Abstract
Background:
Apoptosis is suppressed in cancer tissues and tumor cell lines because anti-apoptosis genes are over- expressed. The inhibitor of apoptosis proteins (IAP) gene family contributes to control of apoptosis. The expression profile of eight genes of the IAP family in biopsies from patients with a history of bladder cancer and normal bladder tissues, as well as a bladder tumor cell line (5637), was assessed in the present study.
Methods:
Cancer tissue samples were obtained at surgery and the 5637 tumor cell line was cultured in RPMI1640 medium. Beyond tumor margins were selected as normal tissue. Expressional profile of interested genes was obtained by using specific primers and the real-time PCR method.
Results:
The results showed that expression of seven of the studied genes was up-regulated in cancer tissues and the cell line whereas BIRC4 (XIAP) was down-regulated in both.
Conclusions:
The results showed that these genes were expressed to a greater extent in cancer tissue and cancer cells than in normal tissues. The data suggested that over-expression of anti-apoptotic genes such as IAP family members, can trigger cells to escape from apoptosis.
Keywords: Bladder cancer, IAP gene family, tumor cell line
Introduction
Bladder cancer is the fourth most common malignancy in the United States and the fifth most common malignancy in Europe with an estimated 430,000 new cases diagnosed in 2012 (Antoni et al., 2017). The peak incidence of bladder cancer is in the fifth and seventh decade and tobacco smoking is the main risk factor for bladder cancer. Smokers are considered to have a 2.5 times higher risk for this cancer than the general population (Cumberbatch et al., 2016).
Bladder cancer divided into different types (based different cell types that are involved in cancer). One of the most common types is urothelial carcinoma or transitional cell carcinoma (TCC). Other types of bladder cancer include Squamous Cell Carcinoma and Aden carcinoma. TCC is more common in older people (The average age of diagnosis is 69 years). There exist two hypotheses about the origin cancer cells and according to the first theory, tissue cells, based ability of cancer, have been homogenized, this means that all of tissue cells, have the ability to induced cancer cell (Kujawa and Lisowska, 2015). The second theory provided according to the findings about normal stem cells and similarity that were seen by cancer cells. Based this hypothesis, tissue-forming cells in terms of ability to convert to cancer cell, have been heterogeneous. In the other words, all tissue cells are not able to form cancer cell. Certain cells known as adult stem cells (ASC) that are present in all tissues with very low frequency (one to one hundred thousand cells), or somatic cells with the changes in gene expression (reprogramming) of certain genes, have earned the ability to divide indefinitely (immortalized), are the origin of the cancer (Chang et al., 2012). Accordingly, the normal stem cells in the tissues, the occurred changes in gene expression profile (that are not yet well known) or disrupt the paths of differentiation, are origin of cancer. The find of cellular and molecular mechanisms that trigger primary cells (Progenitor) to create a cancer stem cell (CSC), could be helpful in identify of diagnose, treat and prevent of cancer. Studied showed that escape from apoptosis is the first alteration in the cells that move toward establish a cancer colony. There are different gene families related to apoptosis pathway such as extrinsic or intrinsic apoptosis pathway (Hosseini et al., 2017; Karimabad et al., 2017a). One of these is IAP gene family.
Inhibitors of apoptosis proteins (IAPs) are a conserved family of proteins identified in species ranging from virus, yeasts, nematodes, fishes, flies and mammals. The common structural feature is the presence of at least one Baculovirus IAP Repeat (BIR) domain. Hence, IAPs are also known as BIR-containing proteins (BIRCs). Most of them display anti-apoptotic properties when over-expressed (Dubrez-Daloz et al., 2008).
IAP gene family can play an important role in inhibiting apoptosis by exerting their negative action on caspases (apoptotic proteins). There are eight proteins in this family: NAIP/BIRC1/NLRB, cellular IAP1 (cIAP1)/human IAP2/BIRC2, cellular IAP2 (cIAP2)/human IAP1/BIRC3, X-linked IAP (XIAP)/BIRC4, survivin/BIRC5, baculoviral IAP repeat (BIR)-containing ubiquitin-conjugating enzyme/apollon/BIRC6, livin/melanoma-IAP (ML-IAP)/BIRC7/KIAP, and testis-specific IAP (Ts-IAP)/hILP-2/BIRC8 (Saleem et al., 2013; Altieri, 2010).
IAPs each contain between one and three regions called baculovirus inhibitor repeat (BIR) domains, which attach (bind) to particular caspases. Some IAPs also have a region called a RING domain (Altieri, 2010). The RING domain, in conjunction with other enzymes, can label target proteins with ubiquitins. This labeling can mark the target protein for destruction by a cellular ubiquitin-proteasome mechanism (Obexer and Ausserlechner, 2014). Genes in the IAP family provide instructions for making inhibitors of apoptosis by help to protect cells from self-destructing (undergoing apoptosis) by blocking (inhibiting) the action of caspases, which are necessary for apoptosis (Byers et al., 2016).
Based the hypotheses about the origin of cancer (specially cancer stem cell hypothesis), it would be presumption an identical gene expression profile of gene families related to apoptosis pathway in stem cells (cancer cell lines) and cancer cell tissues. So, the aim of this study was to compare the expression pattern of IAP gene family member, in tumour cell lines, cancer and healthy tissue of bladder cancer.
Materials and Methods
5637 tumor cell line
The bladder cancer cell line (5637) was purchased from Pasteur Institute of Iran. The cells were cultured in RPMI 1,640 containing 10% fetal calf serum, penicillin and streptomycin antibiotics and incubated at 37 °C with 5% CO2 and 90% humidity. Initially, about 2×106 cells were cultured when the cells occupied 70–80% of the flask, the cells were harvested and used for RNA extraction.
Cancer biopsy tissue
In the present study, five biopsies from patients with a history of bladder cancer who referred to the Ali Ebne Abi Talib hospital in Rafsanjan were collected in sterile and free RNase tubes and stored in nitrogen tank.
RNA extraction and cDNA synthesis
Total RNA were extracted from 5637 tumor cell line, cancer tissues and normal bladder tissues using total RNA extraction Kit (Bioneer, Korea). RNA preparations were also analyzed by agarose gel electrophoresis and with a spectrophotometer, respectively. Then, total RNA was used for cDNA synthesis using the cDNA PCR reverse transcription kit (Bioneer, Korea) according to the manufacturer’s instructions. The synthesized cDNA was stored at -20 °C until use.
Primer design
Forward and reverse primers of studied genes, BIRC1-8 and β-actin (as internal housekeeping control gene) were designed with version 3 design primer software and then control at NCBI Blast software (Table 1).
Table 1.
Sequence of BIRC1-8 (IAP Family Gene) and ß-Actin Genes Primers
| Target genes | Designed Oligo | Relative Sequence 5’→ 3’ | Fragment length |
|---|---|---|---|
| BIRC1 | F | GCCTTAAGCCTAGCAGTCT | 84 |
| R | GCCTAGAACTTACGGATCC | ||
| BIRC2 | F | ACGGATTCAGATGCCATAGTC | 122 |
| R | GGATCATCAGTTACGGATTA | ||
| BIRC3 | F | GCTTAAGTACGGATGCC | 74 |
| R | GCCATAGCAGTCCAGTC | ||
| BIRC4 | F | AGTCCGTAAGTAACCGTAGC | 142 |
| R | GCATGGACCTAGCAGTAGC | ||
| BIRC5 | F | GCATGAAGTCAGTCAATACGT | 146 |
| R | ACCGTACGTAGGTCCAGTTAC | ||
| BIRC6 | F | AGAGAGGAGGTTCCATCTCTGGC | 116 |
| R | GGGACTAGGTACCCATAAGT | ||
| BIRC7 | F | GAAATCTGGTCCTAGCATGG | 98 |
| R | AGCATTGACTGATGGCATG | ||
| BIRC8 | F | GACCTAGTTAACGTACGTAG | 152 |
| R | AAGTCCATGCAATCGATAGTC | ||
| ß-actin | F | CACACCTTCTACAATGAGC | 160 |
| R | ATAGCACAGCCTGGATAG |
Amplification of the desired genes
To detect gene expression rate of studied genes (mRNA expression), quantitative Real-Time PCR (ABI PLUS ONE, Life Technologies, Grand Island, NY, USA) were used. Briefly, 4 µL of specific primers (forward and Reverse), 3 µL of cDNA, 10 µL of prime Q-Master Mix with SYBR Green I GeNet Bio (Chungnam, Korea) and 3 µL DNase free water (final volume of 20 µL) was added to each well of the PCR plate and covered with special tape to prevent evaporation. The Real-time reaction was reproduced with the Company’s proposed synthesis of primers (one cycle of 95 °C for 30 seconds and 45 cycles with the conditions of 95 °C for 10 seconds, 58-62 °C for 20 seconds, and 72 ° C for 30 seconds). Charts and data devices (numbers ct) were analyzed and evaluated; β-actin gene was used as an internal control.
Results
Expression pattern of IAP gene family
Expression profile of eight genes from IAP gene family in 5637 tumor cell line, bladder cancer tissues and bladder normal tissue cells are showed in Figure 1. Expression of seven genes including, BIRC1 (NAIP (, BIRC2 (IAP1 (, BIRC3) IAP2 (, BIRC5) Survivian (, BIRC6 (Apollon/Bruce (, BIRC7 (ML-IAP (and BIRC8) ILP-2 (were up-regulated and expression rate of BIRC4) XIAP (were down-regulated in 5637 tumor cell line and bladder cancer tissues in compared to bladder normal tissues.
Figure 1.
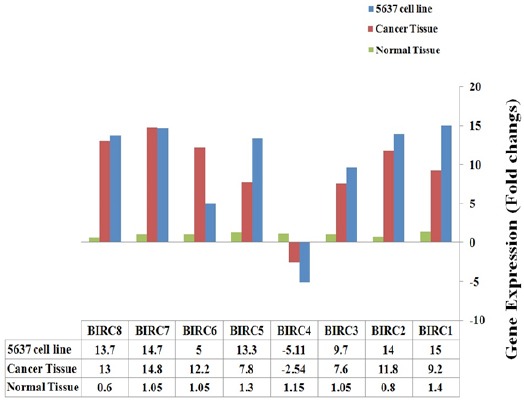
Expressional Profile of IAP Gene Family Members in 5637 Tumor Cell Line and Bladder Cancer Tissue Compared to Normal Tissue of Bladder
Discussion
Our data revealed that IAP gene family were up-regulated in both tumor cell line and cancer tissues of bladder in compared to normal healthy cells of bladder. IAP gene family contains at least eight genes that acts as ani-apoptotic potency (Silke and Vucic, 2013). Apoptosis is one of the critical cell pathway that help tissues to remove the invasive cells such as cancer cells (Sheikhrezaei et al., 2018). Apoptosis pathway regulated by a lot of genes classified in twelve category, one of this gene families named IAP gene family (Schultz and Harringto, 2003). As showed in previous studies apoptosis were supported in tumor cell lines and cancer tissue cells (Mirzaei et al., 2014a; Ramezani et al., 2017; Mirzaei et al., 2014b).
Krepela et al., (2009) Showed IAP gene family were expressed by high level in non-small cell lung carcinoma (NSCLC) cell lines and NSCLC (Non-small cell lung cancer) tumors. The previous study showed that expression profile of IAP gene family in some of human tumor cell lines such as HeLa, KB, HSC-3, H357, H376 and H413, and observed high level expression of mentioned genes (Konopka et al., 2008). Chen and co-workers Detected expression level of IAP gene family in Non-muscle invasive bladder cancer (NMIBC) samples from bladder patients and observed over-expression of IAP gene family in both mRNA and protein level (Chen et al., 2013). Deregulation of these inhibitors of apoptotic proteins (IAPs) may push cell toward cancer and neurodegenerative disorders. Inhibitors of apoptotic proteins (IAPs) may provide new target for anticancer therapy. Drugs may be developed that are inhibiting these IAPs to induce apoptosis in cancerous cells (Saleem et al., 2013). As our data revealed, seven of eight IAP gene family members were up-regulated but BIRC4 (XIAP) showed down-regulation status (Figure 1). Mutation in XIAP/BIRC4 gene caused a syndrome named X-linked lympho-proliferative syndromes (XLP) a rare primary immunodeficiency disease. X-linked inhibitor of apoptosis (XIAP) or BIRC4 gene encodes a protein that functions through binding to tumor necrosis factor receptor-associated factors (TRAF) and suppress apoptosis (Piacentino et al., 2012). In mammals, BIRC4/XIAP, the most studied IAP member can directly inhibit the activity of caspase-3, 7 and 9. However, this activity is not conserved in other IAPs and physiological relevancies of such anti-caspase activities are still discussed (Dubrez-Daloz et al., 2008).
Interestingly, more studies showed in cancer cell line and cancer tissues, BIRC4 were over-expresed (Liston et al., 2003), but in our study BIRC4 was the only gene that showed down-expressed. Because BIRC4 expression is same as all specimens and so 5637 tumor cell line and Real-Time PCR reaction repeated tree times, we suggest for approve the results we need to detect AIP gene family member expression in more tumor cell line as well as cancer tissue specimens.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This project was financially supported by the Rafsanjan University of Medical Sciences.
References
- Altieri DC. Survivin and IAP proteins in cell-death mechanisms. Biochem J. 2010;430:199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality:a global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Byers NM, Vandergaast RL, Friesen PD. Baculovirus inhibitor-of-apoptosis Op-IAP3 blocks apoptosis by interaction with and stabilization of a host insect cellular IAP. J Virol. 2016;90:533–44. doi: 10.1128/JVI.02320-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LY, Lin YC, Mahalingam J, et al. Tumor-derived chemokine CCL5 enhances TGF- β–mediated killing of CD8+T cells in colon cancer by T-regulatory cells. Cancer Res. 2012;72:1092–1102. doi: 10.1158/0008-5472.CAN-11-2493. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang T, Yang D, et al. Expression of the IAP protein family acts cooperatively to predict prognosis in human bladder cancer patients. Oncol Lett. 2013;5:1278–84. doi: 10.3892/ol.2013.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch MG, Rota M, Catto JW, La VC. The role of tobacco smoke in bladder and kidney carcinogenesis:A comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 2016;70:458–66. doi: 10.1016/j.eururo.2015.06.042. [DOI] [PubMed] [Google Scholar]
- Dubrez-Daloz L, Dupoux A, Cartier J. IAPs:more than just inhibitors of apoptosis proteins. Cell cycle. 2008;7:1036–46. doi: 10.4161/cc.7.8.5783. [DOI] [PubMed] [Google Scholar]
- Hosseini FS, Falahati-pour SK, Hajizadeh MR, et al. Persian shallot, Allium hirtifolium Boiss, induced apoptosis in human hepatocellular carcinoma cells. Cytotechnology. 2017;69:551–63. doi: 10.1007/s10616-017-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimabad MN, Mahmoodi M, Jafarzadeh A, et al. The novel Indole-3-formaldehyde (2-AITFEI-3-F) is involved in processes of apoptosis induction? Life Sci. 2017;181:31–44. doi: 10.1016/j.lfs.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Konopka K, Spain C, Yen A, et al. Correlation between the levels of survivin and survivin promoter-driven gene expression in cancer and non-cancer cells. Cell Mol Biol Lett. 2008;14:70. doi: 10.2478/s11658-008-0034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krepela E, Dankova P, Moravcikova E, et al. Increased expression of inhibitor of apoptosis proteins, survivin and XIAP, in non-small cell lung carcinoma. Int J Oncol. 2009;35:1449–62. doi: 10.3892/ijo_00000464. [DOI] [PubMed] [Google Scholar]
- Kujawa KA, Lisowska KM. Ovarian cancer–from biology to clinic. Postep Hig Med Dosw. 2015;1:1275–90. doi: 10.5604/17322693.1184451. [DOI] [PubMed] [Google Scholar]
- Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis:there is more to life than Bcl2. Oncogene. 2003;22:8568–80. doi: 10.1038/sj.onc.1207101. [DOI] [PubMed] [Google Scholar]
- Mirzaei MR, Hassanshahi G, Mahmoodi M, et al. RNA-interference-mediated silencing of OCT4B1, alters expression profile of several TNF ligand/Receptor transcripts in human tumor cell lines. Pharm Sci. 2014a;20:114. [Google Scholar]
- Mirzaei MR, Najafi A, Arababadi MK, Asadi MH, Mowla SJ. Altered expression of apoptotic genes in response to OCT4B1 suppression in human tumor cell lines. Tumor Biol. 2014b;35:9999–10009. doi: 10.1007/s13277-014-2238-9. [DOI] [PubMed] [Google Scholar]
- Obexer P, Ausserlechner MJ. X-linked inhibitor of apoptosis protein-a critical death resistance regulator and therapeutic target for personalized cancer therapy. Front Oncol. 2014;4:197. doi: 10.3389/fonc.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentino V, Milano CA, Bolanos M, et al. X-linked inhibitor of apoptosis protein-mediated attenuation of apoptosis, using a novel cardiac-enhanced adeno-associated viral vector. Hum Gene Ther. 2012;23:635–46. doi: 10.1089/hum.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani M, Ramezani M, Hassanshahi G, et al. Does the novel class of (2R, 4S)-N-(2, 5-Difluorophenyl)-4-Hydroxy-1-(2, 2, 2-Trifluoroacetyl) Pyrrolidine-2-Carboxamide's have any effect on cell viability and apoptosis of human hepatocellular carcinoma cells? Int J Cancer Manag. 2017;10:e8413. [Google Scholar]
- Saleem M, Qadir MI, Perveen N, et al. Inhibitors of apoptotic proteins:new targets for anticancer therapy. Chem Biol Drug Des. 2013;82:243–51. doi: 10.1111/cbdd.12176. [DOI] [PubMed] [Google Scholar]
- Schultz DR, Harringto WJ. Apoptosis:programmed cell death at a molecular level. Semin Arthritis Rheum. 2003;32:345–369. doi: 10.1053/sarh.2003.50005. [DOI] [PubMed] [Google Scholar]
- Sengupta N, Siddiqui E, Mumtaz FH. Cancers of the bladder. J R Soc Promot Health. 2004;124:228–9. doi: 10.1177/146642400412400520. [DOI] [PubMed] [Google Scholar]
- Sheikhrezaei Z, Heydari P, Farsinezhad A, et al. A new indole derivative decreased SALL4 gene expression in acute promyelocytic leukemia cell line (NB4) Iran Biomed J. 2018;22:99–106. doi: 10.22034/ibj.22.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silke J, Vucic D. IAP family of cell death and signaling regulators. Methods Enzymol. 2013;545:35–65. doi: 10.1016/B978-0-12-801430-1.00002-0. [DOI] [PubMed] [Google Scholar]


