Abstract
Background and objectives:
Colorectal cancer (CRC) is the most common gastrointestinal cancer and the second leading cause of cancer death in women in the world. Cancer-Testis Antigens (CTAs) are a group of tumor-associated proteins which typically are expressed in normal reproductive cells of men, but their expression in normal somatic cells is silenced. CTAs, due to their limited expression pattern, are considered as promising targets for cancer diagnosis and immuno-therapy.
Methods:
Expression of AKAP4, SPAG9 and CTAG1B genes from the CTAs family was studied in both tumor and normal tissues of 62 Iranian CRC patients by RT-PCR with the aim of finding biomarkers for early detection and anticipated progression. Statistical analysis was performed SPSS software V22.0 to assess the significance of any associations.
Results:
Elevated expression of SPAG9 and AKAP4 genes was observed in approximately 66% and 44% of tumours, respectively, as compared to adjacent non-cancerous tissues. While a significant association was found between AKAP4 gene expression and metastasis (P-value: 0.045), expression of the CTAG1B (NY-ESO-1) gene was not observed in our cases.
Conclusion:
AKAP4 and SPAG9 genes may find use as diagnostic biomarkers for CRC and AKAP4 may play an important role in progression to metastasis.
Keywords: AKAP4, biomarker, colorectal cancer, CTAs, NY−ESO−1- SPAG9
Introduction
Colorectal cancer (CRC) is the fourth most common cancer in the world. Colorectal cancer is one of the most common cancers in the world, accounting for nearly 10 percent of new cases of all cancer (Gou et al., 2014). Each year 1.5 million people worldwide are diagnosed with CRC. In America around 60,000 deaths due to CRC happens. Men and women are almost equally affected, and its prevalence increases gradually from age 40 to 50 years. In Iran the incidence of this cancer is on the rise and in now CRC is the fourth most common cancer among men (5%) and women (5.5%), respectively. Surprisingly, in Iran prevalence of CRC in men under 50 years old is remarkable.
Due to the slow development of CRC and the ability to treat at the early stages, screening for CRC can reduce the incidence of death and mortality in this disease. Therefore, a noninvasive biomarker for early detection of the disease can be useful (Kanojia et al., 2011).
Cancer-Testis antigens (CTAs) are molecules characterized by expression restricted to normal testis tissue but aberrant expression in a variety of cancer types (John et al., 2013). Although the biological role of CTA are not fully understood, but their association with cell proliferation, migration and invasion are well documented (Agarwal et al., 2013b). CT antigens have been proposed to play pivotal role in various malignant properties of cancer cells (Sinha et al., 2013). Recently, some investigations have been carried out using RT-PCR and statistical analysis to reveal the correlation of some CTA genes expression and clinical risk factors with malignancy in CRCs (Almanzar et al., 2009; Chen et al., 2010). In theory CTA can be detected only in cancer cells and not normal cells. Therefore, if a CTA proven that is detected in cancer cells eg. Breast cancer cells, it may have the potential to be considered as diagnostic biomarker for breast cancer. However, a specific CTA can be expressed in an eg. Breast cancer tissues but not in colorectal tissues. Thus some of the CTA are cancer specific.
AKAP4 is a member of CTA family encoded by X-chromosome (Saini et al., 2013a). AKAP4 is a member of the A-kinase anchor proteins which bind the protein kinase A (PKA) regulatory subunit and functions to anchor PKA to specific cellular locations. Previous studies demonstrated that AKAP4 plays a role in tumor development and progression, including esophageal cancer, ovarian cancer, breast cancer and lung cancer (Han et al., 2017). SPAG9 is a cytoplasmic enzyme and initially found as a scaffolding protein that bring MAPKs and their target transcription factors (Wang et al., 2013). SPAG9 is a member of the CTA family that is highly expressed in many types of cancers such as in 88% of breast cancer, 82% of cervical cancer, 74% of colorectal cancer, and 60% of astrocytoma that causes a strong immune response (Ren et al., 2016; Yan et al., 2016). Studies of SPAG9 suggest that it promotes proliferation and invasion (Ren et al., 2016). CTAG1B, the gene for NY-ESO-1, is located at Xq28 and codes for an intracellular, 18-kDa protein(Hemminger et al., 2013). The function of NY-ESO-1 is unknown; however, it has been postulated that cancer-testis antigens in general are involved in germ cell self-renewal or differentiation, conferring qualities such as immortality, self-renewal, migratory ability, and capacity to transform to cancer cells upon expression (Hemminger et al., 2013). The CT antigen NY-ESO-1/CTAG1/CT6 was first identified by SEREX in esophageal squamous cell carcinoma (Pagotto et al., 2013). NY-ESO-1 exhibits a relatively unique architecture, with a Pcc-1 domain in the C-terminus (89-164 aa) homologous to a yeast transcription factor involved in cell cycle progression and polarized growth(Pagotto et al., 2013). Its aberrant expression has been observed in a variety of neoplasms, including esophageal carcinoma, hepatocellular carcinoma, melanoma, and synovial sarcoma(Lai et al., 2012).
In the current study, we aimed to investigate the expression of these three CTA genes in Iranian CRC patients and their possible associations with some clinical risk The selection of these three CTA genes was based on two key reasons: (1) their presence in colorectal cancer tissue or cell lines and (2) their association with tumor aggressiveness (Almanzar et al., 2009; Chen et al., 2010).
Materials and Methods
Patients
Sixty two newly diagnosed cases with locally advanced colorectal cancer admitted to Rasoul Akram Hospital in Tehran, and willing to collaborate were enrolled in this study during the years 2008 to 2013. Only sporadic CRC patients were enrolled in this study. The samples were collected during surgery. Patients had received no other treatment such as chemotherapy before surgery.
The project was approved by the local ethics committee of Rasoul Akram Hospital and written informed consent was obtained from each case. Clinicopathological characteristics contained demographic variables (age, gender), tumor size, tumor location (colon and rectum), and pathological status classified according to the TNM system. During surgery and before any chemotherapy treatments, fresh tissue specimens and paired adjacent normal tissues were collected by the clinicians in separated sterile tubes. Tissue samples were frozen and stored at −70 °C.
RNA Extraction and cDNA Synthesis
Total RNA extraction was performed from 50–100 mg of each sample with the TriPure Isolation Reagent (Roche Applied Sciences, Germany). For cDNA synthesis, 3 µg of total RNA from each sample was used to synthesize fist-strand cDNA according to the manufacturer protocol (Fermentas, Germany).
Reverse Transcriptase-Polymerase Chain Reaction
To evaluate the expression of individual CTA genes, all reactions were carried out in a peqSTAR 96 Universal Thermal Cycler. The PCR mixture included 1 µM primer, 200 µM of each dNTP (KBC), reaction buffer 1x with 1.5 mMolar Mgcl2, and 1 unit Taq polymerase (5 U/µL, KBC). The PCR analysis was performed on selected genes using the primers and the conditions shown in Table 1. Amplified segments were analyzed by electrophoresis on a 2.5% agarose gel, stained with ethidium bromide, and observed under E box vx2 ultraviolet light system.
Table 1.
Primers and Condition of RT-PCR Analysis
| Genes | Primers sequence (5’→ 3’) | Denaturation | Annealing Extension Cycle (no.) |
|---|---|---|---|
| AKAP4 | TCCAGCTCAGAAGGCAACT | 95 °C--- 30 s | 59 °C---30 s 72°C---25 s 35 |
| CGCCCTCTGTGTCTCCTAC | |||
| SPAG9 | GCAGTAAACAGCGAAGTG | 95 °C---30 s | 64.2 °C---30 s 72 °C ---25 s 40 |
| CTTTTGTAGCCGAATGAGT | |||
| CTAG1B | CGACAACATACTGACTATCCG | 94 °C---30s | 58 °C---30 s 72 °C---45s 35 |
| CCTCCAGCGACAAACAATC |
Statistical Analysis
All the data were tabulated, and the statistical analysis was performed using the SPSS software V22.0 (SPSS, IBM Corp.: Armonk, NY). Association of the frequency of CTA genes expression and clinicopathological markers was analyzed using Fisher’s exact test for a two-by-two contingency table or by the Pearson χ2 test.
In all statistical analyses, a value less than 0.05 was considered to be statistically significant. All of the clinicopathological variables assessed in this study were categorized hence to create the two-by-two contingency table, we divided each of them into two groups according to their calculated means (Table 2 and 3). Finally, the coexpression of the CTA genes as well as the association of CTA genes coexpression was analyzed using Fisher’s exact test.
Table 2.
The Relation between the Percentages of the Positive Samples for SPAG9 Gene Expression and Clinicopathological Data at Diagnosis
| SPAG9 expression percentage | ||||
|---|---|---|---|---|
| Clinical Factor | Number of cases | - | + | P-value |
| Gender | Male (n=27) | 26 | 74 | |
| Female (n=34) | 28 | 62 | 0.412 | |
| Age (years) | >50 (n=39) | 31 | 69 | |
| ≤50 (n=21) | 38 | 62 | 0.579 | |
| T category (A) | TX (n=2) | 100 | 0 | |
| T1 (n=5) | 20 | 80 | ||
| T2 (n=14) | 43 | 57 | 0.261 | |
| T3 (n=30) | 33 | 67 | ||
| T4 (n=9) | 22 | 78 | ||
| T category (B) | TX & T1 & T2 (n= 21) | 41 | 59 | |
| T3 & T4 (n=39) | 32 | 68 | 0.577 | |
| Relapse | + (n=43) | 46 | 54 | |
| - (n=13) | 33 | 67 | 0.51 | |
| N category (A) | N0 (n=31) | 39 | 61 | |
| N1 (n=22) | 27 | 73 | 0.686 | |
| N2 (n=9) | 33 | 67 | ||
| N category (B) | N0 (n=30) | 40 | 60 | |
| N1 & N2 (n=31) | 30 | 70 | 0.426 | |
| M category | M0 (n=51) | 31 | 69 | |
| M1 (n=11) | 45 | 55 | 0.485 | |
| Tumor Size (centimeters) | 1.5-3 (n=22) | 36 | 64 | |
| 3.1-5.5 (n=19) | 32 | 68 | 0.948 | |
| 5.6-12 (n=20) | 35 | 65 | ||
| Stage | Stage 0 (n=6) | 33 | 67 | |
| Stage 1 (n=14) | 36 | 64 | ||
| Stage 2 (n=19) | 47 | 53 | 0.504 | |
| Stage 3 (n=16) | 25 | 75 | ||
| Stage 4 (n=7) | 14 | 86 | ||
p* < 0.05 significance association between gene expression and clinical risk factors; T category, The rate of growth and progression of tumors in the intestinal wall layers based on the pathological division; M, Metastasis to other organs (M0: non metastatic, M: metastatic); N, Lymph node involvement (N0: no involvement); Tumor Size, The length of tumors in centimeters; Relapse, Tumor Recurrence (+: Recurrence, -: no Recurrence); Stage, Stage of cancer according to TNM classification System and pathological data.
Table 3.
The Relation between the Percentages of the Positive Samples for AKAP4 Gene Expression and Clinicopathological Data at Diagnosis
| AKAP4 Expression percentage | ||||
|---|---|---|---|---|
| Clinical Factor | Number of cases | - | + | P-value |
| Gender | Male (n=27) | 33 | 67 | |
| Female (n=34) | 50 | 50 | 0.207 | |
| Age (years) | >50 (n=39) | 62 | 38 | |
| ≤50 (n=21) | 48 | 52 | 0.414 | |
| T category | Tx (n=2) | 50 | 50 | |
| T1 (n=5) | 60 | 40 | ||
| T2 (n=14) | 79 | 21 | 0.301 | |
| T3 (n=30) | 43 | 57 | ||
| T4 (n=9) | 56 | 44 | ||
| T category (B) | TX & T1 & T2 (n= 21) | 73 | 27 | |
| T3 & T4 (n=39) | 45 | 55 | 0.059 | |
| M category | M0 (n=51) | 63 | 37 | |
| M1 (n=11) | 27 | 73 | 0.045* | |
| N category (A) | N0 (n=31) | 61 | 39 | |
| N1 (n=22) | 50 | 50 | 0.715 | |
| N2 (n=9) | 56 | 44 | ||
| N category (B) | N0 (30) | 63 | 37 | |
| N1 & N2 (31) | 52 | 48 | 0.44 | |
| Tumor Size(A) (centimeters) | 1.5-3 (n=22) | 55 | 45 | |
| 3.1-5.5 (n=19) | 53 | 47 | 0.889 | |
| 5.6-12 (n=20) | 60 | 40 | ||
| Relapse | - (n=43) | 61 | 39 | |
| +(n=13) | 38 | 62 | 0.21 | |
| Stage | Stage 0 (n=6) | 50 | 50 | |
| Stage 1 (n=14) | 79 | 21 | ||
| Stage 2 (n=19) | 37 | 63 | 0.192 | |
| Stage 3 (n=16) | 62 | 38 | ||
| Stage 4 (n=7) | 57 | 43 | ||
p < 0.05 significance association between gene expression and clinical risk factors; T category, The rate of growth and progression of tumors in the intestinal wall layers based on the pathological division. M, Metastasis to other organs (M0, non metastati; M, metastatic). N, Lymph node involvement (N0, no involvement); Tumor Size, The length of tumors in centimeters; Relapse, Tumor Recurrence (+: Recurrence, -: no Recurrence); Stage, Stage of cancer according to TNM classification System and pathological data.
Results
Clinical data
Sample tissues were collected from 62 CRC patients including tumoral and adjacent normal tissues (27 male, 34 female; F/M = 1.2/1) and 10 colon tissues from healthy controls from Rasoul Akram Hospital in Tehran.
Result s of RT- PCR Amplification
CTA mRNA expression was investigated in 62 CRC cancer specimens and paired adjacent normal tissues and colon tissues of healthy controls by RT-PCR. None of these genes were expressed in normal mucosal tissues. SPAG9 mRNA expression was found in 41 of 62 (66%) tumoral tissue specimens and AKAP4 SPAG9 mRNA expression was found in 27 of 62 (44%) tumoral tissue specimens irrespective of tumor stages. NY-ES0-1 mRNA expression was not observed in any tumoral tissue specimens. Positive RT-PCR results for each CTA gene studied, was shown in Figure 1.
Figure 1.
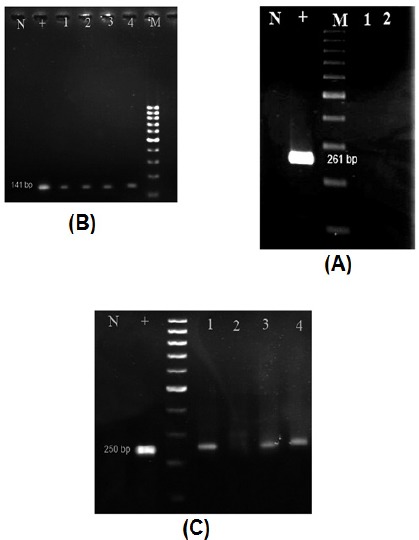
The Result of RT-PCR Analysis of Positive mRNA Expression of CTAG1B (A, 261 bp), SPAG9 (B, 141 bp), AKAP4 (C, 250 bp) Genes in Some Patients, M, Molecular marker; N, Negative control; +, Positive control (cDNA from testis).
Correlation between CTA mRNA expression and clinicopathological markers
The relation between clinical risk factors and the expression frequency of the three CTA genes were examined (Table 2 and 3). Our results revealed that the expression of AKAP4 was significantly correlated with liver metastasis (M category) (< 0.05). The SPAG9 and AKAP4 expressions were revealed to be independent of tumor stages, tumor size, lymph node involvement, age and gender of the patients (p>0.05).
Co-expression of CTA genes in CRC. At least one of the above CT genes was expressed in 40 patients (64%) of all 62 CRC patients
Among them, both of AKAP4 and SPAG9 genes were expressed in 14 patients (23%) and none of CT genes expressed in the other 8 patients (13%). The expression of AKAP4 was significantly associated with SPAG9 gene expression (p< 0.05).
Patients follow-up
Among the patients studied, 14 patients had recurrence, 3 more patients had metastasis to liver, 4 patients passed away; one due to metastasis and 3 for non-cancerous reasons. The rest of the patients are alive and without metastasis (Table 4).
Table 4.
The Coexpression of AKAP4 and SPAG9 in CRC Patients after Follow up
| A KAP4 Expression percentage at diagnosis | SPAG9 expression percentage at diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Factor | Number of cases at diagnosis | Number of cases after follow up | - | + | P-value | + | - | P-value |
| Metastasis to liver | M0 (n=51) | M0 (n=48) | 63 | 37 | 31 | 69 | ||
| M1 (n=11) | M1 (n=14) | 27 | 73 | 0.045* | 45 | 55 | 0.485 | |
| Relapse | - (n=43) | - (n=42) | 61 | 39 | 46 | 54 | ||
| +(n=13) | +(n=14) | 38 | 62 | 0.21 | 33 | 67 | 0.948 | |
Among patients with metastasis and relapse, AKAP4 expression was observed in 21 and SPAG9 gene expression was observed in 26, and both genes were expressed in 14 cases. In fact, the expression of the AKAP4 gene in about 45% of patients and the expression of the SPAG9 gene in about 55% of patients and the expression of both genes in about 30% of the patients studied could be as a prognostic biomarker for the relapse or metastasis of CRC.
Discussion
Colorectal cancer is the third most common cancer in the world, with nearly 1.4 million new cases diagnosed in 2012 (Ferlay et al., 2014). In Iran, CRC, is the most common fatal cancer (5000 new cases per year) (Kanojia et al., 2011). If the CRC diagnosed in early stages, before the lymph-vascular invasion, the survival will be increased. Therefore there is an urgent need to identify biomarkers in the early stages of CRC (Kanojia et al., 2011). Over the last decades, tremendous studies about cancer molecular markers have been accomplished; however, only a few such markers have entered clinical practice. The lack of clinical prognostic markers clearly reflects limitations in prognostic studies. Although the function of CTA genes is still largely unknown partly due to their presence in multiple tumor types, their limited expression in normal tissue has made them ideal molecular markers for cancer prognosis and diagnosis (Odunsi et al., 2007; Atanackovic et al., 2008). To improve the prognosis of CRC, the most significant considerations are the selection of patients at high risk for liver metastasis and subsequently the initiation of suitable adjuvant therapy. Adjuvant therapy in patients with CRC after curative resection has been reported to be useful for improving overall and disease-free survival (Watanabe et al., 2001; Cascinu et al., 2002).
The current study is the first to describe AKAP4, SPAG9 and NY-ESO-1 genes expression in Iranian CRC patients.
Agarwal et al., (2013) study revealed that AKAP4 gene and protein expression was detected in 86% of total patients with cervical cancer. AKAP 4 expression was significantly associated with early grades tumor specimens (P = 0.023). In addition, humoral response was detected in 53% of patients irrespective of stages, lymph node positivity, and grades (Agarwal et al., 2013a). Saini et al., (2013b) concluded that AKAP4 may be used as serum based diagnostic test for an early detection and diagnosis of breast cancer and may be a potential target for immunotherapeutic use. Chiriva -Internati et al., (2012) demonstrated the aberrant expression of AKAP-4 in PC (prostate cancer), which will potentially be developed as a biomarker in PC (Chiriva-Internati et al., 2012). Agarwal et al., (2013) results revealed that AKAP4 was expressed at both the mRNA and protein levels in 89% (34/38) of ovarian carcinoma tissue specimens but not in 21 matched adjacent non-cancerous tissues.
According to our data, a significant association between AKAP4 expression and liver metastasis was shown suggesting that AKAP4 can have a potential to be considered as a prognostic biomarker to predict metastasis in CRC patients. Therefore AKAP4 may have a potential role in disease progression and tumorigenesis.
Wang et al., (2013) demonstrated overexpression of SPAG9 in NSCLC and its direct correlation with tumor differentiation, pTNM stage, nodal status and poor survival. Moreover, their data showed for the first time that SPAG9 could promote lung cancer cell invasion, possibly by enhancing JNK-mediated MMP9 transcriptional activation. Given these findings, SPAG9 might be a potential therapeutic target in the treatment of lung cancer, and future studies may be warranted to investigate these possibilities. Sinha et al., (2013) detected SPAG9 mRNA and protein expression in all breast cancer cells. Their data indicated that down regulation of SPAG9 reduces growth and invasive potential of triple-negative breast cancer cells, suggesting that SPAG9 may be a potential target for therapeutic use. Yu et al., (2011) showed that SPAG9 antibodies were detected in approximately 72% of patients with endometrial cancer but not in healthy controls. A significant difference has been found among pathological types and degrees (P < 0.05), and it was also found to be expressed in transferred lymph nodes. It may serve as a new type of endometrial cancer markers for early detection, diagnosis and treatment (Yu et al., 2012). Xie et al., (2014) analyzed SPAG9 expression in human HCC (Hepatocellular carcinoma) tissues by immunohistochemistry and found that SPAG9 overexpression is correlated with tumor stage (p<0.001), tumor multiplicity (p=0.019), tumor size (p=0.034), AFP levels (p=0.006), and tumor relapse (p=0.0017). Furthermore, they demonstrated that SPAG9 overexpression is correlated with poor overall survival (p<0.001) and relapse-free survival (p=0.002) in HCC. Yi et al., (2013) showed that overexpression of SPAG9 correlated with tumor grade (p<0.001). Li et al., (2014) using Immunohistochemistry showed that SPAG9 was overexpressed in 36.5 % of prostate cancer specimens. There was a significant association between SPAG9 overexpression and tumor stage (p=0.0020) and Gleason score (p=0.0377). Garg et al., (2009) suggested that SPAG9 mRNA and protein expression was detected in 78% of the thyroid cancer patients but not multiple goiters and follicular adenoma disease patients. They concluded SPAG9 expression may play a role in cellular growth and thyroid carcinogenesis (Garg et al., 2009b). RT-PCR, in situ RNA hybridization, and immunohistochemical analyses revealed that SPAG9 expression was significantly associated with tumor grades in 82% of early stage cervical cancer specimens (Garg et al., 2009a). Kanojia et al., (2011) RT-PCR analysis showed that 58 of 78 (74%) CRC patients expressed SPAG9 mRNA. They concluded testing for SPAG9 may be useful for early detection of EC (Esophageal cancer) in asymptomatic high-risk women. Garg et al., (2008) investigated the clinical relevance of SPAG9 in RCC (Renal cell carcinoma) patients. RT-PCR analysis showed expression of SPAG9 transcript in RCC tissues and RCC cell lines. In situ RNA hybridization and immunohistochemistry analyses confirmed the expression of SPAG9 in 88% of cancer patients, suggesting that SPAG9 participates in renal cancer. SPAG9 mRNA and protein expression was detected in 90% of EOC (Epithelial Ovarian Cancer) tissues and in all three human ovarian cancer cell lines by Garg et al., (2007). In the present study, the SPAG9 expression was investigated in various histotypes of CRC by RT-PCR technique. We demonstrated that majority of colorectal cancer patients (66%) showed SPAG9 expression irrespective of clinical stages and histological grades of tumor suggesting that SPAG9 may have a potential role in disease progression and tumorigenesis.
Relationship between expression of AKAP4, SPAG9 and CTAG1B genes with clinical risk factors in CRC patients
Liver metastasis
In this study, the expression of AKAP4 gene in Iranian colorectal cancer patients was investigated and it was observed that the expression of this gene in CRC patients with metastasis of tumor to the liver was significantly different from those with primary tumor of CRC. Therefore, there is a significant relationship between the expression of this gene and metastasis in the liver in patients with colorectal cancer (P-value = 0. 045). In this study, the expression of SPAG9 gene for the first time in Iranian colorectal cancer samples was studied. The qualitative expression of this gene was not significantly different between people with malignant tumor CRC and those with primary tumor of this cancer (P-value = 458).
Metastasis to lymph nodes
Spreading metastases from lymph nodes to other locations is considered as a general feature of carcinoma, and the spread of lymphoid tumor cells occurs fairly early; this process is common in CRC patients. In this study, the qualitative expression of AKAP4 and SPAG9 genes was not significantly associated with metastasis to lymph nodes (state B) and with the number of nodes involved in metastasis (state A) (P value> 0.05). These data indicate that the expression of these genes in individuals with a higher number of metastatic lymph nodes (N2) is no different from those with less metastatic lymph nodes (N1).
Tumor growth rate in the intestinal wall (T factor)
The qualitative expression of AKAP4 and SPAG9 genes did not show any significant relationship with tumor growth in the intestinal wall (T) (P> 0.05). One of the ways in which liver metastases in CRC patients is tumor growth in the intestinal wall and passing through it, and invasion of nearby organs such as the liver around the intestine. Although, according to the obtained data, the expression of AKAP4 gene is associated with liver metastasis, but does not have a significant relationship with the T factor. Perhaps this is due to the fact that the T factor has four grades and just T4 passes through the intestinal wall and metastases to the surrounding organs. The number of T4 patients enrolled in this study is low and is not statistically comparable. On the other hand, although the expression of genes associated with liver metastasis is not accompanied by tumor growth in the intestinal wall, this finding suggests that the metastatic potential has been created from the very early stages of the tumor, and the growth of the tumor in the layers of intestinal wall is an independent factor of the expression of genes. In other words, the tumor growth and expression of genes should be considered simultaneously.
Tumor size
In this study, the relationship between the expression of AKAP4 and SPAG9 genes and the size of the tumor (tumor length) in CRC patients is reported. The qualitative expression of these genes was not significantly correlated with tumor size in three groups (1.5-3), (3.1-5.5) and (5.6-12) cm (P> 0.05). These findings are not unexpected due to the role of AKAP4 and SPAG9 gene products as scaffold proteins.
Age and sex
The qualitative expression of the studied genes in this study was completely independent of the sex and age of the patients and there was no significant relationship between them (p <0.05). These findings indicate that age and gender factors do not play a role in expressing of these genes, and this is the tumor phenotype that determines the expression of genes.
Cancer stage
In this study, there was no significant correlation between qualitative expression of the genes and cancer stage (p >0.05).
Cancer recurrence
In this study, there was no significant correlation between qualitative expression of the genes with cancer recurrence (p >0.05). Thus, in patients whose cancer has recurred, there has not been a steady pattern in the expression of genes.
In conclusion, according to this fact that AKAP4 and SPAG9 gene only expressed in normal testis and tumoral CRC tissues but not in normal colon tissues they can be used as a diagnostic biomarker for colorectal cancer.
From all clinicopathological factors investigated in this study, AKAP4 gene expression was significantly correlated with liver metastasis. Thus AKAP4 gene expression may be used as a risk factor and alarm for metastasis (potential prognostic biomarker).
In this study we observed NYESO-1 gene expression in none of CRC patients. So this gene expression has no role in tumorigenesis of CRC.
Since the useful features of a biomarker should be such that its measurement method be simple, fast, affordable and inexpensive, RT-PCR technique employed in this study has this features and could be applied in CRC specimens in any genetic laboratory.
Acknowledgements
This project was funded by grants from the National Institute of Genetic Engineering and Biotechnology (NIGEB) of Iran. The authors would like to thank all patients who willingly participated in the study.
References
- Agarwal S, Saini S, Parashar D, et al. Expression and humoral response of a-kinase anchor protein 4 in cervical cancer. Int J Gynecol Cancer. 2013a;23:650–8. doi: 10.1097/IGC.0b013e31828a0698. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Saini S, Parashar D, et al. The novel cancer-testis antigen A-kinase anchor protein 4 (AKAP4) is a potential target for immunotherapy of ovarian serous carcinoma. Oncoimmunology. 2013b;2:e24270. doi: 10.4161/onci.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almanzar G, Olkhanud PB, Bodogai M, et al. Sperm-derived SPANX-B is a clinically relevant tumor antigen that is expressed in human tumors and readily recognized by human CD4+and CD8+T cells. Clin Cancer Res. 2009;15:1954–63. doi: 10.1158/1078-0432.CCR-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanackovic D, Altorki NK, Cao Y, et al. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci U S A. 2008;105:1650–5. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascinu S, Georgoulias V, Kerr D, et al. Colorectal cancer in the adjuvant setting:perspectives on treatment and the role of prognostic factors. Ann Oncol. 2002;14:25–9. doi: 10.1093/annonc/mdg725. [DOI] [PubMed] [Google Scholar]
- Chen Z, Li M, Yuan Y, et al. Cancer/testis antigens and clinical risk factors for liver metastasis of colorectal cancer:a predictive panel. Dis Colon Rectum. 2010;53:31–8. doi: 10.1007/DCR.0b013e3181bdca3a. [DOI] [PubMed] [Google Scholar]
- Chiriva-Internati M, Yu Y, Mirandola L, et al. Identification of AKAP-4 as a new cancer/testis antigen for detection and immunotherapy of prostate cancer. Prostate. 2012;72:12–23. doi: 10.1002/pros.21400. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, et al. Globocan 2012 1.0, cancer incidence and mortality worldwide:IARC cancerBase 2014. Vol. 11. Lyon, France: International Agency for Research on Cancer; 2013. Visit: http://globocan.iarc.fr . [Google Scholar]
- Garg M, Chaurasiya D, Rana R, et al. Sperm-associated antigen 9, a novel cancer testis antigen, is a potential target for immunotherapy in epithelial ovarian cancer. Clin Cancer Res. 2007;13:1421–8. doi: 10.1158/1078-0432.CCR-06-2340. [DOI] [PubMed] [Google Scholar]
- Garg M, Kanojia D, Khosla A, et al. Sperm-associated antigen 9 is associated with tumor growth, migration, and invasion in renal cell carcinoma. Cancer Res. 2008;68:8240–8. doi: 10.1158/0008-5472.CAN-08-1708. [DOI] [PubMed] [Google Scholar]
- Garg M, Kanojia D, Salhan S, et al. Sperm-associated antigen 9 is a biomarker for early cervical carcinoma. Cancer. 2009a;115:2671–83. doi: 10.1002/cncr.24293. [DOI] [PubMed] [Google Scholar]
- Garg M, Kanojia D, Suri S, et al. Sperm-associated antigen 9:a novel diagnostic marker for thyroid cancer. J Clin Endocrinol Metabol. 2009b;94:4613–8. doi: 10.1210/jc.2009-0703. [DOI] [PubMed] [Google Scholar]
- Gou W-f, Sun H-z, Zhao S, et al. Downregulated inhibitor of growth 3 (ING3) expression during colorectal carcinogenesis. Indian J Med Res. 2014;139:561. [PMC free article] [PubMed] [Google Scholar]
- Han J, Gao W, Su D, Liu Y. Silencing of A-kinase anchor protein 4 (AKAP4) inhibits proliferation and progression of thyroid cancer. Oncology research featuring preclinical and clinical cancer therapeutics. Oncol Res. 2017;25:873–8. doi: 10.3727/096504016X14783701102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminger JA, Toland AE, Scharschmidt TJ, et al. The cancer-testis antigen NY-ESO-1 is highly expressed in myxoid and round cell subset of liposarcomas. Mod Pathol. 2013;26:282–8. doi: 10.1038/modpathol.2012.133. [DOI] [PubMed] [Google Scholar]
- John T, Starmans MH, Chen Y-T, et al. The role of Cancer-Testis antigens as predictive and prognostic markers in non-small cell lung cancer. PLoS One. 2013;8:e67876. doi: 10.1371/journal.pone.0067876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanojia D, Garg M, Gupta S, et al. Sperm-associated antigen 9 is a novel biomarker for colorectal cancer and is involved in tumor growth and tumorigenicity. Am J Pathol. 2011;178:1009–20. doi: 10.1016/j.ajpath.2010.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J-P, Robbins PF, Raffeld M, et al. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors:significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod Pathol. 2012;25:854–8. doi: 10.1038/modpathol.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Peng Y, Niu H, et al. SPAG9 is overexpressed in human prostate cancer and promotes cancer cell proliferation. Tumor Biol. 2012;35:6949–54. doi: 10.1007/s13277-014-1947-4. [DOI] [PubMed] [Google Scholar]
- Odunsi K, Qian F, Matsuzaki J, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:12837–42. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagotto A, Caballero OL, Volkmar N, et al. Centrosomal localisation of the cancer/testis (CT) antigens NY-ESO-1 and MAGE-C1 is regulated by proteasome activity in tumour cells. PLoS One. 2013;8:e83212. doi: 10.1371/journal.pone.0083212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Wei X, Zou G, et al. Cancer testis antigen SPAG9 is a promising marker for the diagnosis and treatment of lung cancer. Oncol Rep. 2016;35:2599–605. doi: 10.3892/or.2016.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S, Agarwal S, Sinha A, et al. Gene silencing of A-kinase anchor protein 4 inhibits cervical cancer growth in vitro and in vivo. Cancer Gene Ther. 2013a;20:413–20. doi: 10.1038/cgt.2013.32. [DOI] [PubMed] [Google Scholar]
- Saini S, Jagadish N, Gupta A, et al. A novel cancer testis antigen, A-kinase anchor protein 4 (AKAP4) is a potential biomarker for breast cancer. PLoS One. 2013b;8:e57095. doi: 10.1371/journal.pone.0057095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Agarwal S, Parashar D, et al. Down regulation of SPAG9 reduces growth and invasive potential of triple-negative breast cancer cells:possible implications in targeted therapy. J Exp Clin Cancer Res. 2013;32:69. doi: 10.1186/1756-9966-32-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dong Q, Miao Y, et al. Clinical significance and biological roles of SPAG9 overexpression in non-small cell lung cancer. Lung Cancer. 2013;81:266–72. doi: 10.1016/j.lungcan.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Wu T-T, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Fu L, Liu N, et al. Overexpression of SPAG9 correlates with poor prognosis and tumor progression in hepatocellular carcinoma. Tumor Biol. 2014;35:7685–91. doi: 10.1007/s13277-014-2030-x. [DOI] [PubMed] [Google Scholar]
- Yan Q, Lou G, Qian Y, et al. SPAG9 is involved in hepatocarcinoma cell migration and invasion via modulation of ELK1 expression. OncoTargets Ther. 2016;9:1067. doi: 10.2147/OTT.S98727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Ni W, Liu W, et al. SPAG9 is overexpressed in human astrocytoma and promotes cell proliferation and invasion. Tumor Biol. 2013;34:2849–55. doi: 10.1007/s13277-013-0845-5. [DOI] [PubMed] [Google Scholar]
- Yu P, Yan L, Zhang H, et al. Expression and clinical significance of sperm-associated antigen 9 in patients with endometrial carcinoma. Int J Gynecol Cancer. 2012;22:87–93. doi: 10.1097/IGC.0b013e3182370f2e. [DOI] [PubMed] [Google Scholar]


