | 25.00% | 2.25/7.5 ml | NR | NR | 25 | Al Ustwani O et al., 2012 | ||
| Density gradient centrifugation | Colorectal cancer | 36/63 | 57.00% | NR/10 ml | NR | NR | Rahbari NN et al., 2011 | ||
| Real Time PCR | Density gradient centrifugation | Colorectal Cancer | 25/42 | 59.50% | NR/5 ml | NR | NR | Xu D et al., 2006 | |
| Red blood cell lysis | Colorectal cancer | 24/36 | 66.70% | NR/5 ml | NR | NR | 57.65 | Teama SH et al., 2010 | |
| Density gradient centrifugation &Immunomagnetic bead based separation | Colorectal cancer | 74/156 | 47.40% | NR/5ml | NR | NR | Shen C et al., 2008 | ||
| Red blood cell lysis | Pancreatic cancer | 10/40 | 25.00% | NR/10 ml | NR | NR | Sergeant G et al., 2011 | ||
| Density gradient centrifugation &Immunomagnetic bead based separation | Pancreatic cancer | 21/25 | 84.00% | NR/10 ml | NR | NR | Zhou J et al., 2011 | ||
| Real Time PCR | Immunomagnetic enrichment | Pancreatic cancer | 16/34 | 47.10% | NR/ 10 ml | NR | NR | 52.03 | de Albuquerque A et al., 2011 |
| Red blood cell lysis&immunomagnetic bead based separation | Pancreatic cancer | 24/25 | 96.10% | NR | 3/7.5ml | 0-13 | Gao Y et al., 2016 | ||
| Red blood cell lysis&immunomagnetic bead based separation | Pancreatic cancer | 33/41 | 80.50% | 16.8±16.0/7.5 ml | NR | 0–59 | Ren C et al., 2011 | ||
| Immunomagnetic enrichment | Pancreatic cancer | 15/22 | 68.20% | NR | 3 | 0-60 | Zhang Y et al., 2015 | ||
| Immunocyto | |||||||||
| Chemistry/ | Filtration | Pancreatic cancer | 39/50 | 78.00% | NR | 30/ml | 1–251 | Poruk KE et al., 2016 | |
| Immuno fluorescence | (ISET) | ||||||||
| ScreenCell filtration | Pancreatic cancer | 51/105 | 49.00% | NR | NR | NR | 70.26 | Cauley CE et al., 2015 | |
| Microfluidic GEM chip | Pancreatic cancer | 17/18 | 94.00% | 2.8±1.8/ml | NR | 0-7 | Sheng W et al., 2014 | ||
| Nycoprep based centrifugation | Pancreatic cancer | 28/105 | 26.00% | NR/10-20 ml | NR | NR | Z graggen K et al., 2001 | ||
| Red blood cell lysis | Gastric Cancer | 31/57 | 54.40% | NR | NR | NR | Pituch-Noworolska A et al., 2007 | ||
| Flowcytometry | Immunomagnetic separation | Colorectal cancer | 38/49 | 77.00% | 2/7.5 ml | NR | NR | 74.63 | Cohen SJ et al., 2006 |
| Immunomagnetic separation | Gall Bladder Cancer | 25/27 | 92.50% | 5±5/4ml | 3 | 0-20 | Current study |
CTCs are generally heterogeneous in both phenotype and genotype, and only 2.5% of CTCs develop micrometastasis and only 0.01% develop macrometastasis (O’Flaherty et al., 2012). In a large study by Allard WJ et al on blood samples from 344 healthy and non-malignant disease subjects and 964 metastatic cancer subjects, less than 1% of the subjects without cancer (n=1) had a positive CTC ≥2/7.5ml of blood (Allard et al., 2004). It is relevant here to mention that different studies use varying quantities of blood for CTC count hence total number of CTC are not comparable across studies and no standard cut-offs have been defined. It may be a good idea to introduce a convention to give CTC is a specific quantify to peripheral blood, for example 7.5 ml, as taken in the USFDA approved CellSearch methods, which may help establish cut offs. Through an ROC curve analysis (Table 2), we have found a cut off of 1 CTC/4ml peripheral blood to give a diagnostic sensitivity, specificity and accuracy of 92.59%, 91.67% and 92.31% respectively in GBC. Various thresholds have been used in studies examining the predictive performance of CTC in different cancers, for example studies have put values of 3CTC/7.5 ml for metastatic colorectal cancer (Cohen et al.,2008), 5CTC/7.5 ml for breast and prostate cancers (Liu et al., 2009; de Bono et al., 2008), which may be related to the variation or loss in the expression of EpCAM. We observed that at a cut-off of CTC ≥6, CTC count showed significant diagnostic discrimination of GBC with metastasis from cases with no metastasis with sensitivity, specificity and diagnostic accuracy of 55.56%, 100.0% and 85.18 respectively. The current study reports the role of CTCs in predicting distant metastasis (AUC-ROC, 0.772; p=0.002). Mean (±SD) of CTC count was significantly higher in metastatic phenotype as compared to localized phenotype and was able to also segregate early stage from stage IV GBC. Similarly diagnostic discrimination of early and late T stges, lymph node metastases, stage and grade could be achieved at different cut off values (Table 2).
Biliary tract cancer cells over express EpCAM (Kawashima et al., 2011). Ustwani and colleagues, reported detectable CTCs in patients with advanced biliary cancer (Al Ustwani et al., 2012). Assays were performed in 16 patients including 13 with cholangiocarcinoma (CCA) and 3 with gallbladder cancer. Three of 13 patients with cholangiocarcinoma and one of the 3 with gallbladder cancer were found to have 2 CTC’s per 7.5 mL of blood at baseline. All of the patients with detectable CTCs had stage III or IV disease, while 0/3 patients with Stage I-II disease had detectable CTCs (Al Ustwani et al., 2012). In cholangiocarcinomaYang et al. reported an association of the number of CTCs with more aggressive tumor characteristics and lower survival in CCA patients in a large cohort of 88 cases, which also included correlation with clinical outcome (Yang et al., 2016). CTC enumeration was done using the semi automatedCellSearch (Janssen Diagnostics). The authors reported an association between CTC number and aggressive tumor characteristics including increased tumor burden such as size, multinodularity, and loco-regional lymph node invasion (Yang et al., 2016). On the basis of ROC curves different cut-off values were used in our study for discrimination of tumor stage, nodal status, stage, grade and metastasis. We have observed a significant difference of CTC level between T stage (T1andT2 vs. T3andT4), systemic metastasis (M0 vs. M1), tumor stage (IandII vs. IIIandIV) and nodal metastasis viz N0 vs. N1 +N2. Median CTC level was higher in cases with well differentiated and poorly differentiated histological grade which was associated at a borderline p value of 0.051.
Uchikura K et al. reported CTC in biliary cancer using a completely different technique based on reverse transcriptase polymerase chain reaction (RT-PCR) for carcinoembryonic antigen (CEA) (Uchikura et al.,2002). Molecular techniques targeting CK19 and hTERT mRNA has also been used by Kim et al to detect CTC in biliary carcinoma (Kim et al., 2012). The authors found CTCs in 45% patients of cholangiocarcinoma and positive cases were associated with worse overall survival. CTC were utilized to detect recurrence post curative surgical resection (n=53) in cases with pancreaticobiliary cancer using RT-PCR to detect CEA mRNA. Positive CTC status was observed in 75% of relapsed patients but only in 5.4% in disease-free patients, suggesting that it might indicate early relapse (Mataki et al., 2004). Intraoperative detection of CEA mRNA in cholangiocarcinoma has also been reported to be associated with recurrence and worse survival (Uchikura et al.,2002).
CTCs are derived from both primary and metastatic sites of tumors in the blood and are therefore more likely to be detected in advanced tumors with multiple sites and increased tumor burden. CTCs have also been detected in early disease, after primary radical surgery, and even in follow-up when clinically detectable tumor or recurrence is not detectable (Gao et al., 2016). Preoperative CTC values however do not identify patients at risk for early disease recurrence after curative resection of colorectal liver metastases (Lalmahomed et al., 2015). The half-life of CTCs is 1-2.4 hours (Meng et al., 2004), they hence reflect the current status of both primary tumors and secondary deposits. Markers may be used to identify tumor cells for example EGFR is expressed in the majority of tumor cells in squamous cell carcinoma of head and neck at the primary site, detection of EGFR expression has been observed in 100% of the CTC. In contrast, phospho-EGFR expression is observed in 36% of the CTC (Hristozova et al., 2012). Genetic heterogeneity has also been studied in CTC. Subsets of CTCs have been reported to have phenotypes of cancer stem cells (CSCs), which may initiate tumor formation and drug resistance (López-Gómez et al., 2016). Transformation between CTCs and CSCs has been linked to epithelial-mesenchymal transition (EMT) (Mani et al., 2008). Future perspectives, in view of the new sensitive detection methods, are bright and CTC may find an application in cancer diagnosis in terms of screening, prediction of aggressive nature of disease, prognosis and prediction. In clinical practice CTC evaluation may be useful for differentiating xanthogranulomatous cholecystitis and chronic cholecystitis from GBC as all these may show overlapping radiological features. In the current study we have also observed a significant difference of CTC level between T stage , systemic metastasis, tumor stage and nodal metastasis suggesting its role in prognostication of GBC. We propose that future studies serially quantifying change in CTC during treatment follow up in cases of GBC undergoing treatment may predict response to therapy.
Detection of static and dynamic marker expression in CTC before and after therapy can be investigated in association with disease progression or response to therapy and overall survival (Khan et al., 2016).
High purity isolation of CTCs and its utility in various downstream genomic analyses, including comparative genomic hybridization, polymerase chain reaction and next generation sequencing (NGS) has been reported. Isolation and characterization of cancer cells can afford a better understanding of genomic changes with cancer progression, address issues of tumor heterogeneity (Premasekharan et al., 2016). Selection of new targets for personalized therapy, studying tumor sensitive and resistance profiles, NGS, mutational analysis and global gene expression profiling would help is specific diagnosis as well as profiling of driver mutations.
The current study has some limitations relating to the capture method, which relies on EpCAM expression and could miss non-EpCAM CTCs, so the actual CTC number could be underestimated. Paired analysis of EpCAM expression in tumor tissue with CTC enumeration could help us to understand the extent of this limitation. A cocktail of antibodies in flowcytometry including cytokeratin 8, 18, 19 along with EpCAM and CD45 may also help overcome this limitation.
We have earlier looked at circulating free DNA (cfDNA) in peripheral blood of cases with GBC and found high diagnostic sensitivity and specificity in diagnosis of GBC as well as prediction of aggressive behavior of the tumor. Our study using cfDNA levels reported a sensitivity and specificity of 100 % to distinguish cases with GBC from healthy controls while a sensitivity and specificity of 88.24 % and 100 % respectively was seen in discriminating cholecystitis from cases with GBC (Kumari et al., 2017). Although cfDNA values were several times higher in inflammation as compared to controls they were significantly lower that cases of GBC. In preoperative cases CTC analysis along with data coupled with cfDNA level may increase the sensitivity of GBC diagnosis. EpCAM +ve cells and high cfDNA levels are expressed in all carcinomas. The circulating tumor DNA and DNA from CTC can also be utilized for specific molecular tests like p53 mutation detection thereby increasing the specificity of diagnosis.
Detection and quantification of CTC may serve as a non invasive biomarker for diagnosis of GBC in correlation with radiological studies thereby providing the oncologist with preoperative near definite idea of presence of malignancy with appropriate treatment planning.
Acknowledgments
Authors wish to acknowledge Department of Science and Technology (DST), New Delhi, India for providing INSPIRE Research Fellowship (IF120759) support to Swati Kumari and for Ph.D registration at Integral University, Lucknow.
References
- Al Ustwani O, Iancu D, Yacoub R, Iyer R. Detection of circulating tumor cells in cancers of biliary origin. J Gastrointest Oncol. 2012;3:97–104. doi: 10.3978/j.issn.2078-6891.2011.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- Autebert J, Coudert B, Champ J, et al. High purity microfluidic sorting and analysis of circulating tumor cells:towards routine mutation detection. Lab Chip. 2015;15:2090–101. doi: 10.1039/c5lc00104h. [DOI] [PubMed] [Google Scholar]
- Bidard FC, Huguet F, Louvet C, et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma:the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24:2057–61. doi: 10.1093/annonc/mdt176. [DOI] [PubMed] [Google Scholar]
- Bobek V, Gurlich R, Eliasova P, Kolostova K. Circulating tumor cells in pancreatic cancer patients:enrichment and cultivation. World J Gastroenterol. 2014;20:17163–70. doi: 10.3748/wjg.v20.i45.17163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley CE, Pitman MB, Zhou J, et al. Circulating epithelial cells in patients with pancreatic lesions:clinical and pathologic findings. J Am Coll Surg. 2015;221:699–707. doi: 10.1016/j.jamcollsurg.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Alpaugh RK, Gross S, et al. Isolation and characterization of circulating tumor cells in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2006;6:125–32. doi: 10.3816/CCC.2006.n.029. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J ClinOncol. 2008;26:3213–21. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- de Albuquerque A, Kubisch I, Breier G, et al. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients:a feasibility study. Oncology. 2012;82:3–10. doi: 10.1159/000335479. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- Deneve E, Riethdorf S, Ramos J, et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin Chem. 2013;59:1384–92. doi: 10.1373/clinchem.2013.202846. [DOI] [PubMed] [Google Scholar]
- Diehl AK. Epidemiology of gallbladder cancer:a synthesis of recent data. J Natl Cancer Inst. 1980;65:1209–14. [PubMed] [Google Scholar]
- Gao Y, Zhu Y, Yuan Z. Circulating tumor cells and circulating tumor DNA provide new insights into pancreatic cancer. Int J Med Sci. 2016;13:902–13. doi: 10.7150/ijms.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristozova T, Konschak R, Budach V, Tinhofer I. A simple multicolor flow cytometry protocol for detection and molecular characterization of circulating tumor cells in epithelial cancers. Cytometry A. 2012;81:489–95. doi: 10.1002/cyto.a.22041. [DOI] [PubMed] [Google Scholar]
- Iwatsuki M, Kurashige J, Ishimoto T, et al. The clinical significance of circulating tumor cells in gastrointestinal cancer. J Cancer Metastasis Treat. 2015;1:130–7. [Google Scholar]
- Kawashima R, Abei M, Fukuda K, et al. EpCAM- and EGFR-targeted selective gene therapy for biliary cancers using Z33-fiber-modified adenovirus. Int J Cancer. 2011;129:1244–53. doi: 10.1002/ijc.25758. [DOI] [PubMed] [Google Scholar]
- Khan MS, Kirkwood AA, Tsigani T, et al. Early changes in circulating tumor cells are associated with response and survival following treatment of metastatic neuroendocrine neoplasms. Clin Cancer Res. 2016;22:79–85. doi: 10.1158/1078-0432.CCR-15-1008. [DOI] [PubMed] [Google Scholar]
- Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumor cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106:508–16. doi: 10.1038/bjc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YD, Hwang S, Lee YJ, et al. Preoperative peripheral blood human telomerase reverse transcriptase mRNA concentration is not a prognostic factor for resection of hepatocellular carcinoma. Hepatogastroenterology. 2012;59:1512–5. doi: 10.5754/hge10342. [DOI] [PubMed] [Google Scholar]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non small-cell lung cancer. J ClinOncol. 2011;29:1556–63. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- Kumari S, Tewari S, Husain N, et al. Quantification of circulating free DNA as a diagnostic marker in gall bladder cancer. Pathol Oncol Res. 2017;23:91–7. doi: 10.1007/s12253-016-0087-0. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Itoi T, Sofuni A, et al. Detection of circulating tumor cells in patients with pancreatic cancer:a preliminary result. J Hepatobiliary Pancreat Surg. 2008;15:189–95. doi: 10.1007/s00534-007-1250-5. [DOI] [PubMed] [Google Scholar]
- Lalmahomed ZS, Mostert B, Onstenk W, et al. Prognostic value of circulating tumor cells for early recurrence after resection of colorectal liver metastases. Br J Cancer. 2015;112:556–61. doi: 10.1038/bjc.2014.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MC, Shields PG, Warren RD, et al. Circulating tumor cells:a useful predictor of treatment efficacy in metastatic breast cancer. J ClinOncol. 2009;27:5153–9. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gómez M, Casado E, Mu˜nozf M, et al. Current evidence for cancer stem cells in gastrointestinal tumors and future research perspectives. Crit Rev Oncol Hematol. 2016;107:54–71. doi: 10.1016/j.critrevonc.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataki Y, Takao S, Maemura K, et al. Carcinoembryonic antigen messenger RNA expression using nested reverse transcription-PCR in the peripheral blood during follow-up period of patients who underwent curative surgery for biliary-pancreatic cancer:longitudinal analyses. Clin Cancer Res. 2004;10:3807–14. doi: 10.1158/1078-0432.CCR-03-0130. [DOI] [PubMed] [Google Scholar]
- Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–62. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- Nurwidya F, Zaini J, Putra AC, et al. Circulating tumor cell and cell-free circulating tumor DNA in lung cancer. Chonnam Med J. 2016;52:151–8. doi: 10.4068/cmj.2016.52.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty JD, Gray S, Richard D, et al. Circulating tumor cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer. 2012;76:19–25. doi: 10.1016/j.lungcan.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Pituch-NoworolskaA Kolodziejczyk P, Kulig J, et al. Circulating tumor cells and survival of patients with gastric cancer. Anticancer Res. 2007;27:635–40. [PubMed] [Google Scholar]
- Poruk KE, Valero V, Saunders T, et al. Circulating tumor cell phenotype predicts recurrence and survival in pancreatic adenocarcinoma. Ann Surg. 2016;264:1073–81. doi: 10.1097/SLA.0000000000001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premasekharan G, Gilbert E, Okimoto RA, et al. An improved CTC isolation scheme for pairing with downstream genomics:Demonstrating clinical utility in metastatic prostate, lung and pancreatic cancer. Cancer Lett. 2016;380:144–52. doi: 10.1016/j.canlet.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Reissfelder C, Mühlbayer M, et al. Correlation of circulating angiogenic factors with circulating tumor cells and disease recurrence in patients undergoing curative resection for colorectal liver metastases. Ann Surg Oncol. 2011;18:2182–91. doi: 10.1245/s10434-011-1761-9. [DOI] [PubMed] [Google Scholar]
- Ren C, Han C, Zhang J, et al. Detection of apoptotic circulating tumor cells in advanced pancreatic cancer following 5-fluorouracil chemotherapy. Cancer Biol Ther. 2011;12:700–6. doi: 10.4161/cbt.12.8.15960. [DOI] [PubMed] [Google Scholar]
- Sastre J, Maestro ML, Puente J, et al. Circulating tumor cells in colorectal cancer:correlation with clinical and pathological variables. Ann Oncol. 2008;19:935–8. doi: 10.1093/annonc/mdm583. [DOI] [PubMed] [Google Scholar]
- Sergeant G, Roskams T, van Pelt J, et al. Perioperative cancer cell dissemination detected with a real-time RT-PCR assay for EpCAM is not associated with worse prognosis in pancreatic ductal adenocarcinoma. BMC Cancer. 2011;11:47. doi: 10.1186/1471-2407-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Hu L, Xia L, Li Y. Quantitative real-time RT-PCR detection for survivin, CK20 and CEA in peripheral blood of colorectal cancer patients. Jpn J Clin Oncol. 2008;38:770–6. doi: 10.1093/jjco/hyn105. [DOI] [PubMed] [Google Scholar]
- Shen H, Yang J, Chen Z, et al. A novel label-free and reusable electrochemical cytosensor for highly sensitive detection and specific collection of CTCs. Biosens Bioelectron. 2016;81:495–502. doi: 10.1016/j.bios.2016.03.048. [DOI] [PubMed] [Google Scholar]
- Sheng W, Ogunwobi OO, Chen T, et al. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip. 2014;14:89–98. doi: 10.1039/c3lc51017d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teama SH, Sara HAAgwa. Detection of circulating tumor cells by nested RT-PCR targeting EGFR/CEA/CK20mRNAs in colorectal carcinoma patients. Egypt J Med Human Genet. 2010;11:173–80. [Google Scholar]
- Uchikura K, Takao S, Nakajo A, et al. Intraoperative molecular detection of circulating tumor cells by reverse transcription-polymerase chain reaction in patients with biliary-pancreatic cancer is associated with hematogenous metastasis. Ann Surg Oncol. 2002;9:364–70. doi: 10.1007/BF02573871. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang Y, Liu Y, et al. Flow cytometric analysis of CK19 expression in the peripheral blood of breast carcinoma patients:relevance for circulating tumor cell detection. J Exp Clin Cancer Res. 2009;28:57. doi: 10.1186/1756-9966-28-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GA, Samuel ST, Al Ustwani O, Iancu D. Circulating tumor cells in gastrointestinal malignancies. Transl Gastrointest Cancer. 2013;2:118–29. [Google Scholar]
- Welinder C, Jansson B, Lindell G, Wenner J. Cytokeratin 20 improves the detection of circulating tumor cells in patients with colorectal cancer. Cancer Lett. 2015;358:43–6. doi: 10.1016/j.canlet.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Xu D, Li XF, Zheng S, Jiang WZ. Quantitative real-time RT-PCR detection for CEA, CK20 and CK19 mRNA in peripheral blood of colorectal cancer patients. J Zhejiang Univ Sci B. 2006;7:445–51. doi: 10.1631/jzus.2006.B0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JD, Campion MB, Liu MC, et al. Circulating tumor cells are associated with poor overall survival in patients with cholangiocarcinoma. Hepatology. 2016;63:148–58. doi: 10.1002/hep.27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Z graggen K, Centeno BA, Fernandez-del Castillo C, et al. Biological implications of tumor cells in blood and bone marrow of pancreatic cancer patients. Surgery. 2001;129:537–46. doi: 10.1067/msy.2001.113819. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang F, Ning N, et al. Patterns of circulating tumor cells identified by CEP8, CK and CD45 in pancreatic cancer. Int J Cancer. 2015;136:1228–33. doi: 10.1002/ijc.29070. [DOI] [PubMed] [Google Scholar]
- Zhou J, Hu L, Yu Z, et al. Marker expression in circulating cancer cells of pancreatic cancer patients. J Surg Res. 2011;171:631–6. doi: 10.1016/j.jss.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Zhu J, Strickler JH. Clinical applications of liquid biopsies in gastrointestinal oncology. J Gastrointest Oncol. 2016;7:675–86. doi: 10.21037/jgo.2016.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]


