Abstract
Background:
Copper transporter 1 (CTR1) is a critical determinant of the uptake and cytotoxic effect of the platinum drugs carboplatin and cisplatin. Thymidylate synthase (TS) is an enzyme involved in DNA synthesis and is associated with resistance of tumor cells to 5-fluorouracil. We investigated the correlation between CTR1 and TS expression levels and treatment outcomes in patients with advanced non-small-cell lung cancer (NSCLC) treated with S-1/carboplatin doublet chemotherapy.
Methods:
Twenty-nine patients were enrolled in this study. Tumor expression of CTR1 and TS was measured immunohistochemically and analyzed for correlation with tumor response, progression-free survival (PFS), and overall survival (OS).
Results:
Tumor response was significantly better in patients with CTR1High tumors than in patients with CTR1Low tumors (64% vs. 18%, P = 0.02). Patients with TSLow tumors had a significantly longer OS (median 21.2 vs. 8.5 months, P = 0.02), but not PFS, than patients with TSHigh tumors. When CTR1 and TS co-expression was analyzed, patients with either CTR1High or TSLow tumors showed a significantly better tumor response (50% vs. 0%, P = 0.01), longer PFS (median 4.2 vs. 2.1 months, P = 0.03), and longer OS (median 21.2 vs. 8.5 months, P = 0.01) than patients with both CTR1Low and TSHigh tumors.
Conclusions:
Our study suggests that combined CTR1/TS expression status has the potential to be an important predictor of good treatment outcomes in patients with advanced NSCLC treated with S-1/carboplatin doublet chemotherapy.
Keywords: Copper transporter 1, thymidylate synthase-non-small cell lung cancer, S-1, carboplatin
Introduction
Lung cancer is one of the most common cancers worldwide and is a major cause of cancer-related death (Torre et al., 2015). Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases. Platinum-based doublet chemotherapy is the standard first-line treatment for advanced NSCLC and shows response rates of approximately 20–40% and a median survival of 8–10 months (Grossi et al., 2007).
S-1 is an oral fluoropyrimidine agent that consists of tegafur, 5-chloro-2,4-dihydroxypyridine (CDHP), and potassium oxonate (Shirasaka et al., 1996; Malet-Martino and Martino, 2002). Tegafur is a prodrug of fluorouracil (5-FU), and CDHP is an inhibitor of dihydropyrimidine dehydrogenase, an enzyme involved in the degradation of 5-FU. Thus, CDHP allows 5-FU to be maintained at higher levels in the plasma and tumor tissue than when 5-FU is intravenously infused. S-1 is active against a wide range of solid tumors, including NSCLC. Although many treatment options are available for patients with advanced NSCLC, S-1–containing regimens offer several potential clinical benefits, such as oral administration and well-tolerated toxicity (Takeda, 2013).
Carboplatin (CBDCA) is a cisplatin (CDDP) analog that has lower nonhematological toxicity than CDDP. Clinical trials have shown that combination therapy with S-1 and CBDCA is feasible and well tolerated in patients with advanced NSCLC and has efficacy similar to that of other platinum doublet regimens (Kaira et al., 2007; Tamura et al., 2009; Yoshioka et al., 2013; Kuyama et al., 2017). However, as with other treatment regimens, individual patients vary in their response to S-1/CBDCA doublet chemotherapy. Therefore, biomarkers that identify subgroups of patients who will benefit from S-1/CBDCA doublet chemotherapy would be useful for guiding treatment decisions.
Cellular uptake of anticancer drugs is mediated by facilitated transport systems, suggesting that expression of transport-related molecules may predict the response to chemotherapy. Copper transporter 1 (CTR1; SLC31A1) controls the intracellular accumulation and cytotoxic effects of CBDCA and CDDP (Holzer et al., 2004; Song et al., 2004; Holzer et al., 2006). Thymidylate synthase (TS) catalyzes methylation of deoxyuridine monophosphate to deoxythymidine monophosphate (Malet-Martino and Martino, 2002; Pinedo and Peters, 1988). 5-FU is metabolized to 5-fluorodeoxyuridine monophosphate (FdUMP) by thymidine kinase, thymidine phosphorylase, and other enzymes. In turn, FdUMP forms a covalent ternary complex with TS and reduced folic acid, inhibiting TS activity. Multiple investigators have reported that high TS expression is associated with resistance to 5-FU (Beck et al., 1994; Fukushima et al., 2001; Miyoshi et al., 2007).
Based on these findings, we considered that the expression of CTR1 and TS might influence treatment outcomes of S-1/CBDCA-treated NSCLC patients. Here, we investigated the relationship between CTR1 and TS expression levels and tumor response, progression-free survival (PFS), and overall survival (OS) in patients with advanced NSCLC treated with S-1/CBDCA doublet chemotherapy.
Materials and Methods
Patients and chemotherapy
This was a retrospective observational study. A total of 31 consecutive patients with advanced NSCLC were treated with S-1/CBDCA doublet chemotherapy between March 2011 and March 2015 at the Japanese Red Cross Kobe Hospital and the Kobe City Medical Center General Hospital. Inclusion criteria were as follows: histological diagnosis of NSCLC; measurable patient prognosis; clinical stage of III or IV; adequate hematologic, hepatic, and renal function; treatment with S-1/CBDCA doublet chemotherapy; and sufficient availability of tissue samples in paraffin blocks. Two patients were excluded because the tissue samples were too small for immunohistochemical analysis, and consequently, the remaining 29 patients were included in this study.
All patients received S-1/CBDCA doublet chemotherapy as first- or subsequent-line treatment. The S-1 dose was based on body surface area (BSA) as follows: BSA <1.25 m2, 80 mg/day; BSA 1.25 m2 to <1.50 m2, 100 mg/day; and BSA ≥1.5 m2, 120 mg/day. Patients received S-1 in two divided doses on days 1–14. CBDCA (area under the curve = 5) was administered intravenously on day 1 and then again every 3−4 weeks. This treatment cycle was repeated until disease progression, development of unacceptable toxicity, or patient refusal. The median number of cycles administered was 3 (range 1–6). After the failure of S-1/ CBDCA chemotherapy, docetaxel- or gemcitabine-containing regimens were given as standard therapies based on the Japanese Lung Cancer Clinical Practice Guidelines. A subset of patients received palliative radiotherapy to reduce the uncontrolled pain that is associated with bone metastasis or to control brain metastasis. There were no patients who received surgery. This study was approved by the ethics committees of Kobe University Graduate School of Health Sciences, Japanese Red Cross Kobe Hospital, and Institute of Biomedical Research and Innovation (reference numbers 470, 38, and 15-15, respectively). All patients provided written informed consent for chemotherapy and for the use of their tissue samples for this study.
Formalin-fixed, paraffin-embedded tissue blocks were available for diagnostic purposes. Sections (3-μm-thick) were cut and mounted on aminopropyltriethoxysilane-coated slides, and hematoxylin and eosin staining was performed to assess histopathological features. Clinical tumor size (T), lymph node metastasis (N), and distant metastasis (M) were categorized according to the tumor-node-metastasis (TNM) classification of the International Union against Cancer (Sobin et al., 2009). Histological classification was determined using the criteria of the World Health Organization (Travis et al., 2015).
Assessment of treatment outcomes
Computerized tomography scanning was performed before treatment, every 3 months during treatment until completion, and every 3–6 months thereafter until disease progression. Tumor response to chemotherapy was assessed according to the criteria of the response evaluation criteria for solid tumors (RECIST) (Eisenhauer et al., 2009) and was classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). CR was defined as disappearance of all target lesions, PR as ≥30% decrease in the sum of target lesion diameters, SD as <20% increase or <30% decrease in the sum of diameter of target lesions, and PD as ≥20% increase in the sum of target lesion diameters and at least a 5 mm absolute increase or appearance of new lesions. Patients who achieved CR or PR were considered to have good tumor responses, whereas SD or PD were considered poor tumor responses.
PFS was measured from the date of chemotherapy initiation to either the date of disease progression or the date of death from any cause. OS was measured from the date of chemotherapy initiation to the date of death or last follow-up.
Immunohistochemistry
Sections were deparaffinized in xylene, rehydrated through a graded series of ethanol, and rinsed with tap water. CTR1 and TS antigen retrieval was performed using protease-induced and heat-induced methods, respectively. Protease-induced retrieval was performed by incubating sections with pronase solution (Nichirei Bioscience, Tokyo, Japan) for 3 min at room temperature (RT). For heat-induced retrieval, sections were placed in 10 mM Tris base containing 1 mM ethylenediaminetetraacetic acid (pH 9.0) and incubated in a pressure cooker for 8 min at 120°C.
The sections were rinsed with 10 mM phosphate-buffered saline (PBS, pH 7.2), and nonspecific binding sites were blocked with 0.25% casein solution (Dako, Glostrup, Denmark) for 5 min at RT. Sections were then incubated overnight at RT with rabbit polyclonal anti-CTR1 (1:100, Abnova, Taipei, Taiwan) or mouse monoclonal anti-TS (1:40, Santa Cruz Biotechnology, Dallas, TX, USA) primary antibodies.
After rinsing with PBS, sections for CTR1 staining were incubated with anti-rabbit horseradish peroxidase (HRP) polymer (Histofine Simple Stain MAX-PO; Nichirei Bioscience) for 1 h at RT. Sections for TS staining were incubated with MACH3 mouse probe for 30 min, rinsed, and then incubated with MACH3 mouse micro-polymer HRP (MACH3 Mouse HRP-Polymer Detection; Biocare Medical, Concord, CA, USA) for 30 min at RT. Sections were then rinsed again with PBS, and the reaction products were developed with a diaminobenzidine solution (Dako). Finally, sections were lightly counterstained with Mayer’s hematoxylin, dehydrated in an ethanol gradient, treated with xylene, and coverslipped. Positive controls were sections of serous ovarian cancer for CTR1 and gastric cancer for TS. Negative controls were NSCLC tumor sections processed as above except the primary antibody was replaced with PBS containing 1% bovine serum albumin.
Assessment of CTR1 and TS levels
The immunostained sections were reviewed by three investigators (MK, AI, and SK) who were blinded to the clinical characteristics and outcome data. Expression levels of CTR1 and TS were semiquantitatively assessed by scoring the staining intensity (0, no staining; 1, weakly positive; 2, moderately positive; and 3, strongly positive) and the percentage of positively stained tumor cells (0, none; 1, 1–10%; 2, 11–49%; 3, ≥50%). A composite score (0–6) was obtained for each sample by adding together the intensity and percentage scores. Differences in scores were adjudicated by consensus among the three investigators.
Receiver operating characteristic curve analysis showed that the best sensitivity and specificity cut-off values for CTR1 and TS were 4.0. Tumors were then classified into low (CTR1Low, TSLow) and high (CTR1High, TSHigh) expression groups.
Statistical analysis
All statistical analyses were performed using SPSS 23.0 software (SPSS Inc., Chicago, IL, USA). Correlations between CTR1 and TS expression levels or clinicopathological parameters and tumor response were examined using Fisher’s exact test. Kaplan–Meier curves were constructed to estimate PFS and OS, and statistical significance was analyzed using the log-rank test. A P < 0.05 was considered to be statistically significant.
Results
Patient characteristics
The clinicopathological characteristics of the 29 patients are summarized in Table 1. The group comprised 19 men and 10 women with a median age of 68 years (range 48–84 years). Seven patients were diagnosed with adenocarcinoma (one lepidic, 2 acinar, 2 papillary, and 2 solid), 20 with squamous cell carcinoma (8 keratinizing and 12 non-keratinizing), and two with pleomorphic carcinoma. The clinical tumor stages were T1 in 5 patients, T2 in 7 patients, T3 in 6 patients, and T4 in 11 patients. Sixty-two percent of the patients had advanced lymph node stage N2 or N3. Two patients had stage IIIA disease, 5 patients had stage IIIB, and 22 patients had stage IV. All patients had epidermal growth factor receptor wild type tumors. Ninety percent of the patients had an Eastern Cooperative Oncology Group performance status of 0–1. All patients received S-1/CBDCA doublet chemotherapy: first line in 14 patients, second line in 9 patients, and third or later line in 6 patients.
Table 1.
Clinicopathological Characteristics of Patients
| Variables | n | |
|---|---|---|
| All patients | 29 | |
| Age | <65 | 8 |
| ≥65 | 21 | |
| Sex | Male | 19 |
| Female | 10 | |
| Tumor histology | Adenocarcinoma | 7 |
| Non-adenocarcinoma | 22 | |
| T stage | T1/T2 | 12 |
| T3/T4 | 17 | |
| N stage | N0/N1 | 11 |
| N2/N3 | 18 | |
| Clinical stage | III | 7 |
| IV | 22 | |
| Performance status | 0/1 | 26 |
| 2 | 3 | |
| Smoking status | Never | 4 |
| Ever | 25 | |
| Line | 1st | 14 |
| 2nd and later | 15 |
Correlation of CTR1 and TS levels with tumor response
Representative staining patterns of CTR1 and TS in NSCLC tissues are shown in Figure 1. CTR1 was detected in the cell membrane, whereas TS was observed in the nucleus and cytoplasm. Of the 29 patients, 11 (38%) and 18 (62%) patients had CTR1High and CTR1Low tumors, respectively, and 16 (55%) and 13 (45%) patients had TSHigh and TSLow tumors, respectively.
Figure 1.
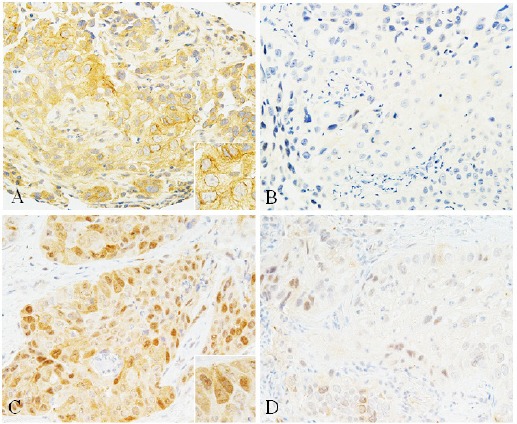
Representative Immunostaining Patterns of CTR1 and TS in NSCLC Tissue Samples. (A) High CTR1 expression, (B) low CTR1 expression, (C) high TS expression, (D) low TS expression. Insets show high-power views of positive cells. A number of tumor cells showed strong positive staining for CTR1 in the cell membrane (A) and for TS in the nucleus and cytoplasm (C).
Twenty-eight of the 29 patients were evaluable for tumor response. The overall response rate was 36% and the disease control rate was 75%. One (4%) patient achieved CR, 9 patients (32%) achieved PR, 11 patients (39%) had SD, and 7 (25%) had PD. Table 2 shows the correlations between CTR1 and TS levels or clinicopathological parameters and tumor response. CTR1High was significantly correlated with a good tumor response. Indeed, 7 of the 11 (64%) patients with CTR1High tumors but only 3 of the 17 (18%) patients with CTR1Low tumors showed a good tumor response (P = 0.02). However, no correlations were noted between tumor response and any other variables, including TS level.
Table 2.
Correlations between Clinicopathological Parameters or Expression Levels of CTR1 and TS and Tumor Response in NSCLC Patients Treated with S-1/carboplatin Doublet Chemotherapy
| Variables | na | Good response (%) | Poor response (%) | P value | |
|---|---|---|---|---|---|
| Age | <65 | 8 | 5 (63) | 3 (78) | 0.08 |
| ≥65 | 20 | 5 (25) | 15 (75) | ||
| Sex | Male | 18 | 8 (44) | 10 (56) | 0.41 |
| Female | 10 | 2 (20) | 8 (80) | ||
| Tumor histology | Adenocarcinoma | 6 | 2 (33) | 4 (67) | 1.00 |
| Non-adenocarcinoma | 22 | 8 (36) | 14 (64) | ||
| Clinical stage | III | 7 | 4 (57) | 3 (43) | 0.19 |
| IV | 21 | 6 (29) | 15 (71) | ||
| Performance status | 0/1 | 26 | 10 (38) | 16 (62) | 0.53 |
| 2 | 2 | 0 (0) | 2 (100) | ||
| Smoking status | Never | 4 | 0 (0) | 4 (100) | 0.27 |
| Ever | 24 | 10 (42) | 14 (58) | ||
| CTR1 | High | 11 | 7 (64) | 4 (36) | 0.02b |
| Low | 17 | 3 (18) | 14 (82) | ||
| TS | High | 15 | 3 (20) | 12 (80) | 0.06 |
| Low | 13 | 7 (54) | 6 (46) | ||
| CTR1/TS | Either CTR1High or TSLow | 20 | 10 (50) | 10 (50) | 0.01b |
| Both CTR1Lowand TSHigh | 8 | 0 (0) | 8 (100) |
Tumor response was evaluable in 28 of the 29 patients;
Statistically significant; CTR1, copper transporter 1; NSCLC, non-small cell lung cancer; TS, thymidylate synthase
When CTR1 and TS co-expression was analyzed, a good tumor response was detected in 10 of the 20 (50%) patients with either CTR1High or TSLow tumors but none of the 8 patients with both CTR1Low and TSHigh tumors (P = 0.01).
Correlation of CTR1 and TS levels with PFS and OS
Figures 2 and 3 show Kaplan–Meier survival curves for PFS and OS of patients stratified by tumor expression of CTR1 and TS. All patients were evaluable for PFS and OS, and the median follow-up time was 9.0 months. The median PFS and OS were 4.0 months (range 0.5–40.4) and 9.2 months (range 0.9–47.8), respectively. Patients with TSLow tumors had a significantly longer OS (median 21.2 vs. 8.5 months, P = 0.02) but not PFS (median 4.2 vs. 3.2 months, P = 0.11) than patients with TSHigh tumors (Table 3). A performance status of 0/1 also correlated with a longer OS (P = 0.02) but not PFS (P = 0.16). However, no other variables, including CTR1 level, were significantly correlated with PFS and OS.
Figure 2.
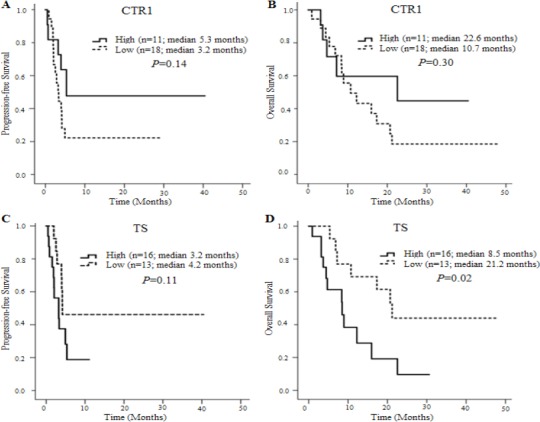
Kaplan–Meier Survival Curves for PFS and OS of Patients Stratified by CTR1 or TS Expression. (A) PFS Stratified by CTR1 Expression level (5.3 vs. 3.2 months, P = 0.14). (B) OS stratified by CTR1 expression level (22.6 vs. 10.7 months, P = 0.30). (C) PFS stratified by TS expression level (median 3.2 vs. 4.2 months, P = 0.11). (D) OS stratified by TS expression level (median 8.5 vs. 21.2 months, P = 0.02).
Figure 3.
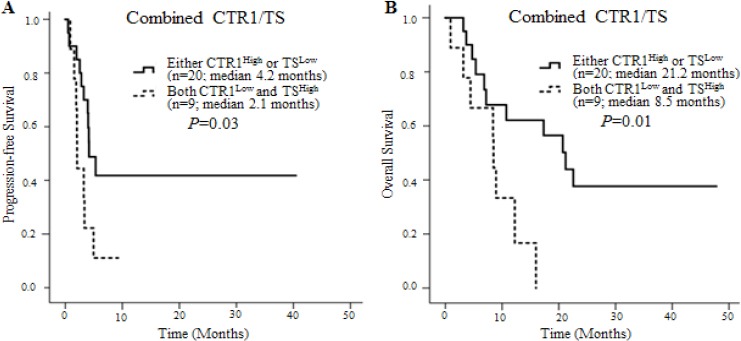
Kaplan–Meier Survival Curves for PFS and OS of Patients Stratified by Co-expression of CTR1 and TS. (A) PFS stratified by CTR1 and TS co-expression level (median 4.2 vs. 2.1 months, P = 0.03). (B) OS stratified by CTR1 and TS co-expression level (median 21.2 vs. 8.5 months, P = 0.01).
Table 3.
Correlations between Clinicopathological Parameters or Expression Levels of CTR1 and TS and PFS or OS in NSCLC Patients Treated with S-1/carboplatin Doublet Chemotherapy
| Variables | n | Median PFS (95% CI)a | P value | Median OS (95% CI)a | P value | |
|---|---|---|---|---|---|---|
| Age | <65 | 8 | 5.3 (3.3−7.4) | 0.47 | 22.6 (0.0−55.1) | 0.68 |
| ≥65 | 21 | 3.4 (2.0−4.7) | 12.2 (2.5−22.0) | |||
| Sex | Male | 19 | 4.0 (2.7−5.3) | 0.87 | 16.0 (4.2−27.7) | 0.88 |
| Female | 10 | 3.9 (1.0−6.9) | 10.7 (7.8−13.7) | |||
| Tumor histology | Adenocarcinoma | 7 | 4.2 (0.7−7.6) | 0.98 | 12.2 (8.4−16.1) | 0.99 |
| Non-adenocarcinoma | 22 | 3.9 (2.8−5.1) | 17.3 (1.7−32.9) | |||
| Clinical stage | III | 7 | 5.3 (2.3−8.4) | 0.25 | 22.6 (0.0−51.6) | 0.35 |
| IV | 22 | 3.4 (2.0−4.7) | 10.7 (5.3−16.2) | |||
| Performance status | 0/1 | 26 | 4.0 (3.0−5.0) | 0.16 | 16.0 (3.2−28.7) | 0.02b |
| 2 | 3 | 2.1 (0.2−4.0) | 3.2 (0.0−6.8) | |||
| Smoking status | Never | 4 | 1.9 (0.7−3.1) | 0.37 | 10.7 (3.4−18.1) | 0.92 |
| Ever | 25 | 4.1 (3.8−4.5) | 12.2 (0.0−25.2) | |||
| CTR1 | High | 11 | 5.3 (2.0−16.0) | 0.14 | 22.6 (0.0−58.1) | 0.30 |
| Low | 18 | 3.2 (2.1−4.4) | 10.7 (4.5−17.0) | |||
| TS | High | 16 | 3.2 (1.0−5.5) | 0.11 | 8.5 (3.7−13.2) | 0.02b |
| Low | 13 | 4.2 (3.8−16.9) | 21.2 (15.0−27.3) | |||
| CTR1/TS | Either CTR1High or TSLow | 20 | 4.2 (2.5−5.8) | 0.03b | 21.2 (14.0−28.4) | 0.01b |
| Both CTR1Low and TSHigh | 9 | 2.1 (2.0−2.1) | 8.5 (8.2−8.7) |
In months;
Statistically significant; CI, confidence interval; CTR1, copper transporter 1; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; TS, thymidylate synthase
When CTR1 and TS co-expression was analyzed, patients with either CTR1High or TSLow tumors had a significantly longer PFS (median 4.2 vs. 2.1 months, P = 0.03) and OS (median 21.2 vs. 8.5 months, P = 0.01) than patients with both CTR1Low and TSHigh tumors (Table 3).
Discussion
S-1/CBDCA doublet chemotherapy is feasible and well tolerated in patients with advanced NSCLC (Kaira et al., 2007; Tamura et al., 2009; Yoshioka et al., 2013; Kuyama et al., 2017), although individual patients vary in their responses to the chemotherapy. Therefore, biomarkers with predictive and prognostic value for S-1/CBDCA doublet chemotherapy would be of considerable help in selecting the optimal treatment regimen for each patient. CTR1 has been shown to control the accumulation and cytotoxic effect of CBDCA and CDDP in tumor cells (Holzer et al., 2004; Song et al., 2004; Holzer et al., 2006). TS is involved in DNA synthesis, and TSHigh is reportedly associated with resistance to 5-FU (Beck et al., 1994; Fukushima et al., 2001; Miyoshi et al., 2007). In this study, we investigated the predictive value of CTR1 and TS expression levels for treatment outcomes of advanced NSCLC patients treated with S-1/CBDCA doublet chemotherapy.
We found that a good tumor response was significantly more common in patients with CTR1High tumors than in patients with CTR1Low tumors (P = 0.02), but we failed to detect an association between CTR1 level and either PFS or OS. This result contrasts with the finding of Chen (2012), who found that CTR1High is associated with a longer PFS and OS in NSCLC patients treated with first-line chemotherapy consisting of a platinum (CBDCA or CDDP) plus a non-platinum (gemcitabine or taxane) agent; however, they also noted a positive association between CTR1High and tumor response. In addition, Kim (2014) reported that NSCLC patients whose tumors showed undetectable CTR1 levels also had reduced tissue concentrations of platinum and showed poor responses to neoadjuvant chemotherapy with platinum (CBDCA or CDDP) plus taxane or other drugs. Taken together, these and our results suggest that the level of CTR1 in NSCLC tumors may have value in predicting tumor response to platinum-containing chemotherapy.
S-1 is an oral fluoropyrimidine that combines the 5-FU prodrug tegafur as the effector drug with two modulators, CDHP and potassium oxonate (Shirasaka et al., 1996). Several in vitro and in vivo studies with tumor cell lines have implicated TSHigh as a mechanism of resistance to 5-FU (Beck et al., 1994; Fukushima et al., 2001; Miyoshi et al., 2007), suggesting that TS expression may influence the effectiveness of S-1–containing chemotherapy. In our study, TS levels, unlike CTR1 levels, did not correlate with tumor response. However, patients with TSLow tumors had a significantly longer OS (P = 0.02), but not PFS, than did patients with TSHigh tumors. Takeda (2011) found that TSLow predicted better tumor response in patients with advanced NSCLC treated with S-1/CBDCA doublet chemotherapy, which contrasts with our findings; however, they, like us, observed a significant association between TSLow and longer OS. The conflicts between the results of our and other studies might be due to a variety of factors, including differences in the sample size, clinical stage, treatment line, chemotherapy regimen, and immunostaining method.
Tumor response to chemotherapy is a complex process involving many factors. Thus, it seems reasonable that a combination of biomarkers might be more accurate and/or reliable than single markers in predicting the effectiveness of chemotherapy in NSCLC. Indeed, when we performed the analyses using CTR1 and TS co-expression levels, patients with either CTR1High or TSLow tumors showed a significantly better tumor response (P = 0.01), longer PFS (P = 0.03), and longer OS (P = 0.01) than did patients with both CTR1Low and TSHigh tumors. These results indicate that combining CTR1 and TS can significantly increase the predictive power for tumor response, PFS, and OS, compared with CTR1 or TS alone. Thus, S-1/CBDCA doublet chemotherapy might be particularly beneficial for advanced NSCLC patients with either CTR1High or TSLow tumors.
Our study has several limitations: (1) the line of S-1/CBDCA chemotherapy was not constant (i.e., first, second, and later line), (2) the patients were at different disease stages, (3) the study was retrospective, and (4) the sample size was small, which resulted in low statistical power and prevented multivariate analysis. Therefore, our results are still exploratory, and they will need to be confirmed in prospective studies of large patient cohorts at the same stage of disease and receiving the same line of treatment.
In conclusion, this study suggests that combined CTR1/TS expression status has the potential to be an important predictor of good treatment outcomes in patients with advanced NSCLC treated with S-1/CBDCA doublet chemotherapy.
References
- Beck A, Etienne MC, Cheradame S, et al. A role for dihydropyrimidine dehydrogenase and thymidylate synthase in tumour sensitivity to fluorouracil. Eur J Cancer. 1994;30:1517–22. doi: 10.1016/0959-8049(94)00216-r. [DOI] [PubMed] [Google Scholar]
- Chen HH, Yan JJ, Chen WC, et al. Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer. 2012;75:228–34. doi: 10.1016/j.lungcan.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours:revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Fujioka A, Uchida J, Nakagawa F, Takechi T. Thymidylate synthase (TS) and ribonucleotide reductase (RNR) may be involved in acquired resistance to 5-fluorouracil (5-FU) in human cancer xenografts in vivo. Eur J Cancer. 2001;37:1681–7. doi: 10.1016/s0959-8049(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Grossi F, Aita M, Follador A, et al. Sequential, alternating, and maintenance/consolidation chemotherapy in advanced non-small cell lung cancer:a review of the literature. Oncologist. 2007;12:451–64. doi: 10.1634/theoncologist.12-4-451. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Samimi G, Katano K, et al. The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol Pharmacol. 2004;66:817–23. doi: 10.1124/mol.104.001198. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70:1390–4. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- Kaira K, Sunaga N, Yanagitani N, et al. A phase I dose-escalation study of S-1 plus carboplatin in patients with advanced non-small-cell lung cancer. Anticancer Drugs. 2007;18:471–6. doi: 10.1097/CAD.0b013e32801265eb. [DOI] [PubMed] [Google Scholar]
- Kim ES, Tang X, Peterson DR, et al. Copper transporter CTR1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer. 2014;85:88–93. doi: 10.1016/j.lungcan.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyama S, Ochi N, Bessho A, et al. A phase II trial of carboplatin plus S-1 for elderly patients with advanced non-small-cell lung cancer with wild-type epidermal growth factor receptor:The Okayama Lung Cancer Study Group Trial 1202. Lung Cancer. 2017;112:188–94. doi: 10.1016/j.lungcan.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1):a review. Oncologist. 2002;7:288–323. doi: 10.1634/theoncologist.7-4-288. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Kondo K, Toba H, et al. Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase expression in tumor tissue, regarding the efficacy of postoperatively administered UFT (tegafur+uracil) in patients with non-small cell lung cancer. Anticancer Res. 2007;27:2641–8. [PubMed] [Google Scholar]
- Pinedo HM, Peters GFJ. Fluorouracil:biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–64. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Nakano K, Takechi T, et al. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996;56:2602–6. [PubMed] [Google Scholar]
- Sobin LH, Gospodarowicz MK, Wittekind C. UICC TNM Classification of Malignant Tumours. 7th edn. New York: Wiley-Liss; 2009. pp. 136–48. [Google Scholar]
- Song IS, Savaraj N, Siddik ZH, et al. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther. 2004;3:1543–9. [PubMed] [Google Scholar]
- Takeda K. Clinical development of S-1 for non-small cell lung cancer:a Japanese perspective. Ther Adv Med Oncol. 2013;5:301–11. doi: 10.1177/1758834013500702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Okamoto I, Hirabayashi N, Kitano M, Nakagawa K. Thymidylate synthase and dihydropyrimidine dehydrogenase expression levels are associated with response to S-1 plus carboplatin in advanced non-small cell lung cancer. Lung Cancer. 2011;73:103–9. doi: 10.1016/j.lungcan.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Tamura K, Okamoto I, Ozaki T, et al. Phase I/II study of S-1 plus carboplatin in patients with advanced non-small cell lung cancer. Eur J Cancer. 2009;45:2132–7. doi: 10.1016/j.ejca.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA-Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. WHO/IARC Classification of Tumours. 4th edn 7. Lyon: IARC; 2015. pp. 10–151. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Okamoto I, Morita S, et al. Efficacy and safety analysis according to histology for S-1 in combination with carboplatin as first-line chemotherapy in patients with advanced non-small-cell lung cancer:updated results of the West Japan Oncology Group LETS study. Ann Oncol. 2013;24:1326–31. doi: 10.1093/annonc/mds629. [DOI] [PubMed] [Google Scholar]


