Abstract
This research work was initiated to explore the efficacy of ellagic acid in mitigation of hepatocarcinogenesis in rats. Rats were distributed into 4 groups; negative control, hepatocellular carcinoma (HCC), doxorubicin and ellagic acid. Serum alpha-fetoprotein (AFP), glypican-3 (GPC-3), signal transducer and activator of transcription 3 (STAT3) and suppressors of cytokine signaling 3 (SOCS3) levels were assayed by ELISA. Immunohistochemical examination of hepatic VEGF expression was also conducted, along with histological procedures for examination of liver tissue sections. Significant elevation in serum AFP, GPC-3 and STAT3 levels with a significant drop in SOCS3 were observed in the HCC group. In contrast, the treated groups showed significant decline in serum AFP, GPC-3 and STAT3 levels and significant increase in SOCS3. Additionally, the HCC group declared mild positive immunoreaction for VEGF in hepatocytes while treatment with doxorubicin or ellagic acid was associated with a negative immunoreaction for VEGF. These results were supported by histological examination of liver tissue. The obtained findings suggested that ellagic acid may have beneficial chemopreventive role against hepatocarcinogenesis through its apoptotic, antiangiogenic and antiproliferative activities.
Keywords: Hepatocellular carcinoma, N−nitrosodiethylamine, ellagic acid, doxorubicin, rats
Introduction
Hepatocellular carcinoma (HCC) is standout amongst the most widely recognized disease around the world, it positions as the fifth in men and seventh in women (El-Serag, 2012). Also, it is a main reason of cancer related death globally. The major risk factors of hepatocellular carcinoma include hepatitis B virus (HBV) and hepatitis C virus (HCV) infection in addition to alcoholic liver disease, nonalcoholic steatohepatitis, and aflatoxin-contaminated food (El-Serag, 2012).
N-nitrosodiethylamine (NDEA) is considered as one of the most common environmental chemicals that are known to be metabolized to carcinogenic and pro-oxidant agents. It usually exists in processed meats, tobacco products, alcoholic beverages and agricultural chemicals (Amin et al., 2011). The carcinogenic effect of NDEA is associated with a highly generation of reactive oxygen species (ROS) which could damage biomolecules such as DNA, proteins and lipids. NDEA could cause the formation of large amounts of 8-hydroxy-2-deoxyguanosine in rat liver at very low dose, which could then initiate carcinogenesis. Hepatocarcinogenesis induced by NDEA is a well-known animal model commonly used for the screening of the hepatoprotective activity of natural compounds (Yamada et al., 2006).
The response rate for HCC therapeutic regimen is unsatisfactory because of late diagnosis and poor treatment, particularly resistance to chemotherapeutic medications and metastasis to different organs. Thus, seeking for novel and potential therapeutic approaches for HCC is of great importance and need. During the last decade, the utilization of natural products has gained a great consideration in view of their capacity to provide prevention and therapeutic efficacy against number of cancers (Amin et al., 2009).
Ellagic acid (4,4′,5,5′,6,6′-Hexahydroxydiphenic acid 2,6,2′,6′-dilactone), a polyphenolic compound, is a dimeric derivative of gallic acid found in grapes, nuts, pomegranates, and berries. It is mentioned that the biological activities of ellagic acid displayed antioxidant, antivirus, anticarcinogenic, antifibrosis, and chemopreventive activities (Seeram et al., 2005).
The current investigation was organized to assess the chemopreventive efficiency of ellagic acid against NDEA-induced hepatocarcinogenesis in rats.
Materials and Methods
Extraction and isolation of ellagic acid from Punica granatum peel
Punica granatum peels were extracted three times at room temperature with methanol (CH3OH or MeOH): water (H2O) (7:3). The extract was filtered, evaporated under reduced pressure and lyophilized. Then, the extract was loaded on a polyamide 6S column chromatography (80 x 3 cm). The column was eluted with H2O, and then H2O-CH3OH mixtures of decreasing polarity and 10 fractions (1 L, each) were collected. The major phenolic fractions obtained were combined into two fractions after chromatographic analysis. The first fraction was subjected to column chromatography on cellulose and n-butanol (n-BuOH) saturated with H2O as an eluent to give two major subfractions, then one of them was separately fractionated on a Sephadex LH-20 to yield pure compound; ellagic acid. The chemical structure of ellagic acid was identified using proton nuclear magnetic resonance (1H NMR) spectroscopy and carbon-13 nuclear magnetic resonance (13C NMR) spectroscopy.
Chemicals and Drugs
N-nitrosodiethylamine (NDEA) was supplied from Sigma-Aldrich Chemicals Co. (St Louis, MO, USA). While, doxorubicin (Adricin) was purchased from EIMC united Pharmaceuticals, Egypt. All the other chemicals were of high purity grade.
Animals
Forty adult male rats of Wistar strain weighing 170-200 g were supplied from the Animal House Colony of the National Research Centre, Giza, Egypt and housed in polypropylene cages in an environmentally controlled clean air room with a temperature of 25±1°C, an alternating 12h light/12h dark cycle, a relative humidity of 60±5% and ad libitum access to tap water and a standard rodent chow consisted of 10% casein, 4% salt mixture, 1% vitamin mixture, 10% corn oil, 5% cellulose and completed to 100 g with corn starch (Wadi El Kabda Co., Cairo, Egypt). The study protocol complied with the guidelines for animal experiment, which was approved by the Ethical Committee of the Medical Research of the National Research Centre, Giza, Egypt.
Experimental set-up
The animals were randomly divided into 4 groups, with 10 rats in each group. Group (1): set as healthy control animals [negative control] received orally 0.9 % normal saline daily during the experimental period. Group (2): set as hepatocellular carcinoma-induced group [HCC untreated group] in which the rats were orally administered with N-nitrosodiethylamine (NDEA) (dissolved in 0.9% normal saline), in a dose of 20 mg/kg b. wt. five times weekly for five weeks according to the method of Darwish and El-Boghdady (2011). Group (3): Doxorubicin-treated group [HCC + Doxorubicin] in which HCC bearing rats were intraperitoneally (i.p.) treated with doxorubicin as reference drug in a dose of 0.72 mg/rat which is equivalent to the human dose of 20 mg/m2 according to Barnes and Paget (1965) once weakly for 5 weeks. Group (4):Ellagic acid-treated group [HCC + Ellagic acid] in which HCC bearing rats were treated orally with ellagic acid compound in a dose of 50 mg/kg b.wt (Umesalma and Sudhandiran, 2011) daily for 5 weeks.
After the last treatment, the animals were fasted overnight and the blood samples were collected, under diethyl ether anesthesia, from the retro-orbital venous plexus in a dry clean centrifuge tubes and allowed to coagulate for 45 minutes at room temperature to obtain sera for various biochemical measurements. Serum samples were separated by centrifugation at 1800 x g for 15 minutes at 4°C using cooling centrifuge and stored at -20°C until analyzed. After collection of the blood samples, the animals were sacrificed by cervical dislocation and the whole liver of each rat was rapidly and carefully dissected then, fixed in formalin saline (10%) for immunohistochemical examination and histopathological investigation.
Biochemical determinations
Serum alpha-fetoprotein (AFP), glypican-3 (GPC-3), signal transducer and activator of transcription 3 (STAT3) and suppressors of cytokine signaling 3 (SOCS3) levels were assayed by enzyme-linked immunosorbent assay (ELISA) using the kits purchased from Glory Science Co., Ltd (USA), according to the manufacturer’s instructions provided with kits.
Immunohistochemical examination of hepatic VEGF expression
Samples were taken from liver tissue of rats of the different groups and fixed in 10% formalin saline for 24 hours. Washing was done in tap water then ascending grade of ethyl alcohol (30, 50, 70, 90% and absolute) was used for dehydration. Specimens were cleared in xylene and embedded in paraffin (melting point 58-60 ºC) for 24 hours. Sections were cut into 4 µ thick by slidge microtome then fixed on positive slides in a 65 °C oven for 1 hr. Slides were placed in a coplin jar filled with 200 mL of triology working solution (Cell Marque, CA-USA, Cat. #920P-04) which combines the three pretreatment steps: deparaffinization, rehydration and antigen unmasking. Then, the jar is securely positioned in the autoclave which was adjusted so that temperature reached 120°C and maintained stable for 15 min after which pressure is released. Thereafter, the coplin jar is removed to allow slides to cool for 30 min. Sections were then washed and immersed in Tris-buffer saline (TBS) to adjust the pH and these were repeated between each step of the IHC procedure. Quenching endogenous peroxidase activity was performed by immersing slides in 3% hydrogen peroxide for 10 min. Broad spectrum LAB-SA detection system (Invitrogen, Cat. #85-8943) was used to visualize any antigen-antibody reaction in the tissue. Background staining was blocked by putting 3 drops of 10% goat non immune serum blocker on each slide and incubating them in a humidity chamber for 10 min. Without washing, excess serum was drained, three drops of vascular endothelial growth factor (VEGF) rabbit polyclonal antibody (Thermo Scientific, USA, Cat. #RB-222-R7) were applied and slides were incubated in the humidity chamber overnight at 4 °C. Henceforward, biotinylated secondary antibody from ultravision detection system anti-polyvalent HRP/DAB (Thermo Scientific, Cat. #TP-015-HD) was applied on each slide for 20 min followed by 20 min incubation with the streptavidin HRP enzyme conjugate (Thermo Scientific, Cat. #TP-015-HD). Then, 3,3´-diaminobenzidine (DAB) chromogen (Thermo Scientific, Cat. #TP-015-HD) was prepared and 3 drops were applied on each slide for 2 min. DAB was rinsed, after which counterstaining with Mayer hematoxylin and cover slipping were performed as the final steps before slides were examined under the light microscope (Bancroft and Gamble, 2008). Image analysis was performed using the image J, 1.41a NIH, (USA) analyzer.
Histopathological investigation of liver sections
After fixation of the liver specimens in formalin saline (10%) for 24 hours, the tissues were washed in running tap water and then ascending grade of ethyl alcohol (30, 50, 70, 90% and absolute) was used for dehydration. Specimens were cleared in xylene and embedded in paraffin (melting point 58-60 ºC) for twenty four hours. Paraffin wax tissue blocks were prepared for sectioning at 4 µ by slidge microtome. The obtained tissue sections were collected on glass slides, deparffinized and stained by hematoxylin and eosin (H and E) stain (Banchroft et al., 1996) for histopathological examination through the electric light microscope.
Statistical analysis
The obtained results are represented as the means ± standard errors. Data were analyzed by one way analysis of variance (ANOVA) using the Statistical Package for the Social Sciences (SPSS) program, version 14 followed by least significant difference (LSD) to compare significance between groups (Armitage and Berry, 1987). The level of significance was set at P<0.05.
Results
Identification of ellagic acid
The isolated compound exhibited shiny buff fluorescence under UV light changed to dull yellow fluoresence after exposure to ammonia vapors. Spraying with FeCl3 gave blue color indicating its phenolic nature. The UV spectral data exhibited two absorption bands (λ max. 255, 364) characteristic for ellagic acid (Tanaka et al., 1986).
1H NMR spectrum showed a singlet signal at δ H7.46 integrated for two aromatic protons assigned for H-5 and H-5`. 13C NMR spectrum showed resonances for fourteen carbons appeared as seven signals due to the existence of a symmetric plane in the molecule. The signal at δ c 159.61 was assigned to the lactone carbonyl carbon (C-7, 7`). The spectrum showed signals of aromatic carbon atoms at δ c 108.04(C-6, 6`), 110.67 (C-5, 5`), 112.39 (C-1, 1`), 136.84 (C-2, 2`), 140.12 (C-3, 3`), 148.59 (C-4, 4`). From the previous data and by comparison with reported ones (Nawwar et al., 1994, Fathalla et al., 2014) the isolated compound could be identified as ellagic acid (Figure 1).
Figure 1.
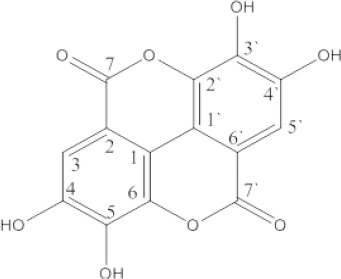
Chemical Structure of Ellagic Acid
Biochemical analysis
Figure 2 lighted the effect of treatment with doxorubicin or ellagic acid on serum tumor markers level in HCC rat model. The untreated HCC group recorded significant elevation (P> 0.05) in AFP and GPC-3 serum levels versus the negative control group. On the opposite side, the treated groups elicited significant depletion (P> 0.05) in serum AFP and GPC-3 levels in regard to the untreated HCC group.
Figure 2.
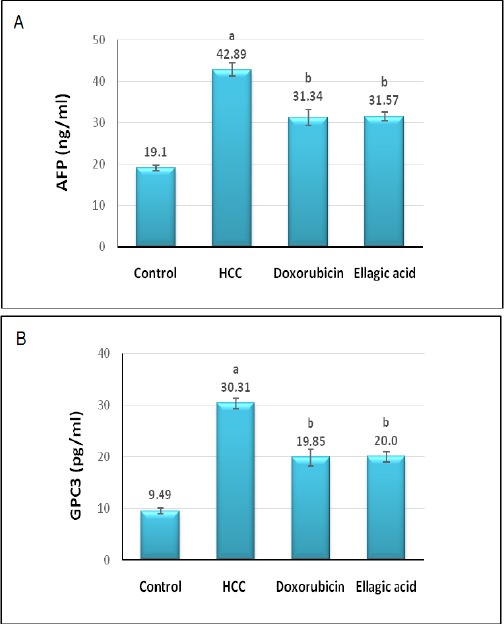
Effect of Treatment with Doxorubicin or Ellagic Acid on Serum AFP (A) and GPC-3 (B) Levels in HCC Rat Model. (a) Significant change at P>0.05 in comparison with the negative control group, (b) Significant change at P>0.05 in comparison with the HCC untreated group.
Figure 3A and 3B delineated the influence of treatment with doxorubicin or ellagic acid on serum STAT3 and SOCS3 levels in HCC rat model. The untreated HCC group demonstrated significant amplification (P> 0.05) in serum STAT3 level paralleled by with significant depression (P> 0.05) in serum SOCS3 level relative to the negative control group. Conversely, the treated groups elicited significant decline (P> 0.05) in serum STAT3 level in accompanied with significant rise in serum SOCS3 level in respect to the untreated HCC group.
Figure 3.
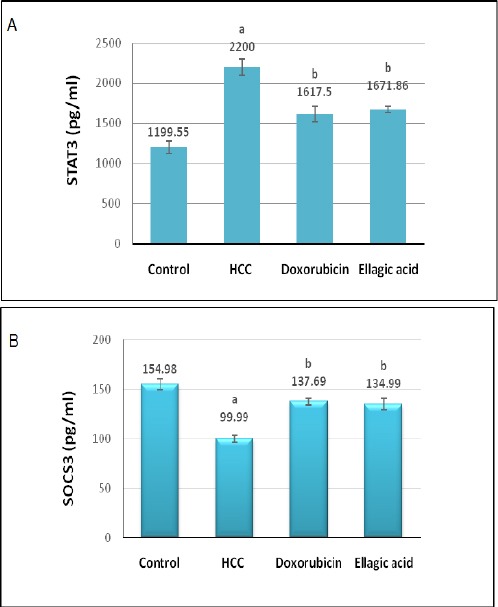
Effect of Treatment with Doxorubicin or Ellagic Acid on Serum STAT3 (A) and SOCS3 (B) Levels in HCC Rat Model. (a) Significant change at P>0.05 in comparison with the negative control group, (b) Significant change at P>0.05 in comparison with the HCC untreated group.
Immunohistochemical examination of hepatic VEGF expression
Optical micrograph for immunohistochemical staining of negative control liver tissue using antibody against VEGF shows negative immunoreaction for VEGF in hepatocytes (Figure 4A). While, optical micrograph for immunohistochemical staining of untreated HCC liver tissue shows mild positive immunoreaction for VEGF in hepatocytes (Figure 4B). Furthermore, optical micrograph for immunohistochemical staining of liver tissue sections of HCC induced rats treated with doxorubicin or ellagic acid show negative immunoreaction for VEGF in hepatocytes (Figure 4C and D respectively).
Figure 4.
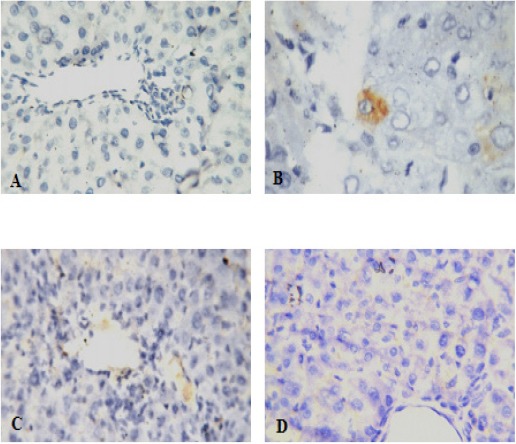
Immunohistochemical Examination of VEGF Expression in Liver Tissues of the Studied Groups. (A)Negative control, (B) HCC untreated, (C) HCC + Doxorubicin, (D) HCC + Ellagic acid
Histopathological findings
Photomicrograph of liver tissue section of negative control group reveals normal histological structure of the central vein and encompassing hepatocytes in the hepatic parenchyma as recorded in Figure 5A. While, the photomicrograph of liver tissue section of untreated HCC group indicates group of dysplastic hepatocytes with prominent nucleolus (Figure 5B). The photomicrograph of liver tissue section of HCC rat treated with doxorubicin shows sever congestion in the portal vein (Figure 5C). Furthermore, the photomicrograph of liver tissue section of HCC rat treated with ellagic acid compound shows the hepatic parenchyma with focal degeneration in the dysplastic hepatocytes which had prominent nucleoli (Figure 5D).
Figure 5.
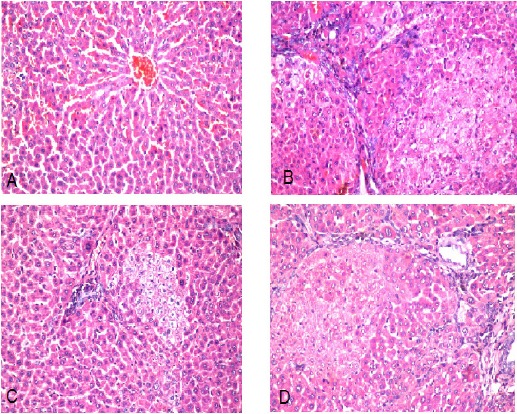
Photomicrograph of Liver Section of (A) negative control group shows normal histological structure of central vein (cv) and surrounding hepatocytes in the parenchyma. (H and E x 10), (B) HCC untreated group shows group of dysplastic hepatocytes with prominent nucleolus. (H and E x 40), (C) HCC + Doxorubicin group shows sever congestion in portal vein. (H and E x 40), (D) HCC + Ellagic acid group shows focal area of degenerated dysplastic with prominent nucleoli hepatocytes. (H and E x 40)
Discussion
A large amount of reactive oxygen species (ROS) has been shown to be generated during the metabolic biotransformation of NDEA bringing about oxidative stress. Oxidative stress prompts to carcinogenesis by more than one mechanism including DNA, lipid and protein deterioration, alteration in the intracellular signaling axis and even changes in gene expression (Devipriya et al., 2007).
The current information demonstrated that administration of NDEA expanded serum AFP level significantly. This finding is similar to the results of the previous studies reported by Zhang et al., (2015). Grizzi et al., (2007) reported that AFP is elevated in HCC, gastric and lung cancer and in embryonic carcinomas. Moreover, AFP is an indicator of HCC risks mostly in patients with HBV/HCV infections and cirrhosis. The re-initiation of AFP synthesis by neoplastic hepatocytes might be credited to the raised transcription of AFP gene (Motalleb et al., 2008).
On the other hand, treatment of HCC group with ellagic acid significantly diminished the rise of AFP level. Polyphenols possessed antitumor activity for various tumor cell lines. It has been reported that these compounds have antiproliferative activity. It has been reported that ellagic acid could exert its anti-cancer effect by modulating oxidative stress (Devipriya et al., 2007).
Our data indicated that NDEA administration brought about significant rise in serum glypican-3 level. The current observation is in accordance with the past investigation of Shahat et al., (2015). GPC3 can be detected in about 50% of HCC patients, and is absent in hepatocytes of healthy patients with nonmalignant hepatopathy. GPC3 has been shown to promote the growth of HCC cells by amplifying autocrine/paracrine canonical Wnt signaling. GPC3 binds to fibroblast growth factor 2 (FGF 2) and functions as a co receptor for it. Lai et al., (2008) indicated that up-regulation of GPC3 by sulfatase 2, promotes FGF signaling, and reduces survival in HCC.
Treatment of HCC bearing rats with ellagic acid decreased serum glypican-3 level significantly. Hussein and Khalifa (2014) investigation proved that the oxidative stress is the main reason behind the elevated lipid peroxidation level in the liver of NDEA administered rats. Reactive oxygen species are created along with the metabolism of NDEA or during the process of carcinogenesis. Also, ellagic acid was shown to inhibit N-nitrosodiethylamine metabolism in rats. Ellagic acid retracted the tumor incidence basically by acting at the initiation phase of the carcinogenesis. In order to seeking for its mode of action, ellagic acid evoked a significant enhancement in GSH while suppressing NADPH and ascorbate-dependent lipid peroxidation. Ellagic acid was reported to be more powerful in diminishing lipid peroxidation and amplifying GSH. This may be one important reason in favoring of the observed anti-carcinogenic potential.
The present data demonstrated that, NDEA administration elicited significant increase in serum STAT3 level. STAT3 has been involved in intracellular signaling transduction by different cytokines and growth factors. In normal cells, activation of STAT3 is tightly controlled to block dysregulated gene transcription, whereas hyper activation of STAT3 plays an important role in tumor cell proliferation, invasion, survival and immunosuppression in several types of human cancers such as HCC. He and Karin (2011) suggested that activation of STAT3 in tumor cells is mediated by cytokines and/or growth factors such as (IL-6) synthesized within the tumor microenvironment. After activation by Janus-activated kinases, STAT3 translocate from cytoplasm to nucleus to stimulate expression of target genes such CyclinD1. Likewise, Subramaniam et al., (2013) cited that IL-6/ STAT3 signaling advances progression of HCC through preventing the expression of anti-apoptotic factors of Bcl-2 family such as Bcl-xl and Mcl-1.
The present observations indicated that the treatment of HCC bearing rats with ellagic acid brought about significant decline in serum STAT3 level. Ellagic acid probably exerted this effect by modulating oxidative stress and NF-κB (Devipriya et al., 2007). NF-κB plays a key role in managing the expression of genes in charge of inflammation, proliferation and apoptosis. A generous number of evidence is estimating that NF-κB regulated the expression of inducible enzymes and cytokines including iNOS, COX-2, TNF-α, IL-2 and IL-6. In particular, ellagic acid could proficiently downregulate TNF-α and IL-6 expression (Chao et al., 2009). Along these lines, the anti-inflammatory property of ellagic acid may be related to the downregulation of IL-6 expression, which is one of the significant mechanisms for the reduction of STAT3 activity.
The present results recorded significant reduction in serum SOCS3 level in hepatocellular carcinoma bearing rats. SOCS3 is a member of the suppressor of cytokine signaling family that could repress several cytokines such as IL-6, IL-11and LIF. Expression of SOCS3 is induced by JAK/STAT signal transduction pathway and it then binds to specific cytokine receptors. Wu et al., (2011) found that diminished expression of SOCS3 was correlate with expression of Ki67, VEGF and pSTAT3, suggesting the role of SOCS3 in HCC. SOCS3 is involved in the suppression of tumor growth and metastasis of HCC. Deletion of SOCS3 gene in liver parenchymal cells enhances hepatitis-induced hepatocarcinogenesis.
Treatment of HCC group with ellagic acid compound increased serum SOCS3 level significantly. It has been shown that overexpression of SOCS3 has an anti-proliferative effect (Barclay et al., 2009). SOCS3 regulates cytokine pathway activity stimulated by the IL-6 family, which signals through the IL-6R – gp130 to activate the STAT3 transcription factor. IL-6 is a key player of the acute-phase response to inflammation, and by controlling the IL-6 – STAT3 pathway, SOCS3 acts as a controller of this response (Campbell et al., 2001). Chao et al., (2009) cited that, ellagic acid proficiently diminished the expressions of TNF-α and IL-6 and NF-κB. Thus, the anti-inflammatory activity of ellagic acid might be correlated with the reduced expression of NF-κB and IL-6 in hepatocellular carcinoma, which is one of the significant mechanisms for attenuation of HCC.
VEGF is one of the most important angiogenic cytokines. VEGF overexpression has been shown to allow tumor growth and its expression is associated with poor prognosis in various types of tumors including HCC (Liu et al., 2010). Data in the present study showed mild positive immunoreaction for VEGF in hepatocytes in HCC group and negative expression of VEGF was found in the negative control group. Liu et al., (2010) attributed the expression of VEGF in NDEA-induced hepatocarcinoma rats to the high angiogenic activity and the increase of nitric oxide activity, thus promoting the angiogenesis by inducing VEGF synthesis (Liu et al., 2010).
The present finding indicated that the treatment of HCC group with ellagic acid showed negative immunoreaction for VEGF in hepatocytes. Wang et al., (2012) observed that ellagic acid fundamentally inhibited a series of VEGF-induced angiogenesis processes including proliferation, tube formation of endothelial cells and migration. Likewise, it specifically hindered VEGFR-2 tyrosine kinase activity and its downstream signaling pathways including PI3K/Akt and MAPK in endothelial cells.
Photomicrograph of liver tissue section of untreated HCC group demonstrated group of dysplastic hepatocytes with noticeable nucleolus. These remarkable features of hepatocellular carcinoma are in concurrence with the investigations of Seufi et al., (2009) who mentioned that the histological examination of liver tissue of HCC group indicated inflammatory cells infiltration and fibroblastic cells proliferation that divided the cancer and necrosed hepatocytes of the parenchyma into nodules with hyperchromatic nuclei as well as cellular pleomorphism and polarity.
Photomicrograph of liver tissue section of HCC rats treated with ellagic acid revealed the hepatic parenchyma with focal degeneration in the dysplastic hepatocytes which had prominent nucleoli. These effects could be ascribed to the antioxidant, chemopreventive, anti-inflammatory and anti-proliferative potentials of ellagic acid (Seeram et al., 2005).
The present results revealed that the treatment of HCC group with doxorubicin showed significant depletion in serum AFP, GPC-3 and STAT3 levels associated with significant elevation in serum SOCS3 level in respect with the untreated HCC group. While, the histopathological investigation of liver tissues of HCC group treated with doxorubicin showed sever congestion in the portal vein. These findings may be ascribed to the antiproliferative and apoptotic effects exerted by doxorubicin (Czeczuga-Semeniuk et al., 2004). The intimate mode of action of doxorubicin is confounded and remains poorly unknown, in spite of the fact that it is thought to interact with DNA by intercalation and suppression of macromolecular biosynthesis. Li et al., (2016) stated that doxorubicin can downregulate the expression of epithelial cell adhesion molecule (EpCAM) and diminish EpCAM-positive cell amounts in human HCC cell lines Hep3B, HepG2, and HuH-7. EpCAM is expressed in liver, kidney, pancreas, fetal lung, skin, germ cells, and in adult epithelia. EpCAM upregulates the proto-oncogene cycles A/E and c-Myc which play an important role in the cell cycle and proliferation. Over-expression of EpCAM had been found to be linked with cancer malignancy and with poor survival in ovarian, breast, esophageal squamous cell carcinoma, colon, and squamous head and neck carcinoma cells. The function and the regulatory mechanism of EpCAM are unclear in HCC. Li et al., (2016) demonstrated that EpCAM is the target of doxorubicin.
Conclusively, the outcomes of the present investigation emphasize the chemopreventive function of ellagic acid against heptocarcinogenesis. Ellagic acid could promote apoptosis, hamper angiogenesis and abrogate proliferation of hepatic cancer cells in vivo. The amelioration of these key events by applying ellagic acid may provide important point of view for treating hepatocellular carcinoma.
Conflict of interest statement
We declare that we have no conflict of interest.
References
- Amin A, Hamza AA, Bajbouj K, Ashraf SS, Daoud S. Saffron a potential candidate for a novel anticancer drug against heptocellular carcinoma. Hepatology. 2011;54:857–67. doi: 10.1002/hep.24433. [DOI] [PubMed] [Google Scholar]
- Amin AR, Kucuk O, Khuri ER, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–25. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage P, Berry G. statistical method in medical research. 2nd ed. Oxford Blockwell significant publication; 1987. Comparison of several groups; pp. 186–213. [Google Scholar]
- Banchroft JD, Stevens A, Turner DR. Theory and practice of histological techniques. 4th ed. Philadelphia PA USA: Churchill Livingstone; 1996. pp. 25–90. [Google Scholar]
- Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. Churchill Livingstone-Elsevier; 2008. pp. 433–69. [Google Scholar]
- Barclay JL, Anderson ST, Waters MJ, Curlewis JD. SOCS3 as a tumor suppressor in breast cancer cells, and its regulation by PRL. Int J Cancer. 2009;124:1756–66. doi: 10.1002/ijc.24172. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Paget GE. Mechanisms of toxic action. Prog Med Chem. 1965;4:18–38. doi: 10.1016/s0079-6468(08)70166-8. [DOI] [PubMed] [Google Scholar]
- Campbell JS, Prichard L, Schaper F, et al. Expression of suppressors of cytokine signaling during liver regeneration. J Clin Invest. 2001;107:1285–92. doi: 10.1172/JCI11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PC, Hsu CC, Yin MC. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab (Lond) 2009;6:33. doi: 10.1186/1743-7075-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeczuga-Semeniuk E, Wołczyński S, Dabrowska M, Dziecioł J, Anchim T. The effect of doxorubicin and retinoids on proliferation, necrosis and apoptosis in MCF-7 breast cancer cells. Folia Histochem Cytobiol. 2004;42:221–7. [PubMed] [Google Scholar]
- Darwish HA, El-Boghdady N. Possible involvement of oxidative stress in diethylnitrosamine induced hepatocarcinogenesis, chemopreventive effect of curcumin. J Food Biochem. 2011;37:353–61. [Google Scholar]
- Devipriya N, Srinivasan M, Sudheer AR, Menon VP. Effect of ellagic acid, a natural polyphenol, on alcohol-induced prooxidant and antioxidant imbalance:A drug dose dependent study. Singapore Med J. 2007;48:311–8. [PubMed] [Google Scholar]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathalla AA, Ahmed HA, Salah ME, Khaled NR, Nabil HA. Phytochemical and biological investigations of Terminalia bellericaRoxb Leaves. J Pharm Res. 2014;8:500–10. [Google Scholar]
- Grizzi F, Colombo P, Taverna G, et al. Geometry of human vascular system:is it an obstacle for quantifying antiangiogenic therapies? Appl Immunohistochem Mol Morphol. 2007;15:134–9. doi: 10.1097/01.pai.0000213105.18569.fa. [DOI] [PubMed] [Google Scholar]
- He G, Karin M. NF-κB and STAT3-key players in liver inflammation and cancer. Cell Res. 2011;21:159–68. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein RH, Khalifa FK. The protective role of ellagitannins flavonoids pretreatment against N-nitrosodiethylamine induced-hepatocellular carcinoma. Saudi J Biol Sci. 2014;21:589–96. doi: 10.1016/j.sjbs.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JP, Sandhu DS, Yu C, et al. Sulfatase 2 upregulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma. Hepatol. 2008;47:1211–22. doi: 10.1002/hep.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Farmer RW, Yang Y, Martin RC. Epithelial cell adhesion molecule in human hepatocellular carcinoma cell lines:a target of chemoresistence. BMC Cancer. 2016;16:228. doi: 10.1186/s12885-016-2252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JG, Zhao HJ, Liu YJ, Wang XL. Effect of selenium-enriched malt on VEGF and several relevant angiogenic cytokines in diethylnitrosamine-induced hepatocarcinomarats. J Trace Elem Med Biol. 2010;24:52–7. doi: 10.1016/j.jtemb.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Motalleb G, Hanachi P, Fauziah O, Asmah R. Effect of Berberis vulgaris f ruit extract on alpha-fetoprotein gene expression and chemical carcinogen metabolizing enzymes activities in hepatocarcinogenesis rats. Iran J Cancer Prev. 2008;1:33–44. [Google Scholar]
- Nawwar M, Hussein SA, Merfort I. NMR spectral analysis of polyphenols from Punica granatum. Phyochemistry. 1994;36:793–8. [Google Scholar]
- Seeram NP, Adams LS, Henning SM, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–7. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Seufi AM, Ibrahim SS, Elmaghraby TK, Hafez EE. Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/ antioxidant activity, molecular and histological evidences. J Exp Clin Canc Res. 2009;28:80–6. doi: 10.1186/1756-9966-28-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahat AA, Alsaid MS, Kotob SE, Ahmed HH. Significance of Rumexvesicarius as anticancer remedy against hepatocellular carcinoma:a proposal-based on experimental animal studies. Asian Pac J Cancer Prev. 2015;16:4303–10. doi: 10.7314/apjcp.2015.16.10.4303. [DOI] [PubMed] [Google Scholar]
- Subramaniam A, Shanmugam MK, Perumal E, et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Bio chim Biophys Acta. 2013;1835:46–60. doi: 10.1016/j.bbcan.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Nonaka G, Nishioka I. Tannins and related compounds XLII. Isolation and characterization of four new hydrolysable tannins, terflavins A and B, tergallagin and tercatain from leaves of Terminalia catappa L. Chem Pharm Bull. 1986;34:1039–49. [Google Scholar]
- Umesalma S, Sudhandiran G. Ellagic acid prevents rat colon carcinogenesis induced by 1, 2 dimethyl hydrazine through inhibition of AKT-phosphoinositide-3 kinase pathway. Eur J Pharmacol. 2011;660:249–58. doi: 10.1016/j.ejphar.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Wang N, Wang ZY, Mo SL, et al. Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Res Treat. 2012;134:943–55. doi: 10.1007/s10549-012-1977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WY, Li J, Wu ZS, et al. Prognostic significance of phosphorylated signal transducer and activator of transcription 3 and suppressor of cytokine signaling 3 expression in hepatocellular carcinoma. Exp Ther Med. 2011;2:647–53. doi: 10.3892/etm.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Yamamiya I, Utsumi H. In vivo detection of free radicals induced by diethylnitrosamine in rat liver tissue. Free RadicBiol Med. 2006;40:2040–6. doi: 10.1016/j.freeradbiomed.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zeng T, Zhao XL, Xie KQ. Garlic oil suppressed nitrosodiethylamine-induced hepatocarcinoma in rats by inhibiting PI3K-AKT-NF-κB pathway. Int J Biol Sci. 2015;11:643–51. doi: 10.7150/ijbs.10785. [DOI] [PMC free article] [PubMed] [Google Scholar]


