Abstract
Objective:
This study was carried out to investigate the antimigration activity of Zanthoxylum acanthopodium DC. in the 4T1 breast cancer cell line.
Methods:
Zanthoxylum acanthopodium DC. fruit powder was extracted by maceration method with n-hexane and ethylacetate solvents. Cytotoxicity and proliferation were assessed using the MTT method and the cell cycle by flow cytometry. In addition, wound healing assays were conducted by a microscopic method, and expression of COX-2 and VEGFR-2 were determined using qRT-PCR.
Results:
The IC50 of the ethylacetate fraction (EAF) was 48.1 ± 1.06 µg/mL. The EAE at a concentration 10 µg/mL with viable cells was 62.3 ± 0.28% after 72 h incubation, with accumulation in the G2-M phase, inhibition of cell migration in the wound healing assay, and decrease in expression of COX-2 (0.62 ± 0.01) and VEGFR-2 (0.39 ± 0.003).
Conclusion:
The results reveal that an ethylacetate fraction of Zanthoxylum acanthopodium DC. fruits provides effective antimigration effects. Further studies are now planned to assess the potential of the ethylacetate fraction to inhibit angiogenesis in breast cancer and determine underlying mechanisms.
Keywords: Antimigration, Zanthoxylum acanthopodium DC. fruits, ethylacetate
Introduction
Cancer cells migration is necessary for tumor development. The spread of cancer in our body is a multistep phenomenon which cancer cells invade surrounding tissues and blood or lymphatic vessel (Lirdprapamongkol et al., 2008; Mao et al., 2016). All solid tumours are dependent on angiogenesis for growth and metastasis. A recent study reported that breast cancer is leading in the estimated new cancer cases, and the second most common death cause of women suffering from cancer in the USA (Siegel et al., 2015).
Traditionally, andaliman fruits (Zanthoxylum acanthopodium DC.) has been used as aromaticum substances, tonicum, and treat dysentery. Indian people have used andaliman to treat paralyzed and skin disease such as abscess and leprosy. Andaliman has been used as spices at North Sumatera especially at North Tapanuli (Suryanto et al., 2004; Hynniewta and Kumar, 2008; Sirait et al., 2001). The plants from Zanthoxylum genus contain many compounds such as phenol hydroquinones, flavonoids, steroids/ triterpenoids, tannins, glycosides, volatile oils, alkaloids, coumarines, lignans, amides and terpenes (Parhusip, 2006; Fernandez et al., 2009; Yao-Kuassi et al., 2015; Hu et al., 2006; Hu et al., 2014; Yang et al., 2004; Cui et al., 2008; Chen et al., 2015). Ethylacetate extract of andaliman fruits (EAF) was showed to have cytotoxicity effect against MCF-7 and T47D cell lines. EAF was found to have synergistic effect when combined with doxorubicin. EAF was showed to have anticancer activity towards mices induced with benzo (a) pyrene, having cardioprotective effect and active on T47D resistance cells (Sihotang, 2015; Anggraeni et al., 2014; Hasibuan et al., 2016). However, the antimigration activity EAF of Zanthoxylum acanthopodium DC. have yet to be elucidated.
The aim of this study was to determine cytotoxic and migration inhibition activity of ethylacetate fraction of Zanthoxylum acanthopodium DC. fruits on 4T1 cells.
Materials and Methods
Fractions Preparation
Fresh fruits of Zanthoxylum acanthopodium DC. was collected from Onan Rungu village, Samosir regency, Sumatera Utara Province, Indonesia. The air-dried and powdered fruits of Zanthoxylum acanthopodium DC. (1 kg) were repeatedly extracted by cold maceration with n-hexane (3x3 d, 7.5 L). The powder was dried in the air and extracted with ethylacetate (3x3 d, 7.5 L) at room temperature on a shake. The filtrate was collected, and then evaporated under reduced pressure to give a viscous extract and then freeze dried to give a dried extract (Anggraeni et al., 2014; Hasibuan et al., 2016; Satria et al., 2015; Hasibuan et al., 2015).
Cytotoxicity assay
EAF were submitted for cytotoxicity test. In that way, 4T1 cell line was grown in DMEM medium containing 10% Fetal Bovine Serum (Gibco), 1% penicillin-streptomycine (Gibco), and fungizone 0.5% (Gibco) in a flask in a humidified atmosphere (5% CO2) at 37 °C. The inoculums seeded at 1 x 104 cells/mL at an optimal volume of 0.1 mL per well. After 24 h incubation, the medium was discharged and treated by fractions. After incubation for 24 h, the cells were incubated with 0.5 mg/mL MTT for 4 h at 37 °C. Viable cells reacted with MTT to produce purple formazan crystals. After 4 h, SDS 10% as stopper (Sigma) in 0.01N HCl (Merck) was added to dissolve the formazan crystals. The cells were incubated for 24 h in room temperature and protected from light. After incubation, the cells were shaken, and absorbance was measured using ELISA reader at λ 595 nm. The data which were absorbed from each well were converted to percentage of viable cells (Hasibuan et al., 2015; Satria et al., 2014; Nurrochmad et al., 2014).
Cell cycle inhibition assay
4T1 cells (5x105 cells/well) were seeded into 6-well plate and incubated for 24 h. After that, the cells were treated with EAF and then incubated for 24 h. Both floating and adherent cells were collected in conical tube using tripsin 0.025%. The cells were washed thrice with cold PBS and centrifuged at 2500 rpm for 5 min. The supernatant was separated, while the sediment was collected and fixed in cold 70% ethanol in PBS at 4 °C for 1 h. The cells were washed thrice with cold PBS and resuspended then centrifuged at 3000 rpm for 3 min and PI kit (containing PI 40 µg/mL and RNAse 100 µg/mL) added to sediment and resuspended and incubated at 37 °C for 30 min. The samples were analysed using FACScan flow cytometer. Based on DNA content, percentage of cells in each of stage in cell cycle (G1, S and G2/M) were calculated using ModFit Lt. 3.0.s (Anggraeni et al., 2015; Satria et al., 2015; Satria et al., 2017).
Antiproliferative Activity
EAF (10 µg/mL) was submitted for antiproliferative test. In that way, 4T1 cell line (2.5 x 103cells/mL) was grown in DMEM complete medium. After 24; 48 and 72 h treatment, MTT assay was performed and cell viability was counted to calculate the antiproliferative activity (Zihlif et al., 2013).
Wound Healing Migration Assay
The migration assay was carried out with 4T1 cells were seeded at 5x104 cells/well in 24-well plates and incubated for 24 h at 37°C. Cultured cells were washed with PBS and added culture media which contining 0.5% FBS and incubated for 24 h. Scratch was done in the bottom center of the well within cell layer using yellow tip. Residues cell in the plate were washed with PBS and treated with EAF and incubated for 72 h at 37°C and documented under inverted microscope against cell migration rapidity after 0, 24, 48 and 72 h. The space from scratch treatment between control and treatment cultur cell was quantified using Image J software and defined as cell migration area (Zihlif et al., 2013; Wang et al., 2012).
Expression of COX-2 and VEGFR-2
4T1 cells (5x105 cells/well) were seeded into 6-well plate and incubated for 24 h. After that, the cells were treated with EAF and then incubated for 24 h. Both floating and adherent cells were collected in conical tube using tripsin 0.025%. The cells were washed thrice with cold PBS and centrifuged at 2500 rpm for 5 min. The supernatant was separated and used for RNA extraction (Genaid, USA) and RNA concentration was determined by spectrophotometric method (Nanodrop) and stored at -80°C until used. Complementary DNA (cDNA) was synthesized from 3.0 µg total RNA using RT-PCR kit (Toyobo, Japan) in a final volume of 20 µL using random primers based on the manufacturer’s instructions. qRT-PCR was carried out in AB 7500 Fast (ABI, USA). The reaction mixture consisted of SensiFASTTM SYBR®Lo-ROX kit (10 µL) (Bioline, USA), 1.0 µL of cDNA and 0.8 µL primers in a total volume 19 µL. β-actin was used as internal reference control. The PCR primers were used for β-actin (F: 5’-gtc gta cca ctg gca ttg t-3’; R: 5’-cag ctg tgg tga agc t-3’), Cox-2 (F: 5’-cca gca ctt cac gca tca gt-3’; R: 5’-acg ctg tct agc cag agt ttc ag-3’) and VEGFR-2 (F: 5’-gtg tca gaa tcc ctg cga agt a-3’; R: 5’-gaa atg gga ttg gta agg atg-3’). The PCR condition were comprised of first incubation at 95°C for 10 minutes, 40 cycles of denaturation at 95°C for 15 sec, annealing/ extension at 60°C 30 sec. Fluoresence was recorded at the end of extension. A negative control (NTC) was run simultaneously with every assay. Quantification of expression was determined by relative method using cycle threshold value (Zihlif et al., 2013; Abdolmaleki et al., 2016; Wang et al., 2012).
Statistical Analysis
The results were presented as means ± SD. The statistical analysis was carried out by using SPSS edition 21.
Results
Inhibitory Concentration 50% (IC50)
MTT method was used to determine cell viability after incubation for 24 h (Figure 1). In every treatment EAF was shown to inhibit cells growth. The IC50 value of EAF was 48.06 ± 1.06 µg/mL.
Figure 1.
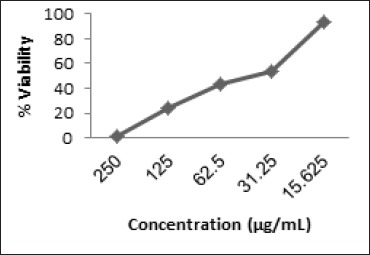
Percentage of Viable Cells VS Concentration of EAF
Effect on Cell Cycle
To evaluate the effect of EAF to increase cell death by modulating cell cycle, we concentrated on it for further studies using flow cytometry method. The effect of EAF is given in Figure 2. Whereas treatment of EAF in 10 µg/mL caused cell accumulation at G2/M phase (38.90%) and for control cell (17.70%).
Figure 2.
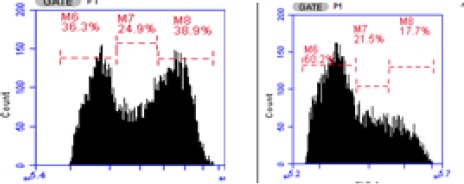
Percentage Phases on Cell Cycle of 4T1 Cells were Treated by (a) Control Cells (b) EAF 10 µg/mL
Antiproliferative Activity
To evaluate the effect of EAF to decrease the number of cells by inhibiting cell proliferation. The percentage of viable cells after treatment and incubation for 24, 48 and 72 h (54.08 ± 1.16; 59.88 ± 0.24 and 62.26 ± 0.28) showed the inhibition effect of EAF towards proliferation of 4T1 cells. The effect of EAF is given in Figure 3.
Figure 3.
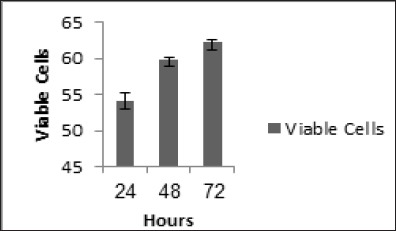
Percentage of Viable Cells of 4T1 Cells were Treated by EAF 10 µg/mL for 24; 48 and 72 h and Measured Viable Cells
Wound Healing Migration Assay
The scratch wound healing assay was performed to evaluate the effect of EAF on 4T1 migration. The wound healing migration of EAF is given in Figure 4. A little wound repair was observed in wells with EAF at 10 µg/mL after 24; 48 and 72 h incubation with 10.23 ± 1.49%; 47.46 ± 1.46% and 64.15 ± 1.13% respectively closure area.
Figure 4.
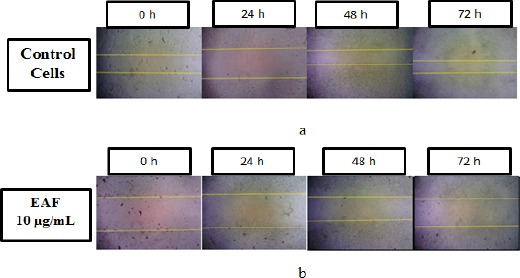
Wound Healing Migration Assay. 4T1 cells were treated by EAF for 24; 48 and 72 h and measured the closure area. (a) control cells; (b) EAF 10 µg/mL. EAF extended migration of 4T1 cells.
COX-2 and VEGFR-2 Expression
Two steps qRT-PCR were used to evaluated COX-2 and VEGFR-2 expression in 4T1 cells after the treatment with EAF. EAF were showed a significant down-regulatory effect on the expression of COX-2 (0.62 ± 0.01) and VEGFR-2 (0.39 ± 0.003) after treatment at 10 µg/mL. The inhibition of EAF towards COX-2 and VEGFR-2 expression are given in Figure 5.
Figure 5.
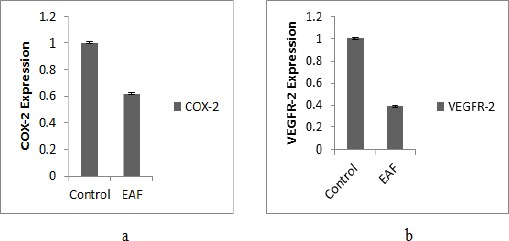
Gene Expression after Treatment with EAF 10 µg/mL. (a) COX-2 expression; (b) VEGFR-2 expression
Discussion
The cytotoxicity estimate of natural product is related to content of active compound in these plants including Zanthoxylum acanthopodium DC. Flavonoids, alkaloids and triterpenoids/steroids estimated as active compounds (Yadav et al., 2010). Other phytochemicals such as resveratrol, salvianolic acid B, and ginseng saponins were found to exert inhibitory effect on the vascularization and migration (Fan et al., 2006). This fact was to indicate that EAF can inhibite cell grow that G2/M. EAF were contained polyphenol compound such as flavonoids and tannins (Satria et al., 2015). The isolated polyphenols from plants including kaemferol, quercetin, anthocyanins, coumarin acid, and ellagic acid were shown to inhibit the growth (inhibit cell cycle and induce apoptosis) of human breast (MCF-7), oral (KB, Cal-27), colon (HT-29, HCT-116), and prostate (LNCaP, DU-145) tumor cell lines (Zhang et al., 2008; Daminiaki et al., 2000; Lim et al., 2006; Tang et al., 2007). VEGFR-2 is a transmembrane receptor that plays an important role in endothelial cell development (Risau, 1997; Shalaby et al., 1995) and is thought to mediate the key effect of the endothelial-specific mitogen VEGF on cell proliferation and permeability. Therefore, the majority of VEGFR-2 actions are related to angiogenesis and migration (Ferrara et al., 2003; Shibuya and Claesson, 2006). VEGFR-2 receptors and VEGFR-2 mRNA are largely expressed in breast cancer (Aesoy et al., 2008; Svensson et al., 2005; Weigand et al., 2005; Carino et al., 2008). Cyclooxygenase-2 (COX-2) is an inducible enzyme which plays a critical role in multiple pathophysiological process including inflammation, atherosclerosis, tissue injury, angiogenesis and tumorigenesis (Howe 2007; Sinicrope and Gill, 2004; Singh and Lucci, 2002; Castellone et al., 2005). Flavonoids such as quercetin, genistein kaemferol inhibited expression of VEGFR-2 and COX-2 which mediated angiogenesis and migration in human breast cancer cells (Xiao et al., 2011). Based on the results and discussion that EAF of Zanthoxylum acanthopodium DC. has antimigration activity through inhibition of cell cycle on G2/M phase, wound healing assay, proliferation of cells, and expression of Cox-2 and VEGFR-2.
Acknowledgements
We gratefully thank to Research Center University of Sumatera Utara through Hibah Talenta “Hibah Penelitian Unggulan Universitas” Research Grant 2017 “No: 5338/UN5.1.R/PPM/2017” for financial support in the study.
References
- Abdolmaleki Z, Arab HA, Amanpour S, Muhammadnejad S. Anti-angiogenic effects of ethanolic extract of Artemisia sieberi compared to its active substance Artemisinin. Rev Bras Farmacogn. 2016;26:326–33. [Google Scholar]
- Aesoy R, Sanchez BC, Norum JH, et al. An autocrien VEGF/VEGFR2 and p38 signaling loop confers resistance to 4-hydroxytamoxifen in MCF-7 breast cancer cells. Mol Cancer Res. 2008;6:1630–8. doi: 10.1158/1541-7786.MCR-07-2172. [DOI] [PubMed] [Google Scholar]
- Anggraeni R, Hadisahputra S, Silalahi J, Satria D. Combinational effects of ethylacetate extract of Zanthoxylum acanthopodium DC with doxorubicin on T47D breast cancer cells. Int J PharmTech Res. 2014;6:2032–5. [Google Scholar]
- Carino C, Olawaiye AB, Cherfils S, et al. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int J Cancer. 2008;123:2782–90. doi: 10.1002/ijc.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Yang CK, Kuo YH, et al. New coumarin derivatives and other constituents from the stem bark of Zanthoxylum avicennae:Effects of neutrophil pro-inflammatory responses. Int J Mol Sci. 2015;16:9719–31. doi: 10.3390/ijms16059719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XG, Zhao QJ, Chen QL, et al. Two new benzophenantridine alkaloids from Zanthoxylum nitidum. Helvetica Chemica Acta. 2008;91:155–8. [Google Scholar]
- Daminiaki A, Bakogeorgou E, Kampa M, Notas G, Hatzoglou A. Potent inhibitory action of red wine polyphenols on human. JCB. 2000;78:429–41. doi: 10.1002/1097-4644(20000901)78:3<429::aid-jcb8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Fan TP, Yeh JC, Leung KW, Yue PYK, Wong RNS. Angiogenesis:from plants to blood vessels. Trends in. Pharmacol Sci. 2006;6:297–309. doi: 10.1016/j.tips.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Fernandez CC, Vieira PC, daSilva VC, et al. 6-acetonyl-N-methyl-dihydrodecarine, a New Alkaloid from Zanthoxylum riedelianum. J Braz Chem Soc. 2009;20:379–82. [Google Scholar]
- Ferrara N, Gerber HP, LeCounter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Hasibuan PAZ, Harahap U, Sitorus P, Satria D. Ethylacetate extract of Zanthoxylum acanthopodium DC fruit against doxorubicin-resistanced T47D cells. Der Pharma Chemica. 2016;8:172–4. [Google Scholar]
- Hasibuan PAZ, Jessy C, Denny S. Combination effect of ethylacetate extracts of Plectranthus ambonicius (Lour.). Spreng. with doxorubicin againts T47D breast cancer cells. Int J Pharm Pharm Sci. 2015;7:155–9. [Google Scholar]
- Howe LR. Inflamtion and breast cancer. Cyclooxygenase/prosraglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Shi X, Mao X, Chen J, Li H. Cytotoxic mannopyranosides of indole alkaloids from Zanthoxylum nitidum. Chem Biodiversity. 2014;11:970–4. doi: 10.1002/cbdv.201300381. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhang WD, Liu RH, et al. Benzophenantridine alkaloids from Zanthoxylum nitidum (Roxb.) DC, and their analgesic and anti-inflammatory activities. Chem Biodiversity. 2006;3:990–5. doi: 10.1002/cbdv.200690108. [DOI] [PubMed] [Google Scholar]
- Hynniewta SR, Kumar Y. Herbal remedies among the khasi traditional healers and village folks in meghalaya. Indian J Traditional Knowledge. 2008;7:581–6. [Google Scholar]
- Lim JH, Park JW, Min DS, et al. NAG-1 up-regulation mediated by EGR-1 and p53 is critica for quercetin induced apoptosis in HCT-116 colon carcinoma cells. Apoptosis. 2006;12:411–21. doi: 10.1007/s10495-006-0576-9. [DOI] [PubMed] [Google Scholar]
- Lirdprapamongkol K, Kramb JP, Daranee C, Chantragan S, Rudee S. Juice of Eclipta prostrata inhibits cell migration in vitro and exhibits anti-angiogenic activity in vivo. In Vivo. 2008;22:363–8. [PubMed] [Google Scholar]
- Mao P, Ershao Z, Yang C, Likun L, Daqing R. Pinus massoniana bark extract inhibits migration of the lung cancer A549 cell line. Oncol Lett. 2017;13:1019–23. doi: 10.3892/ol.2016.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurrochmad A, Lukitaningsih E, Meiyanto E. Anti cancer activity of rodent tuber (Thyphonium flagelliforme (lodd.). Blume on human breast cancer t47d cells. Int J Phytomed. 2011;3:138–46. [Google Scholar]
- Parhusip A. Kajian Mekanisme Antibakteri Ekstrak Andaliman (Zanthoxylum acanthopodium DC. terhadap bakteri patogen pangan. tesis. Institut pertanian bogor. 2006 [Google Scholar]
- Risau W. Mechanisme of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Satria D, Furqan M, Hadisahputra S, Rosidah F. Combinational effects of ethylacetate extract of Picria fel-terrae Lour and doxorubicin on T47D breast cancer cells. Int J Pharm Pharm Sci. 2015;7:73–6. [Google Scholar]
- Satria D, Pandapotan M, Illyas S. Cytotoxcicity effect of sea horse (Hippocampus trimaculatus Leach.). extract and fractions on MCF-7 cell line. Int J PharmTech Res. 2014;6:212–16. [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–60. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Sihotang YM. Uji Aktivitas antikanker payudara dan krdiopretektif dari ekstrak etilasetat daun poguntano (Picria fel-terrae Lour.). dan buah andaliman (Zanthoxylum acanthopodium DC.) secara In-Vivo. Thesis. Fakultas Farmasi USU. 2015 [Google Scholar]
- Singh B, Lucci A. Role of cyclooxygenase-2 in breast cancer. J Surg Res. 2002;108:173–9. doi: 10.1006/jsre.2002.6532. [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004;23:63–75. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- Sirait M, Siahaan M, Mangkudidjojo . Makalah. Bandung: Farmasi Institut Teknologi Bandung; 1991. Pemeriksaan minyak atsiri dan isolasi senyawa getir dari buah andaliman (Zanthoxylumacanthopodium DC,) p. 11. [Google Scholar]
- Suryanto E, Sastrohamidjojo H, Raharjo S, Tranggongo Antiradical activity of andaliman (Zanthoxylum acanthopodium DC.). Fruit Extract. Indonesian Food Nutr Progress. 2004;11:15–9. [Google Scholar]
- Svensson S, Jirstrom K, Ryden L, et al. ERK phosphorylation is linked to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and is associated with tamoxifen treatnebt resistance and small tumours with good prognosis. Oncogene. 2005;24:4370–9. doi: 10.1038/sj.onc.1208626. [DOI] [PubMed] [Google Scholar]
- Tang F, Chiang E, Shih C. Green tea catechin inhibits ephrin A-1mediated cell migration and angiogenesis of human umbilical vein endhothelial cells. J Nutri Biochem. 2007;18:391–9. doi: 10.1016/j.jnutbio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang N, Huo Q, Yang Q. Anti-angiogenic and antitumor activities of Huaier aquaeous extract. Oncol Rep. 2012;28:1167–75. doi: 10.3892/or.2012.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand M, Hantel P, Kreienberg R, Waltenberger J. Autocrine vascular endothelial growth factor signalling in breast cancer. Evidence from cell lines and primary breast cancer cultures in vitro. Angiogenesis. 2005;8:197–204. doi: 10.1007/s10456-005-9010-0. [DOI] [PubMed] [Google Scholar]
- Xiao X, Shi D, Liu L, et al. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLos One. 2011;6:8. doi: 10.1371/journal.pone.0022934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VR, Sahdeo P, Bokyung S, Ramaswamy K, Bharat BA. Targetting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins. 2010;2:2428–66. doi: 10.3390/toxins2102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Lu FH, Wu JS, Wu CH, Chang CJ. The protective effect of habitual tea consumption on hypertension. Arch Intern Med. 2004;164:1534–40. doi: 10.1001/archinte.164.14.1534. [DOI] [PubMed] [Google Scholar]
- Yao-Kuassi PA, Caron C, Ramiarantosa H, et al. New nitro-benzo[c]phenantridine and indolopyridoquinazoline alkaloids from Zanthoxylum atchoum. C.R Chimie. 2015;18:891–7. [Google Scholar]
- Zhang Y, Seeram NP, Lee R, Feng L, Hebe D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J Agric Food Chem. 2008;56:670–5. doi: 10.1021/jf071989c. [DOI] [PubMed] [Google Scholar]
- Zihlif M, Afifi F, Abu-Dahab R, et al. The antiangiogenic activities of ethanolic crude extracts of four Salvia species. BMC Complement Altern Med. 2013;13:358–65. doi: 10.1186/1472-6882-13-358. [DOI] [PMC free article] [PubMed] [Google Scholar]


