Abstract
Introduction:
Down regulation of CD20 expression has been reported in diffuse large B cell lymphoma (DLBCL)). Therefore, it is important to determine whether chemotherapy with rituximab induces CD20 down regulation and effects survival.
Objectives:
To determine the incidence of down regulation of CD20 expression in relapsed DLBCL after treatment with rituximab and to compare outcomes and assess pattern of relapse between CD20 negative and CD20 positive cases.
Methodology:
We retrospectively reviewed patients with relapsed DLBCL who received rituximab in the first line setting at Aga Khan University Hospital between January 2007 and December 2014. Data were recorded on predesigned questionnaires, with variables including demographics, details regarding date of diagnosis and relapse, histology, staging, international prognostic index, treatment and outcomes at initial diagnosis and at relapse. The Chi square test was applied to determine statistical significance between categorical variables. Survival curves were generated by the Kaplan–Meier method.
Results:
A total of 54 patients with relapsed DLBCL were included in our study, 38 (70 %) males and 16(30%) females. Some 23 (43%) patients were at stage IV at the time of diagnosis and 34 (63%) had B symptoms. The most frequent R-IPI at diagnosis was II in 24 (44%) patients. Only 6 (11%) did not show CD20 expression on re-biopsy for relapsed/refractory disease, 2 with CD20 negative DLBCL responding to second line chemotherapy. A complete response after salvage chemotherapy was noted in 16 (29.6%) cases with relapsed/refractory DLBCL. Seven (13%) patients underwent an autologous bone marrow transplant as consolidation after second line treatment. Median overall survival was 18 months in CD20 positive vs. 13 months in CD20 negative patients.
Conclusion:
This study demonstrated that a small percentage of patients treated with rituximab lose their CD20 expression at the time of relapse. However, it is unclear whether this is associated with an inferior outcome.
Keywords: DLBCL, diffuse large B cell lymphoma, R−IPI-revised international prognostic index -chemotherapy
Introduction
Addition of rituximab to induction chemotherapy in DLBCL has improved prognosis, specially bcl2 positive and non-germinal center subtype of DLBCL (Fenske et al., 2009). Response rates with single agent rituximab in DLBCL at initial diagnosis is approximately 30-35% (Davis et al., 1999; Kewalramani et al., 2004). Combination of rituximab with chemotherapy has improved complete response rates to 75%-80% (Gisselbrecht et al., 2010). Unfortunately, 30-40 % patients relapse after complete response and 10% are refractory to standard anthracycline based regimen (Raut and Chakrabarti, 2014). At relapse, retreatment with chemo immunotherapy shows a response rate of 55% when compared to 28% when treated with regimens without rituximab (Raut and Chakrabarti, 2014; Jiang et al., 2013).
A decrease in response to rituximab at relapse is likely secondary to drug resistance, however, the exact mechanisms are not clearly defined (Rezvani and Maloney, 2011). Possible mechanisms are loss of CD20 expression, inflection of receptor, alteration in signaling pathways and decreased apoptotic and complement activity In low grade lymphoma, after rituximab exposure, it has been observed that loss of CD20 expression lead to conversion of low grade lymphoma to high grade lymphoma and inferior survival (Gisselbrecht et al., 2010). However, there is sparse data on clinical outcomes of CD20 negative relapsed/ refractory DLBCL with prior rituximab exposure. We aim to determine the clinical features and prognosis of DLBCL after loss of CD20 expression.
Materials and Methods
This is a retrospective cohort study. After exemption approval from hospital ethical review committee medical records of patients with relapsed/refractory DLBCL who received treatment at Aga Khan University hospital (AKUH) were reviewed from January 2007 and December 2014. We included only those patients who had received rituximab as part of the first line therapy and had pathological assessment at the time of relapse. Patients who did not have adequate biopsy specimen for review at the time of relapse and those who didn’t receive second line treatment and followup at AKUH were excluded from the study.
Primary objective of the study was to determine the incidence of CD20 expression in patients with relapsed DLBCL who were previously exposed to rituximab. The secondary objectives included disease characteristics, disease free survival and overall survival of CD20 positive and CD20 negative relapsed DLBCL. For our analysis disease free survival was defined as any recurrence after completion of definitive treatment and overall survival was defined as time from initial diagnosis until death from any cause. Patients were followed from the time of initial diagnosis till last follow up if alive or till death.
Statistical analysis: SPSS version 19 was used for statistical analysis. Descriptive statistics were calculated with the help of mean standard deviation and for categorical variables, frequencies and percentages were used. Chi square test was applied to determine statistical significance between categorical variables. Survival curves were calculated by Kaplan–Meier curve. P<0.05 was considered to be statistically significant and P<0.1 showed trend toward significance.
Results
Patient characteristics at initial diagnosis
Fifty four patients were included in the analysis. Among them, 38 (70%) were male and 16 (30%) were female. The mean age at diagnosis was 55.3+/-16.7 years (range, 22-91 years). 34 (63%) patients presented with B symptoms. Most common stage was stage IV in 23(43%) patients. 70% of the patients had low- high intermediate prognosis as classified by Revised International Prognostic Index (R-IPI). (Table 1)
Table 1.
Patient Characteristics at Initial Diagnosis
| Age Groups | <= 50Years | 22(40.7%) |
| >50 Years | 32(59.3%) | |
| Gender | Male | 38 (70%) |
| Female | 16 (30%) | |
| B Symptoms | Yes | 34 (63%) |
| No | 20 (37%) | |
| Stage | Stage I / II | 3 (06%) /17 (32%) |
| Stage III / IV | 11 (20%)/23 (43%) | |
| RIPI | RIPI I / II | 11 (20%)/24 (44%) |
| RIPI III / IV | 14 (26%)/5 (09%) |
SD, standard deviation; RIPI, rituximab International Prognostic Index
The most frequently prescribed first line chemo regimen was R-CHOP in 44 (81.5%) patients. 8 (14.8%) patients received R-CVP and 2 (3.7%) patients were treated with R-EPOCH. 26(48%) patients also received intrathecal chemotherapy for central nervous system prophylaxis. 26(48.1%) showed complete response at end of treatment evaluation. Among 26 patients who showed complete response, 17(31.4%) had low RIPI.
Patient characteristics at relapse
26 patients relapsed after first line therapy. Median time interval between last dose of rituximab and re-biopsy at the time of radiographic disease relapse was 11(6-96) months. At relapse, 2 (3.7%) patients were CD20 negative and 24 patients remained CD20 positive as determined by immunohistochemistry (IHC). PAX-5 was then performed on CD20 negative samples and found to be positive in 1/2 patients. There was no statistically significant difference in the expression on ki67 between the biopsy specimens taken at initial diagnosis and relapse. The most common site of relapse in CD20 negative patients was gastrointestinal tract in both patients. In CD20 positive patients, lymph nodal relapse was most frequently documented in 11 (46%) patients followed by gastrointestinal tract in 4(16.6%) patients. DHAP/R-DHAP was prescribed in 11(45.6%), ICE/R-ICE in 6 (25%) and others in 7(29.4%) patients. Complete response (CR) to salvage chemotherapy was documented by imaging studies in 13 (50%) patients out of which 12 patients were CD20 positive and 1 patient was CD 20 negative. DHAP/RDHAP was the was the most successful regimen, with a CR documented in 8/13(61%) (P-0.11). 5 patients underwent high dose chemotherapy with autologous stem cell bone marrow rescue as consolidation therapy in second complete remission. 4 patients after bone marrow transplant alive and disease free till date. Comparison of CD20 positive and CD20 negative patients have been described in Table 2.
Table 2.
Comparison of Patients According to CD20 Expression
| CD20 positive. n-48 patients | CD20negative .n-6 patients | |
|---|---|---|
| Mean age | 55.3 | 53.6 |
| Gender | ||
| Males | 29(60%) | 5(83.3%) |
| Females | 19(40%) | 1(16.6) |
| RIPI | II/III | II/III |
| Initial regimen | ||
| R-CHOP | 39(69.6%) | 5(83.3%) |
| RCVP | 7(13%) | 1(16.7%) |
| REPOCH | 2(3.7%) | |
| Response to First line treatment | ||
| CR | 26(48%) | 2(33%) |
| Relapsed | 24(50%) | 2(33%) |
| Refractory | 24(50%) | 4(66.6%) |
| Salvage chemo | ||
| RDHAP/DHAP | 8(33.3%) | 3(50%) |
| ICE | 5(21%) | 1(16.6%) |
| Others | 4(16.6%) | 2(33.3%) |
| Site of relapse | ||
| Lymph nodes | 11(46%) | 0 |
| GIT | 4(14.6%) | 2(100%) |
| Response to salvage Chemo | ||
| Complete response | 12(50%) | 2(33%) |
| Refractory | 28(44.4%) | 4(14.3%) |
| Median DFS(Months) | 12 | 9 |
| Median OS (Months) | 17.5 | 13 |
Characteristics of patient’s refractory to first line therapy
28(52%) were refractory to first line chemo-immunotherapy. 19(68%) of the patients were male. Most of the patients had stage III/IV (57%) at time of diagnosis and RIPI of III/IV (53%). The interval between last dose of rituximab and biopsy was 2 months. On re-biopsy 24(86%) remained CD20 positive and 4(14.3%) patients with refractory disease lost CD20 expression (p-0.67). These patients went on to receive second line chemotherapy and DHAP/R-DHAP remained the preferred choice of therapy. 3 (11%) showed CR to salvage chemotherapy and 9(32%) showed stable disease (p-0.001). Out of 4 patients who were CD20 negative, showed only 1 patient demonstrated CR to salvage chemotherapy. Among 3 patients who achieved complete response after second line chemotherapy, 2 underwent bone marrow transplant. 1 patient passed away in a year after bone marrow transplant secondary with relapsed disease. 1 patient is alive till date.
Outcome
Among patients who achieved CR, their time to progression was 15 months in CD20 positive vs.10 months in CD20 negative cohort (95%CI 8.95-21.05, p<0.05). The median disease free survival was 12 months vs. 9 months in the CD20 positive and CD20 negative patients respectively (95%CI: 9.455-14.545, p-0.192). Median overall survival was 17 months for the entire cohort (95% CI 11.58-22.41) (Figure 1). Median overall survival was 17.5 months in CD20 positive vs. 13 months in CD20 negative patients (95% CI: 0.9520 to 1.740,p-0.33). However, patients’ who achieved CR and then went on to receive an autologous stem cell transplant, the OS was increased to 62 months (Figure 2).
Figure 1.
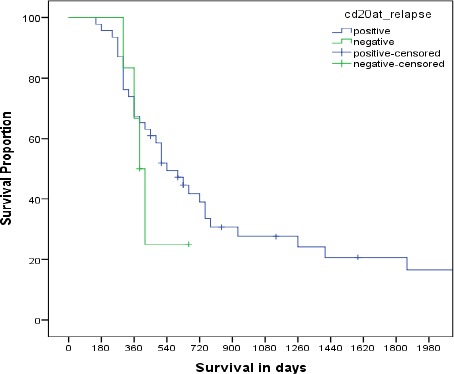
Overall Survival of Patients According to CD20 Expression at Relapse
Figure 2.
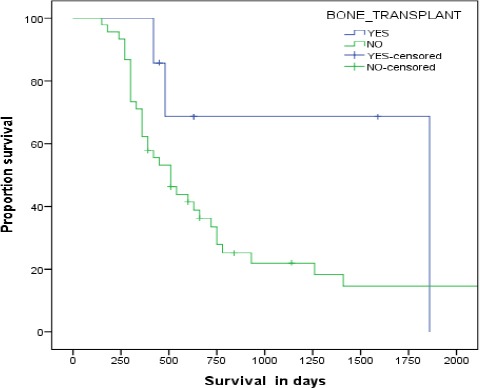
Over all survival of patients underwent bone marrow transplant vs non transplant patients
Discussion
DLBCL with low/ absent CD20 expression at initial diagnosis is a rare phenomenon and has been shown to behave aggressively (Vega et al., 2005). Li et al., (2012) studied 28 patients with CD20 negative DLBCL and his analysis revealed that this cohort of patients had a more aggressive phenotype and an inferior survival then CD20 positive DLBCL counterparts. Loss of CD20 expression has been observed more frequently in non-GC type of DLBCL (Tsutsumi et al., 2016). The outcome of patients with de novo CD20 negative DLBCL is reported to be inferior to the CD20 positive DLBCL (Hiraga et al., 2009). The effect of loss of CD20 expression in relapsed/refractory DLBCL is unclear. We report the results of the largest case series describing the incidence and outcome of patients with CD20 negative DLBCL after treatment with rituximab.
There are several mechanisms that have been proposed to explain loss of CD20 expression in lymphoma cells that might lead to resistance to rituximab. Mechanism of resistance to rituximab beside CD20 receptor loss are separation of CD20 rituximab complexes and neoplasm micro-environment (Czuczman et al., 2008). Possible molecular pathways that leads to resistance to rituximab is down regulation of NF-κB pathway, PI3K/AKT pathway and overexpression of anti-apoptotic gene products (e.g. Bcl2)/ There is preliminary data of Bcl2 pan inhibitor (Oblimersem) that showed clinical response in heavily treated lymphoma patients (Smith, 2003) to overcome NF-κB pathway, trials including combination of rituximab and bortezomib trials are ongoing as salvage treatment in B cell lymphomas (Bonavida, 2014). In Shimizu et al., (2009) Study, treatment with valproic acid and romidepsin(HDAC inhibitor) augmented the expression of CD20 and increased rituximab-mediated CDC cytotoxicity. It has been shown, that CD20 mRNA expression was significantly low in CD20 negative when compared to CD20 positive lymphoma cells (Hiraga et al., 2009). Interesting, when treated with 5’ azacitdine, CD20 mRNA expression was restored in three days (Tsutsumi et al., 2016). This suggests that prior treatment with rituximab can cause epigenetic down regulation of the CD20 gene and hence resulting in decrease protein expression (Wada et al., 2009). CD20 mutations may also have a role, albeit, controversial (Johnson et al., 2009). C terminal deletion of the CD20 molecule has been shown to contribute to decreased CD20 expression in patients with prior exposure to rituximab (Tsai et al., 2012). D20 splice variants which result in the loss of the binding epitope have been shown to cause rituximab resistant, but this finding has not been validated (Smal et al., 2013).
The incidence of loss of CD20 expression is varied. Naoki Wada et al., (2009) reported that, 4(19%) of the 21 cases showed loss of CD20 negative expression after rituximab containing treatment. Kennedy et al., (2002) reported CD20 negativity in 6/10(60%) patients with relapsed DLBCL. Hiagra et al., (2009) reviewed data of 124 patients with relapsed DLBC .19 of 124 were re-biopsied at relapse and 5/19(26%) lost CD20 expression (Hiraga et al., 2009). We observed loss of CD20 expression in 11% of patients. We hypothesize that there are multiple factors contributing to the variability of the reported difference in loss of CD20 expression. One major contributing factor is that very few patients undergo a repeat biopsy at relapse. Indeed, as reported in by Hiraga et al and other studies, only approximately 15% patients undergo histopathological diagnosis at relapse (Tsai et al., 2012). Therefore, it is postulated that this incidence is likely underreported.
Another explanation is that the laboratory tests to check for CD20 expression were different in the reported studies. Immuno- histochemical analysis and flow cytometry are the two methods used, however, immunohistochemistry appears to be method of choice (Tokunaga et al., 2014). Chu et al., (2006) demonstrated that in the case of loss of CD20 expression 88% and 81% expressed Pax5 and CD79a. In the rare instance that these routine markers are not expressed, OCT. 2 and BOB. 1 can be used to determine lineage specification (Small et al., 2013). Flow cytometry identify surface receptors; however, this can be masked by rituximab (Jilani et al., 2003). It also is known that circulating rituximab can be detected in the peripheral blood even 6 months post Rituximab treatment. Therefore, flow cytometry is not considered to be a sensitive test to determine lineage (Berinstein et al., 1998). Given these findings and the relatively ease and reproducibility of IHC, it is now the preferred method to determine lineage specification for inclusion in clinical trials (Jiang et al., 2013; Johnson et al, 2009).
Maeshima et al., (2008) suggested that biopsy site (bone marrow vs. non-bone marrow) might change the CD20 expression. Foran et al., (2001) interesting results of their cohort of 25 patients with relapsed non-Hodgkin’s lymphoma. While bone marrow infiltration with lymphoma of 6 out of 24 patients was CD20 negative, lymph node biopsy remained to be CD20 positive in all patients. This discrepancy, however, did not infer an inferior prognosis. Therefore, it might be important to determine CD20 expression by immunophenotyping both the systemic and involved bone marrow biopsy specimens. However, clinical significance of discrepancy of CD20 expression is not well understood and should not dissuade use of rituximab.
It is also suggested that CD20 negative patients present with more extra nodal disease (23.8% vs. 9.5%) and bone involvement (9.5% vs. 0%) when compared to CD20 positive NHL (Li et al., 2012). Our study also mimics these findings with gastrointestinal tract as the most frequent site of relapse in CD20 negative patients while nodal relapse (46%) was frequently documented in CD20 positive patients.
Response to second line chemotherapy in relapsed DLBCL has been reported as 50% in literature (Alvaro-Naranjo et al., 2003). Our study mirrors this finding with 50% showing complete response to salvage chemotherapy. In our experience, CR rates with DHAP/RDHAP in relapse (61%) and refractory (11%) patients were higher than other regimens. We also observed that re-treatment with rituximab in the second line setting produced better response rates in the relapsed rather than the refractory setting. In our study, 54% patients at relapse received rituximab containing salvage regimen and 50% of them showed complete response. Among refractory patients who received rituximab with chemotherapy as second line, only 7% showed response. This is consistent with question raised in CORAL study, whether patients refractory to rituximab containing induction regimen can be retreated with rituximab (Friedberg, 2011). An interesting finding in our study was that the loss of CD20 expression was more frequently seen in the patients’ refractory to first line therapy (67% vs. 33%).
Our study has some limitations as this is a retrospective study. Even though, biopsy samples were available to be reviewed, we could not perform additional IHC analysis for further classification according to its cell of origin. This limits us in reporting detailed immunophenotype of the cohort. The strength of our study is that we included a relatively homogenous group of patients. Only those patients that had a biopsy at diagnosis and relapse at our institution were included in the study. This allowed us to reconfirm the CD20 expression for inclusion in the analysis. Additionally, most of the reported case series have included all subtypes of NHL. Our cohort was exclusively of patients with relapsed/refractory DLBCL. Therefore, the effect in outcome with regards to loss of CD20 expression in this subset of patients is likely to be clinically valid.
In conclusion, prognosis of patients in DLBCL depends on response to induction chemotherapy. Incidence of CD20 loss after rituximab therapy is low but appears to be more common in the refractory rather than relapsed setting. Patients who respond to second line therapy have a better outcome which is irrespective of CD20 expression. It is important that all patients with relapsed/refractory DLBCL have repeat biopsies at the time of progression. This will aid in better understanding of the cellular and molecular characteristics and tailor treatment and develop novel therapeutic agents.
Competing interests
Authors have no competing interests.
Declarations
Authors have nothing to declare.
References
- Alvaro-Naranjo T, Jaen-Martinez J, Guma-Padro J, et al. CD20-negative DLBCL transformation after rituximab treatment in follicular lymphoma:a new case report and review of the literature. Ann Hematol. 2003;82:585–8. doi: 10.1007/s00277-003-0694-1. [DOI] [PubMed] [Google Scholar]
- Berinstein N, Grillo-Lopez A, White, et al. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- Bonavida B. Postulated mechanisms of resistance of B-cell non-Hodgkin lymphoma to rituximab treatment regimens:strategies to overcome resistance. Semin Oncol Nurs. 2014;41:667–77. doi: 10.1053/j.seminoncol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PG, Loera S, Huang Q, et al. Weiss LM, editor. lineage determination of CD20–B-Cell neoplasms. Am J Clin Pathol. 2006;126:534–44. doi: 10.1309/3WG32YRAMQ7RB9D4. [DOI] [PubMed] [Google Scholar]
- Czuczman MS, Olejniczak S, Gowda A, et al. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res. 2008;14:1561–70. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- Davis TA, Czerwinski DK, Levy R. Therapy of B-cell lymphoma with anti-CD20 antibodies can result in the loss of CD20 antigen expression. Clin Cancer Res. 1999;5:611–5. [PubMed] [Google Scholar]
- Fenske TS, Hari PN, Carreras J, et al. Impact of pre-transplant rituximab on survival after autologous hematopoietic stem cell transplantation for diffuse large B cell lymphoma. Biol Blood Marrow Transplant. 2009;15:1455–64. doi: 10.1016/j.bbmt.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran JM, Norton AJ, Micallef IN, et al. Loss of CD20 expression following treatment with rituximab (chimaeric monoclonal anti-CD20):a retrospective cohort analysis. Br J Haematol. 2001;114:881–3. doi: 10.1046/j.1365-2141.2001.03019.x. [DOI] [PubMed] [Google Scholar]
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. ASH Education Program Book. 2011;1:498–505. doi: 10.1182/asheducation-2011.1.498. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga J, Tomita A, Sugimoto T, et al. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies:its prevalence and clinical significance. Blood. 2009;113:4885–93. doi: 10.1182/blood-2008-08-175208. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhao Y, Dong X, et al. Loss of CD20 expression in relapsed diffuse large B cell lymphoma after rituximab therapy:a case report and review of the literature. J Clin Oncol Res. 2013;12:148–51. [Google Scholar]
- Jilani I, O'Brien S, Manshuri T, et al. Transient down-modulation of CD20 by rituximab in patients with chronic lymphocytic leukemia. Blood. 2003;102:3514–20. doi: 10.1182/blood-2003-01-0055. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Boyle M, Bashashati A, et al. Diffuse large B-cell lymphoma:reduced CD20 expression is associated with an inferior survival. Blood. 2009;113:3773–80. doi: 10.1182/blood-2008-09-177469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Leach S, Woolcock B, et al. CD20 mutations involving the rituximab epitope are rare in diffuse large B-cell lymphomas and are not a significant cause of R-CHOP failure. Haematologica. 2009;94:423–7. doi: 10.3324/haematol.2008.001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GA, Tey SK, Cobcroft R, et al. Incidence and nature of CD20-negative relapses following rituximab therapy in aggressive B-cell non-Hodgkin's lymphoma:a retrospective review. Br J Haematol. 2002;119:412–6. doi: 10.1046/j.1365-2141.2002.03843.x. [DOI] [PubMed] [Google Scholar]
- Kewalramani T, Zelenetz AD, Nimer SD, et al. Title:Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–8. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- Li Y-J, Li Z-M, Rao H-L, et al. CD20-negative de novo diffuse large B-cell lymphoma in HIV-negative patients:A matched case-control analysis in a single institution. J Transl Med. 2012;10:84. doi: 10.1186/1479-5876-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima AM, Taniguchi H, Nomoto J, et al. Histological and immunophenotypic changes in 59 cases of Bâ€-cell nonâ€-Hodgkin's lymphoma after rituximab therapy. Cancer Sci. 2008;100:54–61. doi: 10.1111/j.1349-7006.2008.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut LS, Chakrabarti PP. Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J Cancer. 2014;3:66. doi: 10.4103/2278-330X.126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol. 2011;24:203–16. doi: 10.1016/j.beha.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, McLeod HL, Richards KL. Analysis of innate and acquired resistance to anti-CD20 antibodies in malignant and nonmalignant B cells. PeerJ. 2013;1:e31. doi: 10.7717/peerj.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR. Title:Rituximab (monoclonal anti-CD20 antibody):mechanisms of action and resistance. Oncogene. 22:7359–68. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- Tokunaga T, Tomita A, Sugimoto K, et al. De novo diffuse large B-cell lymphoma with a CD20 immunohistochemistry-positive and flow cytometry-negative phenotype:Molecular mechanisms and correlation with rituximab sensitivity. Cancer Sci. 2014;105:35–43. doi: 10.1111/cas.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P-C, Hernandez-Ilizaliturri FJ, Bangia N, et al. Regulation of CD20 in rituximab-resistant cell lines (RRCL) and B-cell non-Hodgkin lymphoma (B-NHL) Clin Cancer Res. 2012;18:1039–50. doi: 10.1158/1078-0432.CCR-11-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi Y, Ohigashi H, Ito S, et al. 5-Azacytidine partially restores CD20 expression in follicular lymphoma that lost CD20 expression after rituximab treatment:a case report. J Med Case Rep. 2016;10:1–4. doi: 10.1186/s13256-016-0809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi Y, Ohigashi H, Ito S, et al. 5-Azacytidine partially restores CD20 expression in follicular lymphoma that lost CD20 expression after rituximab treatment:a case report. J Med Case Rep. 2016;10:27. doi: 10.1186/s13256-016-0809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega F, Chang C-C, Medeiros LJ, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol. 2005;18:806–15. doi: 10.1038/modpathol.3800355. [DOI] [PubMed] [Google Scholar]
- Wada N, Kohara M, Ogawa H, et al. Change of CD20 expression in diffuse large B-cell lymphoma treated with rituximab, an anti-CD20 monoclonal antibody:a study of the Osaka Lymphoma Study Group. Case Rep Oncol. 2009;2:194–202. doi: 10.1159/000249152. [DOI] [PMC free article] [PubMed] [Google Scholar]


