Abstract
Objective:
This study aimed to investigate the expression of cyclin D1 and hnRNP-K in relation to the pathological findings in bladder cancer including the type, grade, muscle invasion and bilharzial association.
Methods:
We studied the immunoexpression; as regard the percentage, intensity and score of both cyclin D1 and hnRNP-K in different bladder lesions including 10 cases of cystitis; 10 cases of carcinoma insitu (CIS), 20 cases of Squamous cell carcinoma (SCC) and 66 cases of urothelial carcinoma (UC).
Results:
High expression of cyclin D1 was found in UC compared to other groups (p<0.001) and in UC with low grade, non-muscle invasive and papillary tumors compared to their counterparts (p<0.05, <0.01 and <0.05 respectively), however, bilharzial association does not affect cyclin D1 expression. Higher hnRNP-K expression was found in SCC compared to other groups (p <0.001) and in UC with high grade, muscle invasive and non-papillary tumors compared to their counterparts (p<0.001each). Bilharzial-associated UC showed higher expression of hnRNP-K percent (p<0.05) compared to non-bilharzial cases.
Conclusion:
This study elucidated a possible contribution of cyclin D1 and hnRNP-K expression in the initiation and progression of urinary bladder carcinoma, so, both of them can be used in predicting progression of urinary bladder carcinoma and to differentiate between UC and SCC in high grade tumors. The possible role of both markers in immunotherapy deserves supplementary studies.
Keywords: Bladder cancer, cystitis, bilharziasis, cyclin D1, hnRNP−K, immunohistochemistry
Introduction
Bladder cancer is one of the major foundations of morbidity and mortality worldwide, accounting for nearly 429,800 new incidence cases and 165,100 deaths per year (Torre et al., 2015). In Egypt, bladder malignancies are the commonest among urinary system malignant tumors (90.71%) and the third among all malignancies (Harb et al., 2017).
Urothelial carcinoma (UC) is the most frequent histological subtype and accounts for more than 90% of the cases in USA and Europe (Moreira et al., 2010). UC is classified into two major groups for treatment and prognostic purposes, namely: non-muscle invasive bladder cancer NMIBC (Ta/Tis/T1) and muscle invasive bladder cancer (MIBC) (T2-T4). A majority of UC patients present were NMIBC (Isharwal and Konety, 2015), which although clinically heterogeneous, is typically associated with a favorable prognosis and a relatively low risk for recurrence following cystectomy (Nargund et al., 2012). In contrast, MIBC accounts for only 25-30% of cases (Veeratterapillay et al., 2016), yet the five-year survival rate, even following radical cystectomy, is greatly reduced as compared with NMIBC (Proctor et al., 2010; Meeks et al., 2012). In both NMIBC and MIBC, available histopathological parameters, including tumor stage and grade do not always predict the course of disease in individual patients, including those who have an increased risk of post cystectomy recurrence. So there is an urgent need to identify novel biomarkers that indicate UC cases with increased risk for recurrence and metastases. Currently, much interest centers on the molecular processes underlying the development and progression of UC of the bladder (Waddell and Keegan, 1999).
Squamous cell carcinoma (SCC) is the most common type in Egypt due to endemicity of bilharziasis that exposes transitional epithelial lining of bladder to squamous metaplasia and dysplasia (Reuter, 2010). SCC is categorized into two subtypes; SCC associated with bilharziasis (B-SCC) and SCC not associated with bilharziasis (NB-SCC). Both differ in their epidemiology, natural history and clinic-pathological features (Martin et al., 2016).
The cyclin family of proteins, which includes the mitotic cyclins (A and B) and cyclins C, D and E (associated with G/S transition), has a central role in the control of the cell cycle (Sherr, 1996). Cyclin D1 contributes to regulate G1-S phase progression by forming a complex with different cyclin dependent kinases. It has oncogenic properties and is frequently overexpressed in several human tumor types (Kim and Diehl, 2009). A significant proportion of bladder cancer cases showed that increased cyclin D1 expression and overexpression of its gene were associated with poor prognosis and decreased postoperative patient survival (Sherr and Roberts, 1995; Sgambato et al., 1998). Heterozygous deletion of cyclin D1 occurs in approximately 50% of human muscle-invasive bladder cancer. Thus, identifying a new anticancer drug targeting and down regulating cyclin D1 expression and function is one of the first priorities in the field of anticancer research (Fang et al., 2013).
Heterogeneous nuclear ribonucleoprotein K (hnRNP-K), a member of the hnRNP family, is an essential RNA- and DNA-binding protein (Chaudhury et al., 2010). HnRNP-K is multifunctional, involved in the development of heterogeneous nuclear RNA into mature RNA (Dinh et al., 2013). It interacts with diverse molecules involved in gene expression and signal transduction, including chromosome remodelling, DNA transcription, RNA processing, RNA splicing, and RNA stability and translation (Wu et al., 2014). HnRNP-K protein is aberrantly overexpressed in various human cancers including colorectal, pancreatic, hepatic, prostate and renal cancer (Lu and Gao, 2016). It plays a vital role in cancer prognosis and its aberrant expression correlates with unfortunate clinical outcome (Yang et al., 2016).
Early detection and proper typing, grading and staging of bladder cancer are critically important to alleviate morbidity and mortality rate, associated with the disease, and to meliorate the chances of a prosperous outcome. So this study aims to evaluate expression of cyclin D1 and hnRNP-K in bladder lesions and analyze their correlation with the pathological characteristics of bladder cancer including tumor type, grade, stage, as well as bilharzial association and to characterize their potential role as diagnostic markers and/or target therapy for bladder cancer.
Materials ans Methods
Tissue samples
106 archival urinary bladder paraffin blocks from Pathology Department of Theodor Bilharz Research Institute were included in this study; including transurethral resections or radical cystectomy specimens. The study protocol was approved by the Ethics committee of Theodor Bilharz Research Institute, for the protection of human subject and adopted by the 18th world medical assembly, Helsinki, Finland.
Paraffin blocks consisted of 10 cases of chronic cystitis, 10 cases of carcinoma in situ (CIS), 20 cases of squamous cell carcinoma (SCC) and 66 case of urothelial carcinoma (UC).
They belong to 77 males and 29 females (mean age 62.55 ± 6.49 years, range 29– 72years) (Table 1).
Table 1.
Sex and Age Distribution of Studied Cases
| Lesion (n) | Female n (%) | Male n (%) | Age (Mean±S.D.) |
|---|---|---|---|
| Cystitis (10) | 5a (50.0%) | 5a (50.0%) | 63.50 ± 1.73 |
| CIS (10) | 2a (20.0%) | 8b (80.0%) | 64.19 ± 3.34 |
| SCC (20) | 10a (50.0%) | 10b (50.0%) | 67.83 ± 5.06 |
| UC (66) | 12a (18.18%) | 54b (81.82%) | 61.08 ± 6.40 |
| Total (106) | 29 (27.36%) | 77 (72.64%) | 62.54 ± 6.49 |
Each subscript letter denotes a subset of sex categories whose column proportions do not differ significantly from each other at the .05 level. (Chi-Square); CIS, Carcinoma In Situ; SCC, Squamous cell carcinoma; UC, Urothelial carcinoma; n, number of cases
Histological Study
Specimens
Tissue sections were stained by Hematoxylin-eosin and histopathologically diagnosed by expert pathologists, with special reference to type of malignancy, tumor grade and stage as well as positive or negative association with bilharziasis.
Histological grading
Urinary bladder tumors were histologically divided into 3 grades (I–III). Tumors of grade I are considered of low grade, while those in grades II and III are of high grade (Moch et al., 2016).
Pathological staging
Staging of bladder tumors followed WHO classification (Moch et al., 2016). Tumors of pathological stage T1 are considered superficial and that > T1 are muscle invasive.
Diagnosis of bilharziasis
Diagnosis of bilharzial infestation was based on detection of Schistosoma eggs in tissues and/or detection of circulating Schistosoma antibodies in sera of patients by enzyme-linked immunosorbent assay (ELISA).
Immunohistochemical (IHC) technique
Immunohistochemistry for Cyclin D1 and hnRNP-K was performed on tissue sections cut from the paraffin blocks at 4μm onto positively charged slides (Superfrost plus, Menzel-Glaser, Germany) and stained on an automated platform the (Dako Autostainer Link 48) using anti-human hnRNPK and Cyclin D1 monoclonal primary antibodies (Santa Cruz Biotechnology, CA, USA) at 1:100 dilution. Heat induced antigen retrieval was used for 30 min at 97°C in the high-PH EnVision™ FLEX Target Retrieval Solution.
The antigen was localized by the addition of 3,3’diaminobenzidine tetrahydrochloride (DAB) substrate chromogen solution (Universal Detection Kit, Dako, Denmark). Finally, slides were counterstained with hematoxylin, dehydrated in alcohol and mounted.
For each setting, positive and negative control slides were included. As a negative control, bladder tissue was processed in the above mentioned sequences but the primary antibodies were not added and instead add non-immune immunoglobulin G (IgG; DAKO, Glostrup, Copenhagen, Denmark). Colonic mucosa known to express cyclin D1 used as positive control for cyclin D1. Human prostate cancer tissues were used as positive control for hnRNP-K antibody.
Interpretation of immunostaining and scoring analysis
Immunohistochemical analysis of bladder tissue sections was blind-quantified by two pathologists. The sections were examined by using light microscope [Scope A1, Axio, Zeiss, Germay]. Photomicrographs were taken using a microscope-camera [AxioCam, MRc5, Zeiss, Germany].
For Cyclin D1: The expression of cyclin D1 was measured in 10 successive high-power fields (x400). Cyclin D1 expressed as nuclear and cytoplasmic brown color. The immunostaining intensity was graded as negative = 0, weak = 1, moderate = 2, or strong = 3. The fraction of cells stained with cyclin D1 were counted as a percentage and scored (1 <15%, 2=15-50%, 3>50%) (Kopparapu et al., 2013).
For hnRNP-K: The nuclear immunostaining intensity was graded as negative = 0, weak = 1, moderate = 2, or strong = 3. The proportion of positively staining cells was assessed as a percentage. The score was then calculated as the intensity score multiplied by the percentage of cells stained (score = intensity X % of positive cells). The samples were classed as low (score <140) or high (score ≥140) hnRNP-K expression (Chen et al., 2017).
Statistical analyses
The immunohistochemical results were analyzed using SPSS version 20 (IBM Corporation, Armonk, New York, USA). Data are presented as the mean ± S.D. Two-tailed Student’s t-tests and one-way ANOVA were used to evaluate the data. Comparison of difference in percentage between groups was evaluated using two tailed Fischer’s exact test. Differences were considered statistically significant at P < 0.05.
Results
The mean age of different groups was studied statistically and proved by ANOVA test to be significant (p < 0.01). The vast majority of studied cases were males (77 cases) with female patients constitute only 27.36% of cases (29 patients). Age and sex distribution was listed in (Table 1).
All cases of SCC showed high tumor grade and positive muscle invasion, while most cases of UC were of low grade (65.2%) and showed negative muscle invasion (69,6%). The difference was statistically significant (p<0.001). Eighty percent of SCC cases were associated with bilharziasis, while only 4.3% of UC cases were positive for bilharziasis. The difference between groups was statistically significant (p<0.001) (Table 2).
Table 2.
Difference in Tumor Grade, Muscle Invasion and Bilharzial Association between UC and SCC groups
| Tumor Grade | Muscle Invasion | Bilharziasis | ||||
|---|---|---|---|---|---|---|
| Low | High | Negative | Positive | Negative | Positive | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| SCC (20) | 0 (0.0%) | 20 (100.0%) | 0 (0.0%) | 20 (100.0%) | 4 (20.0%) | 16 (80.0%) |
| UC (66) | 40 (60.61%) | 26 (39.39%) | 42 (63.64%) | 24 (36.36%) | 54 (81.82%) | 12 (18.18%) |
| Fisher’s exact test | p<0.0001 | p<0.0001 | p<0.0001 | |||
SCC, Squamous cell carcinoma; UC, Urothelial carcinoma; n, number of cases
Cyclin D1 Immunoexpression
UC showed higher parameters of cyclin D1 expression (percentage, intensity and score) compared to chronic cystitis, CIS and SCC groups, with statistically significant difference (p<0.001) (Table 3).
Table 3.
Difference in Expression Parameters of HnRNP-K and Cyclin D1 in Studied Bladder Lesions
| Diagnosis | Cyclin D1 | HnRNP-K | |||||
|---|---|---|---|---|---|---|---|
| (n) | percent | intensity | score | percent | intensity | score | |
| Cystitis | Mean | 0.5 | 1 | 0.5 | 29 | 1.8 | 53.5 |
| (10) | S.D. | 0.52 | 1.05 | 0.52 | 5.67 | 0.42 | 17.64 |
| CIS | Mean | 25.64 | 1.62 | 1.48 | 90 | 3 | 270 |
| (10) | S.D. | 17.36 | 0.42 | 0.46 | 5.27 | 0 | 15.81 |
| SCC | Mean | 7.85 | 1.4 | 0.85 | 78 | 2.2 | 171 |
| (20) | S.D. | 10.2 | 0.94 | 0.67 | 5.23 | 0.41 | 29.45 |
| UC | Mean | 47.12 | 1.98 | 2.27 | 66.66 | 2.28 | 163.18 |
| (66) | S.D. | 28.56 | 0.71 | 0.66 | 27.1 | 0.67 | 78.59 |
| p value (ANOVA) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
CIS, carcinoma insitu; SCC, squamous cell carcinoma; UC, urothelial carcinoma; S.D, standard deviation; n, number of cases.
UC of high grade, muscle invasion and non-papillary pattern showed significantly lower parameters of cyclin D1 expression compared to their counterparts. UC with bilharzial association show higher cyclin D1 percentage and intensity, while exhibit lower expression score compared to non-bilharzial cancers, however, no significant differences in cyclin D1 expression were achieved between bilharzial and non-bilharzial associated bladder cancer (Table 4).
Table 4.
Difference in Expression of Cyclin D1 and HnRNA Parameters in Urothelial Carcinoma (UC) in Relation to Tumor Grade, Muscle Invasion, Papillary Pattern and Bilharzial Association
| Parameter | Cyclin D1 | HnRNA | |||||
|---|---|---|---|---|---|---|---|
| (n) | percent | intensity | score | percent | intensity | score | |
| Tumor Grade | |||||||
| High grade | Mean | 35.1 | 1.81 | 1.84 | 80.75 | 2.51 | 202.13 |
| (26) | S.D | 29.41 | 0.83 | 0.91 | 10.85 | 0.5 | 46.55 |
| Low Grade | Mean | 50.62 | 2 | 2.37 | 25.12 | 1.5 | 45 |
| (40) | S.D. | 32.5 | 0.63 | 0.71 | 15.49 | 0.52 | 36.15 |
| p value (t-test) | N.S | N.S | <0.05 | <0.00 | <0.00 | <0.00 | |
| Muscle invasion | |||||||
| Negative | Mean | 69.29 | 2.86 | 2.71 | 43.33 | 1.83 | 98.33 |
| (42) | S.D | 20.46 | 0.36 | 0.47 | 29.88 | 0.7 | 90.87 |
| Positive | Mean | 36.88 | 1.63 | 2.06 | 79.35 | 2.44 | 190.8 |
| (24) | S.D. | 26.01 | 0.49 | 0.67 | 10.92 | 0.49 | 36.45 |
| p value (t-test) | < 0.001 | < 0.001 | < 0.01 | <0.00 | <0.00 | <0.00 | |
| Papillary pattern | |||||||
| Non-papillary | Mean | 37.33 | 1.6 | 2.13 | 82.5 | 2.6 | 213.12 |
| (38) | S.D | 27.85 | 0.49 | 0.73 | 13.17 | 0.49 | 47.35 |
| Papillary | Mean | 64.38 | 2.75 | 2.5 | 47.86 | 2.01 | 115.71 |
| (28) | S.D. | 20.89 | 0.45 | 0.52 | 29.42 | 0.77 | 92.23 |
| p value (t-test) | < 0.01 | < 0.001 | < 0.05 | <0.00 | <0.00 | <0.00 | |
| Bilharzial Association | |||||||
| Negative | Mean | 46.59 | 2.05 | 2.27 | 66.05 | 2.32 | 163.42 |
| (54) | S.D | 29.19 | 0.75 | 0.69 | 27.54 | 0.66 | 81.39 |
| Positive | Mean | 50.76 | 2.11 | 2.09 | 78.35 | 2.2 | 171.87 |
| (12) | S.D. | 0.46 | 0.19 | 0.01 | 5.37 | 0.42 | 30.26 |
| p value (t-test) | N.S. | N.S. | N.S. | <0.05 | N.S. | N.S. | |
S.D, standard deviation
HnRNP-K Immunohistochemistry
All cases of cystitis showed lower parameters of hnRNP-K expression compared to cases of CIS, UC, SCC with significant difference (p<0.001). On the contrary, CIS cases show significantly higher parameters of hnRNP-K expression compared to the other groups (p<0.001). SCC cases showed higher hnRNP-K percentage and score compared to UC (p<0.001) (Table 3).
UC cases with high grade, muscle invasion and non-papillary pattern showed high significant difference in all parameters of hnRNP-K expression compared to low grade, non-muscle invasive and papillary counterparts (p<0.001) (Table 4). UC associated with bilharziasis showed significantly higher percentage of hnRNP-K expression (p<0.05) and non-significant higher expression score (p>0.6) compared to non-bilharzial ones, however, they showed non-significant lower hnRNP-K intensity of expression compared to non-bilharzial cancers (p>0.05).
Discussion
In Egypt, bladder cancer accounts for about 30% of all cancers, where it is the most common malignancy in men and the second most common malignancy in women after breast cancer, and has been associated with many pathogenetic factors – most commonly bilharzial infestation, which is an endemic infection in the Nile River Valley (El-Mawla et al., 2001; El-Sebaie et al., 2005). Nonetheless, early diagnosis is critically important to alleviate morbidity and mortality rate, associated with recurrent disease and to meliorate the chances of a prosperous outcome (Ibrahim et al., 2014).
Overexpression of the cyclin D1 gene has been reported in many preneoplastic lesions and human tumors including bladder transitional cell carcinoma (Arber et al., 1996; Oya et al., 1998). Ohtsubo and Roberts (1993) and Quelle et al., (1993) found that alteration in cyclin D1 expression is an early event in bladder tumorigenesis. Cyclin D1 protein expression has been correlated with both poor prognosis (Lopez-Beltran et al., 2004a) and good prognosis (Lopez-Beltran et al., 2004b).
In the present study, cases of chronic cystitis and SCC showed respectively negative and lower scores of cyclin D1 expression compared to cases of UC. This is in accordance with Kopparapu et al., (2013) who stated that cyclin D1 protein expression is higher in UC versus the adjacent non-malignant bladder tissues.
Our results showed also that cyclin D1 expression significantly correlates with low-grade, low-stage and papillary tumor growth. This goes with Levidou et al., (2010); Kopparapu et al., (2013) and Khabaz et al., (2016) who found that high level of cyclin D1 immunoreactivity was more frequent in low grade and NMIBC, while high grade and advanced stage tumors, MIBC tumors and tumors with vascular invasion and lymph node involvement showed lower cyclin D1 score levels. The negative correlation of cyclin D1 expression with tumor grade and stage is in alignment with the experimental data indicating that cyclin D1 expression in urothelial tissue supports not only cell proliferation, but also cell differentiation and raises the hypothesis that cyclin D1 overexpression may play an important role in the early T-categories of bladder tumorigenesis, promoting the acquisition of autonomous growth properties (Proctor et al., 1991).
Nucleus is the ordinary location of cyclin D1 protein in normal cells. A report by Fristrup and colleagues showed that high cyclin D1 expression is predominantly nuclear in tumor cells from NMIBC (Ta/T1) UC, and that this nuclear cyclin D1 expression is associated with poor patient outcome (Fristrup et al., 2012). Controversially, Kopparapu et al., (2013) reported that absence of nuclear cyclin D1 expression in tumor cells was significantly associated with MIBC. Thus, cyclin D1 may have some value as a predictive marker for a sub-group of patients with UC.
No significant difference in cyclin D1 expression was achieved between bilharzial and non-bilharzial associated urothelial carcinoma in our study. This was in agreement with Hammam et al., (2009) who reported that cyclin D1 expression in non-bilharzial and bilharzial associated cancer was almost similar.
Aberrant overexpression of hnRNP-K has been reported in various cancers including those of the colon (Carpenter et al., 2006), lung (Tang et al., 2014), renal (Otoshi et al., 2015) and pancreas (Zhou et al., 2012). Several studies have concluded that hnRNP-K has been robustly involved in the development and the progression of cancer through various biological events including transcriptional regulation, translational control chromatin remodeling, and cellular signal transduction (Lu and Gao, 2016). However, to our knowledge, there is no other studies examined the behavior and expression of hnRNP-K in bilharzial-associated bladder cancer.
Our study detected that, all SCC (100%) and most UC (76.5%) cases show significantly high hnRNP-K expression score compared to cystitis cases. This goes with findings of Guo et al., (2012) who reported high hnRNP-K expression in hepatocellular carcinoma (HCC) tissue compared to the cirrhosis.
CIS shows the highest hnRNP-K expression; a finding that could indicate a critical role of this protein in initiation of bladder carcinogenesis.
We also examined the correlation between hnRNP-K expression and pathological grade and stage in bladder cancer specimens. The expression level of hnRNP-K was positively significantly correlated with higher grade and muscle invasive tumors. This goes with Chen et al., (2017) who reported higher hnRNP-K expression in poorly differentiated and muscle invasive bladder tumors as compared to well-differentiated and non-invasive tumors. This also can parallel findings in studies performed on other organs. Guo et al., (2012) found higher hnRNP-K expression in late compared to early HCC. Additionally, Ayham et al., (2009) found a significant decrease in nuclear hnRNP-K expression levels in primary colon tumor compared to colon cancer with lymph node metastasis. Otoshi et al., (2015) found a significant positive correlation between hnRNP-K staining score and tumor aggressiveness in renal cell carcinoma.
We also observed that the non-papillary bladder cancers exhibited significantly higher value of all parameters of hnRNP-K expression compared to the papillary variants. These findings may suggest that the relative score of hnRNP-K expression proportionate to the degree of invasiveness and aggressiveness of the tumor.
Interestingly, we found that bilharzial-associated bladder cancer showed higher percentage and score of hnRNP-K expression, but lower hnRNP-K expression intensity compared to non-bilharzial associated cases, however, these correlations were statistically insignificant. This is the first study that tries to evaluate the relation between bilharzial associated bladder cancer and expression of hnRNP-K.
In conclusion, the immunohistochemical findings of this study elucidated a possible contribution of cyclin D1 and hnRNP-K expression in the initiation and progression of urinary bladder carcinoma. The lower expression of cyclin D1 and hnRNP-K in cystitis lesions and its higher expression in bladder cancer lesions indicates that changes in these proteins levels represent early events during the development of bladder cancer. In addition, the patterns of cyclin D1 and hnRNP-K expression with tumor grade and stage suggests the involvement of these proteins in bladder cancer progression variably, so, both of them can be used in predicting progression of urinary bladder carcinoma and to differentiate between UC and SCC in high grade tumors. The possible role of both markers in immunotherapy deserves supplementary studies.
Figure 1.
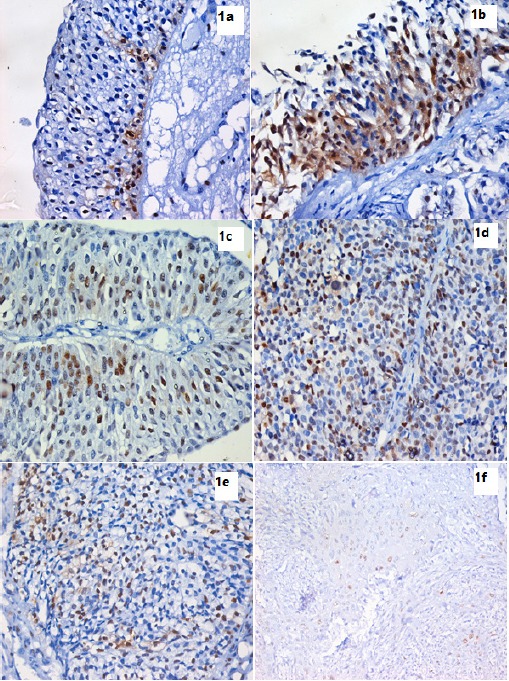
IHC Using Anti-Cyclin D1 Monoclonal Antibody and DAB in Bladder Tissue Sections Expressed as Brown Mostly Nuclear Staining. 1a. Full-thickness urothelium in case of chronic cystitis showing positive nuclear and cytoplasmic expression of cyclin D1 in the basal layer. (IHC stain for cyclin D1, X400). 1b. Full-thickness urothelium in case of CIS showing positive nuclear and cytoplasmic expression of cyclin D1 in all layers. (IHC stain for cyclin D1, X400). 1c. Low grade superficial papillary UC showing moderate positive nuclear expression of cyclin D1. (IHC stain for cyclin D1, X200). 1d. Higher grade of non muscle invasive papillary UC showing increased positive nuclear expression of cyclin D1 compared to previous photo. (IHC stain for cyclin D1, X400). 1e. Non papillary muscle invasive UC showing lower positive nuclear expression of cyclin D1 compared to non invasive UC. (IHC stain for cyclin D1, X400). 1f. Section in a case of invasive SCC showing weak positive nuclear expression of cyclin D1. (IHC stain for cyclin D1, X400).
Figure 2.
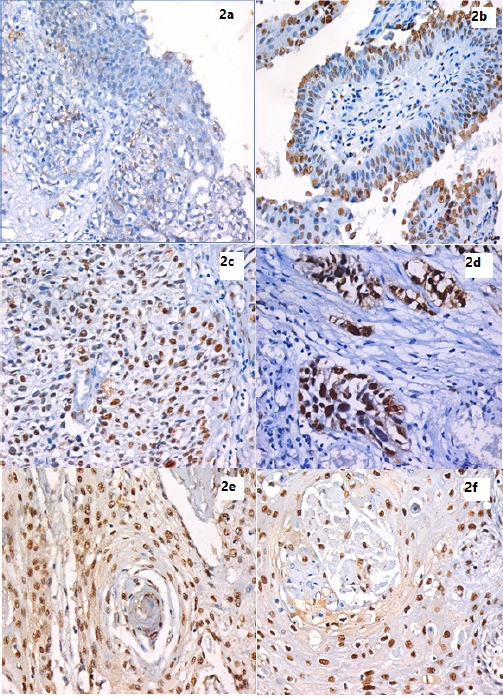
IHC Using Anti-hnRNP-K Monoclonal Antibody and DAB in Bladder Tissue Sections Expressed as Brown Nuclear Staining. 2a. Full-thickness urothelium in case of chronic cystitis showing mild positive nuclear expression of hnRNP-K scattered through all layers. (IHC stain for hnRNP-K, X400). 2b. A case of Low grade superficial papillary UC showing mild positive nuclear expression of hnRNP-K mostly in the superficial layer. (IHC stain for hnRNP-K, X200). 2c. High grade papillary UC showing higher hnRNP-K expression than previous low grade superficial tumor . (IHC stain for hnRNP-K, X400). 2d. High grade non-papillary, muscle invasive UC showing marked hnRNP-K expression. (IHC stain for hnRNP-K, X400). 2e. A case of Bilharzial-associated SCC, showing high hnRNP-K expression. (IHC stain for hnRNP-K, X400). 2f. A case of non-bilharzial-associated SCC, showing high hnRNP-K expression. (IHC stain for hnRNP-K, X400).
References
- Arber N, Hibshoosh H, Moss SF, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–74. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- Ayham A, Brian C, Colin T, Murray GI. Colorectal cancer:Immunohistochemical diagnosis with heterogeneous nuclear ribonucleoprotein K. Colorectal Cancer. 2009;4:25–42. [Google Scholar]
- Carpenter B, McKay M, Dundas SR, et al. Heterogeneous nuclear ribonucleoprotein K is overexpressed, aberrantly localised and is associated with poor prognosis in colorectal cancer. Br J Cancer. 2006;95:921–7. doi: 10.1038/sj.bjc.6603349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A, Chander P, Howe PH. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes:Focus on hnRNP E1's multifunctional regulatory roles. RNA. 2010;16:1449–62. doi: 10.1261/rna.2254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gu P, Xie R, et al. Heterogeneous nuclear ribonucleoprotein K is associated with poor prognosis and regulates proliferation and apoptosis in bladder cancer. J Cell Mol Med. 2017;21:1266–79. doi: 10.1111/jcmm.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh PX, Das A, Franco R, Pattnaik AK. Heterogeneous nuclear ribonucleoprotein K supports vesicular stomatitis virus replication by regulating cell survival and cellular gene expression. J Virol. 2013;87:10059–69. doi: 10.1128/JVI.01257-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mawla NG, El-Bolkainy MN, Khaled HM. Bladder cancer in Africa:update. Semin Oncol. 2001;28:174–8. doi: 10.1053/sonc.2001.21961. [DOI] [PubMed] [Google Scholar]
- El-Sebaie M, Zaghloul MS, Howard G, Mokhtar A. Squamous cell carcinoma of the bladder and nonbilharzial urinary bladder:a review of etiological features, natural history, and management. Int J Clin Oncol. 2005;10:20–5. doi: 10.1007/s10147-004-0457-6. [DOI] [PubMed] [Google Scholar]
- Fang Y, Cao Z, Hou Q, et al. Cyclin D1 downregulation contributes to anti-cancer effect of isorhapontigenin (ISO) on human bladder cancer cells. Mol Cancer Ther. 2013;12:1492–503. doi: 10.1158/1535-7163.MCT-12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristrup N, Birkenkamp-Demtroder K, Reinert T, et al. Multicenter validation of cyclin D1, MCM7, TRIM29, and UBE2C as prognostic protein markers in non-muscle invasive bladder cancer. Am J Pathol. 2012;180:1824–34. doi: 10.1016/j.ajpath.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Guo Y, Zhao J, Bi J, et al. Heterogeneous nuclear ribonucleoprotein K (hnRNP K) is a tissue biomarker for detection of early Hepatocellular carcinoma in patients with cirrhosis. J Hematol Oncol. 2012;5:37–46. doi: 10.1186/1756-8722-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammam O, El Ganzoury H, Abdel Hadi A, AkL M. Immunohistochemical study of caspase 3 and cyclin D1 in bilharzial bladder cancer and their significance. Aust J Basic Appl Sci. 2009;3:1198–205. [Google Scholar]
- Harb OA, Haggag R, Ali MM, et al. The prognostic role of NEDD9 and P38 protein expression levels in urinary bladder transitional cell carcinoma. J Oncol. 2017;2017:6095205. doi: 10.1155/2017/6095205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer incidence in Egypt:results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:1–18. doi: 10.1155/2014/437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isharwal S, Konety B. Non-muscle invasive bladder cancer risk stratification. Indian J Urol. 2015;31:289–96. doi: 10.4103/0970-1591.166445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabaz MN, Buhmeida A, Ghabrah T, et al. Cyclin D1 expression is associated with stage, grade and survival in urinary bladder carcinoma. Int J Clin Exp Med. 2016;9:23482–90. [Google Scholar]
- Kim JK, Diehl JA. Nuclear cyclin D1:An oncogenic driver in human cancer. J Cell Physiol. 2009;220:292–6. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopparapu PK, Boorjian SA, Robinson BD, et al. Expression of cyclin D1 and its association with disease characteristics in bladder cancer. Anticancer Res. 2013;33:5235–42. [PMC free article] [PubMed] [Google Scholar]
- Levidou G, Saetta AA, Karlou M, et al. D-type cyclins in superficial and muscle-invasive bladder urothelial carcinoma:correlation with clinicopathological data and prognostic significance. J Cancer Res Clin Oncol. 2010;136:1563–71. doi: 10.1007/s00432-010-0814-y. [DOI] [PubMed] [Google Scholar]
- Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, et al. Prognostic factors in stage T1 grade 3 bladder cancer survival:The role of G1-S modulators (p53, p21Waf1, p27kip1, Cyclin D1, and Cyclin D3) and proliferation index (ki67-MIB1) Eur Urol. 2004a;45:606–12. doi: 10.1016/j.eururo.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, et al. Prognostic factors in survival of patients with stage Ta and T1 bladder urothelial tumors:The role of G1-S modulators (p53, p21Waf1, p27Kip1, cyclin D1, and cyclin D3), proliferation index, and clinicopathologic parameters. Am J Clin Pathol. 2004b;122:444–52. doi: 10.1309/LTFU-3UUM-BY09-5HUM. [DOI] [PubMed] [Google Scholar]
- Lu J, Gao FH. Role and molecular mechanism of heterogeneous nuclear ribonucleoprotein K in tumor development and progression. Biomed Rep. 2016;4:657–63. doi: 10.3892/br.2016.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JW, Carballido EM, Ahmed A, et al. Squamous cell carcinoma of the urinary bladder. Systematic review of clinical characteristics and therapeutic approaches. Arab J Urol. 2016;14:183–91. doi: 10.1016/j.aju.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JJ, Bellmunt J, Bochner BH, et al. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012;62:523–33. doi: 10.1016/j.eururo.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Moch H, Humphrey PA, Ulbright TM, Reute VE. Tumors of the urinary tract in 'WHO classification of tumors of the urinary system and male genital organs'. 4th Edition. Lyon: Eds international agency for research on cancer; 2016. pp. 78–133. [Google Scholar]
- Moreira JM, Ohlsson G, Gromov P, et al. Bladder cancer-associated protein, a potential prognostic biomarker in human bladder cancer. Mol Cell Proteomics. 2010;9:161–77. doi: 10.1074/mcp.M900294-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargund VH, Tanabalan CK, Kabir MN. Management of non-muscle-invasive (superficial) bladder cancer. Semin Oncol. 2012;39:559–72. doi: 10.1053/j.seminoncol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M, Roberts JM. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–12. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- Otoshi T, Tanaka T, Morimoto K, Nakatani T. Cytoplasmic accumulation of heterogeneous nuclear ribonucleoprotein K strongly promotes tumor invasion in renal cell carcinoma cells. PLoS One. 2015;10:e0145769. doi: 10.1371/journal.pone.0145769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya M, Schmidt B, Schmitz-Drager BJ, Schul WA. Expression of G-S transition regulatory molecules in human urothelial cancer. Jpn J Cancer Res. 1998;89:719–26. doi: 10.1111/j.1349-7006.1998.tb03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor AJ, Coombs JM, Cairns JP, Knowles MA. Amplification at chromosome 11q13 in transitional cell tumours of the bladder. Oncogene. 1991;6:789–95. [PubMed] [Google Scholar]
- Proctor I, Stoeber K, Williams GH. Biomarkers in bladder cancer. Histopathology. 2010;57:1–13. doi: 10.1111/j.1365-2559.2010.03592.x. [DOI] [PubMed] [Google Scholar]
- Quelle DE, Ashmun RA, Shurtleff SA, et al. overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–71. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Reuter VE. The urothelial tract:renal pelvis, ureter, urinary bladder, and urethra. In: Mills SE, Carter D, Greenson JK, Reuter VE, Stoler MH, editors. Sternberg's Diagnostic Surgical Pathology. Philadelphia: Lippin¬cott Williams and Wilkins; 2010. pp. 1830–70. [Google Scholar]
- Sgambato A, Flamini G, Cittadini A, Weinstein B. Abnormalities in cell cycle control in cancer and their clinical implications. Tumori. 1998;84:421–33. doi: 10.1177/030089169808400401. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–63. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Tang F, Li W, Chen Y, et al. Downregulation of hnRNP K by RNAi inhibits growth of human lung carcinoma cells. Oncol Lett. 2014;7:1073–7. doi: 10.3892/ol.2014.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Veeratterapillay R, Heer R, Johnson MI, Persad R, Bach C. High-risk non-muscle-invasive bladder cancer-therapy options during intravesical BCG shortage. Curr Urol Rep. 2016;17:68–75. doi: 10.1007/s11934-016-0625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell GG, Keegan JM. Christian conciliation:An alternative to ordinary ADR. Cumb L Rev. 1999;29:583–89. [Google Scholar]
- Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics:mechanisms and lab approaches. Cancer Lett. 2014;347:159–66. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Yang R, Zeng Y, Xu H, et al. Heterogeneous nuclear ribonucleoprotein K is overexpressed and associated with poor prognosis in gastric cancer. Oncol Rep. 2016;36:929–35. doi: 10.3892/or.2016.4845. [DOI] [PubMed] [Google Scholar]
- Zhou R, Shanas R, Nelson MA, Bhattacharyya A, Shi J. Increased expression of the heterogeneous nuclear ribonucleoprotein K in pancreatic cancer and its association with the mutant p53. Int J Cancer. 2012;126:395–404. doi: 10.1002/ijc.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]


