Abstract
Objective:
To determine the frequency and prognostic significance of vitamin D deficiency in Egyptian women with breast cancer (BC).
Methods:
This prospective study included 50 women with primary invasive, non-metastatic BC. The serum level of 25-hydroxy vitamin D [25(OH)D was measured by ELISA at diagnosis, before any cancer treatment. Vitamin D deficiency was defined as 25(OH) D<20 ng/mL. Patients were followed up for a median of 30 months (range: 18-48).
Results:
The median level of 25(OH)D was 29.0 ng/mL (range: 10.0-55.0 ng/mL). Fifteen patients (30%) had vitamin D deficiency, which was positively associated with larger tumor size (p < 0.001), higher grade (p = 0.014), advanced stage (p = 0.001), lymph node positivity (p = 0.012), and HER2/neureceptor expression (p = 0.002). It was also linked with worse overall survival (OS) and disease free survival (DFS) (p = 0.026, and p = 0.004, respectively). On multivariate analysis, DFS was independently affected by vitamin D deficiency with an HR of 2.8 (95%CI: 1.6-7.0, p = 0.022) and advanced stage, i.e. stage II had worse survival compared to stage I with an HR of 4.8 (95%CI:1.1-21.7, p = 0.042).
Conclusion:
Vitamin D deficiency had a negative effect on overall and disease-free survival in our breast cancer cases, being related to tumor size, stage, grade, nodal status and HER2/neu receptor expression.
Keywords: Vitamine D, cancer breast, prognosis
Introduction
Breast cancer (BC) is the most common cancer worldwide and the leading cause of death and disability among women in developing countries (Butt et al., 2012).In fact, in 2015 BC was the most common cancer overall, with an estimated 2.4 million (95% UI, 2.3-2.5 million) incident cases (Global Burden of Disease Cancer Collaboration et al., 2017).
The known risk factors for breast cancer include age at menarche, parity, age at first full-term birth, family history and body mass index (Yang et al., 2011). The nutritional risk factors have gained considerable concern. Studies suggested that high fruit and vegetable intake with low saturated fats may reduce the risk of breast cancer (freudenheim et al., 1996; Lee and Lin, 2000). Vitamin D is presumed to be one of such factors that can be modified to prevent breast cancer (Shaukat et al., 2017).
Vitamin D is a hormone precursor that maintains calcium homeostasis, and is involved in many vital body functions including bone metabolism, neuromuscular function and immunity. The majority of vitamin D3 is manufactured in the skin by way of ultraviolet (UV) B rays. In the kidney, it is transformed to the active form; 1α,25(OH)2D3 (Kulie et al., 2009). Hypovitaminosis D affects almost 50% of the population worldwide, which can α be attributed mainly to reduced exposure to sunlight (Holick, 2007).
Vitamin D has been investigated in relation to various types of cancers; particularly colorectal, breast and prostate cancers (Jacobs et al., 2016). Previous studies reported reduced incidence of these cancers with higher sun exposure, higher intake or higher serum levels of vitamin D (Lappe et al., 2007; Garland et al., 2009). The incidence of seven types of cancer was studied in the Cohort Consortium Vitamin D Pooling Project of Rare Cancers (VDPP). However, no risk association of these cancers with vitamin D was shown (Helzsouer and VDPP Steering Committee, 2010).
Furthermore, cancer mortality was found to be reduced by solar radiation in North America a long time ago. Later on, it was proposed that vitamin D was responsible for this association in cases of colon cancer (Lappe et al., 2007).
Therefore, the current study aimed to determine the frequency of vitamin D deficiency in women with breast cancer, but it mainly focused on the prognostic significance of vitamin D status in these cases.
Materials and Methods
This prospective study was conducted at the National Cancer Institute (NCI), Cairo University, Egypt, during the period from June 2013 to June 2017. The study was approved by the Institutional Review Board of NCI. Written informed consent was obtained from all participants. The study included 50 women with BC. Inclusion criteria were primary, invasive BC in patients who had primary surgery and pathological confirmation at NCI. Exclusion criteria included recurrent or metastatic cases, hospitalized cases and pregnant or lactating women.
From all eligible patients, data were collected from medical records in the pathology department as well as surgical oncology, medical oncology and clinical pathology. These included the patient’s demographic features –with emphasis on age and menopausal status and pathological characteristics of the disease. In addition, hormone receptor status was determined including estrogen receptor (ER), progesterone receptor (PR) in addition to HER2/neu status. Tumors were typed according to the criteria described by World Health Organization (WHO) (Lakhani et al., 2012).
ER and PR were considered positive if more than 1% of the cells stained positive on immunohistochemistry (IHC). HER2/neu was considered positive if IHC was 3+ while in 2+ tumors, Fish test was performed to show HER2/neu genomic amplification. Histologic grading was carried out using the Nottingham-combined histologic grade [Elston-Ellis modification of Scarff-Bloom-Richardson (SBR) grading system] (Elston and Ellis, 1991). Staging of tumors was carried out according to the American Joint Committee on Cancer (AJCC) TNM staging system of breast cancer, 7th edition (Edge and Compton, 2010).
Systemic and local adjuvant treatments were used according to the NCI guidelines.
Determination of serum 25-hydroxy vitamin D levels
Vitamin D measurement was done for all cases at diagnosis before giving any cancer treatment by ELISA technique according to manufacturer instruction (ALPCO, USA Catalog N.38-25DHU-E01).
Patients with 25(OH) D<20 ng/mL were classified as vitamin D deficiency (Norman and Bouillon, 2010).
Immunohistochemical methods:
Immunohistochemistry was performed for evaluating Estrogen and Progesterone receptors as well as for estimating HER2/neu status. Antibodies used were: primary monoclonal antibody against ER (Dako, mouse monoclonal, clone 1D5, ready to use), PR (Dako, mouse monoclonal, clone PgR 636, ready to use) and HER2/neu (Dako, rabbit polyclonal, clone A0485, dilution 1:250), according to manufacturer’s instruction. Hormone receptors were evaluated according to ASCO/CAP guidelines recommendations with the nuclear staining for ER and PR in ≥ 1% of tumor is considered positive (Hammond et al, 2010). Assessment of HER2/neu state was done according to ASCO/CAP guidelines recommendations (Wolff et al., 2013).
The primary outcome measure was disease outcome expressed as overall and disease free survival. Secondary outcome measures were the relation of vitamin D status with different disease prognostic factors. The overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow up visit. Disease free survival was calculated from the date of surgery to the date of recurrence, death or last follow up visit.
Statistical Analysis:
Statistical analysis was done using IBM© SPSS© Statistics version 22 (IBM© Corp., Armonk, NY, USA). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test (Fisher’s exact test) was used to examine the relation between qualitative variables. Survival analysis was done using Kaplan-Meier method and comparison between two survival curves was done using log-rank test. Multivariate analysis was done using Cox-regression hazard model for the significant factors affecting survival on univariate analysis expressed as Hazard ratio (HR) with it 95% confidence interval (CI). A p-value < 0.05 was considered significant.
Results
The mean age of the studied group was 48.8±13.0 years; ranging from 25 to 80 years. Table 1 shows the demographic and clinical characteristics of the 50 patients. Median level of vitamin D was 29.0 ng/mL (range: 10.0-55.0 ng/mL). Using the 20 ng/mL cut-off value of 25(OH)D, 15 patients (30%) had vitamin D deficiency. Vitamin D deficiency was positively associated with larger tumor size (p < 0.001), higher grade of invasive duct carcinoma (p = 0.014), advanced disease stage (p = 0.001), lymph node positivity (p = 0.012) and overexpression of HER2/neureceptor by tumor cells (p = 0.002). Relation between vitamin D status and clinicopathological characteristics is shown in Table 2.
Table 1.
Demographic and Clinical Characteristics of the Studied Group (n=50)
| Value | |
|---|---|
| Age, mean±SD (years) | 48.8±13.0 |
| Menopausal Status | |
| Premenopausal/ Postmenopausal | 20/30 |
| Pathology | |
| Invasive Duct Carcinoma (IDC) | 37 (74.0%) |
| Invasive Lobular Carcinoma | 9 (18.0%) |
| Mixed | 2 (4.0%) |
| Mucoid Carcinoma | 2 (4.0%) |
| Tumor Size, median (range), (cm) | 3 (1.5-7.0) |
| Grade of IDC (n=37) | |
| 1 | 1 (2.7%) |
| 2 | 30 (81.1%) |
| 3 | 6 (16.2%) |
| Stage | |
| I | 14 (28%) |
| II | 30 (60%) |
| III | 6 (12%) |
| Nodal Status | |
| N0 | 20 (40%) |
| N1 | 10 (20%) |
| N2 | 15 (30%) |
| N3 | 5 (10%) |
| Estrogen Receptors | |
| Positive/Negative | 21/29 |
| Progesterone Receptors | |
| Positive/Negative | 28/22 |
| HER2/neu | |
| 0 | 23 (46%) |
| 1 | 5 (10%) |
| 2 | 9 (18.0%) |
| 3 | 13 (26%) |
Data are expressed as number (%); SD, standard deviation
Table 2.
Relation Between Vitamin D Status and Clinical and Pathological Characteristics of the Studied Group
| Vitamin D Deficiency | p value | ||
|---|---|---|---|
| Yes | No | ||
| n=15 | n=35 | ||
| Menopausal Status | |||
| Premenopausal | 5 (25.0%) | 15 (75.0%) | |
| Postmenopausal | 10 (33.3%) | 20 (66.7%) | 0.529 |
| Pathology | |||
| Invasive Duct Carcinoma | 13 (35.1%) | 24 (64.9%) | |
| Other Types | 2 (15.4%) | 11 (84.6%) | |
| Size | |||
| ≥ 5 cm | 8 (88.9%) | 1 (11.1%) | |
| < 5 cm | 7 (17.1%) | 34 (82.9%) | < 0.001 |
| Grade | |||
| G1 or G2 | 8 (25.8%) | 23 (74.2%) | |
| G3 | 5 (83.3%) | 1 (16.7%) | 0.014 |
| Stage | |||
| I | 0 (0.0%) | 14 (100.0%) | 0.001 |
| II | 10 (33.3%) | 20 (66.7%) | |
| III | 8 (83.3%) | 1 (16.7%) | |
| LN | |||
| Negative | 2 (10.0%) | 18 (90.0%) | |
| Positive | 13 (43.3%) | 17 (56.7%) | 0.012 |
| Estrogen Receptors | |||
| Negative | 11 (37.9%) | 18 (62.1%) | 0.15 |
| Positive | 4 (19.0%) | 17 (81.0%) | |
| Progesterone Receptors | 0.384 | ||
| Negative | 8 (36.4%) | 14 (63.6%) | |
| Positive | 7 (25.0%) | 21 (75.0%) | |
| HER2/neu | |||
| Negative | 2 (8.7%) | 21 (91.3%) | 0.002 |
| Positive | 13 (48.1%) | 14 (51.9%) | |
Data are expressed as number (%)
The median follow up time was 30 months (range: 18-48). The overall survival (OS) of the whole studied group was 82% at 36 months and the disease-free survival (DFS) of the whole studied group was 63% at 24 months. Vitamin D deficiency was associated with worse OS and DFS proportions (p = 0.026, and p = 0.004, respectively) (Figures 1 and 2). The relation of OS and DFS with clinical and pathological characteristics in the studied group is shown in tables 3 and 4, respectively.
Figure 1.
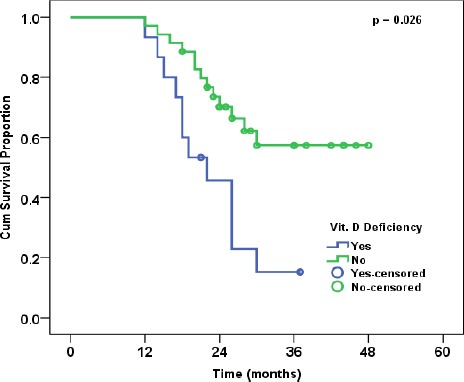
Overall Survival in Relation to Vitamin D Status
Figure 2.
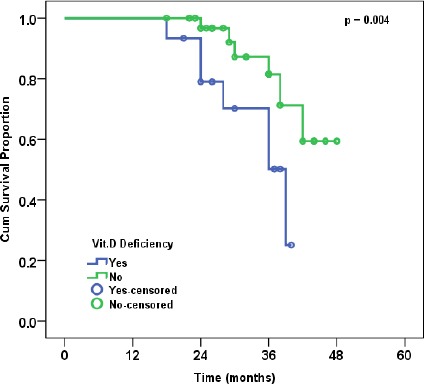
Disease-free Survival in Relation to Vitamin D Status
Table 3.
Cumulative Overall Survival Proportion in the Studied Group and its Relation to Clinical and Pathological Characteristics
| n | Cumulative Overall Survival (%) | p value | |
|---|---|---|---|
| All cases | 50 | 82.00% | |
| Vitamin D Deficiency | |||
| Yes | 15 | 70.20% | 0.026 |
| No | 35 | 87.20% | |
| Menopausal Status | |||
| Premenopausal | 20 | 89.40% | |
| Postmenopausal | 30 | 75.60% | 0.475 |
| Pathology | |||
| Invasive Duct Carcinoma | 37 | 71.80% | 0.777 |
| Other Types | 13 | 79.50% | |
| Size | |||
| ≥ 5 cm | 9 | 88.90% | |
| < 5 cm | 41 | 79.20% | 0.442 |
| Grade | |||
| G1 or G2 | 31 | 93.20% | |
| G3 | 6 | 26.70% | 0.01 |
| Stage | |||
| I | 14 | 100.00% | |
| II | 30 | 73.50% | 0.137 |
| III | 6 | 83.30% | |
| LN | |||
| Negative | 20 | 100% | 0.033 |
| Positive | 30 | 74.30% | |
| Estrogen Receptors | |||
| Negative | 29 | 78.80% | |
| Positive | 21 | 85.20% | 0.518 |
| Progesterone Receptors | |||
| Negative | 22 | 73.10% | |
| Positive | 28 | 89.80% | 0.01 |
| HER2/neu | |||
| Negative | 23 | 89.50% | 0.1 |
| Positive | 27 | 76.90% |
Table 4.
Cumulative Disease-free Survival Proportion in the Studied Group and its Relation to Clinical and Pathological Characteristics
| n | Cumulative Disease-free Survival (%) | p value | |
|---|---|---|---|
| All cases | 50 | 63.00% | |
| Vitamin D Deficiency | |||
| Yes | 15 | 45.70% | 0.004 |
| No | 35 | 70.20% | |
| Menopausal Status | |||
| Premenopausal | 20 | 58.90% | |
| Postmenopausal | 30 | 65.80% | 0.844 |
| Pathology | |||
| Invasive Duct Carcinoma | 37 | 61.80% | 0.967 |
| Other Types | 13 | 66.10% | |
| Size | |||
| ≥ 5 cm | 9 | 77.80% | |
| < 5 cm | 41 | 59.60% | 0.871 |
| Grade | |||
| G1 or G2 | 31 | 70.80% | |
| G3 | 6 | 16.70% | 0.001 |
| Stage | |||
| I | 14 | 100.00% | |
| II | 30 | 42.10% | 0.012 |
| III | 6 | 83.30% | |
| LN | |||
| Negative | 20 | 77.90% | 0.066 |
| Positive | 30 | 53.30% | |
| Estrogen Receptors | |||
| Negative | 29 | 56.80% | |
| Positive | 21 | 71.10% | 0.259 |
| Progesterone Receptors | |||
| Negative | 22 | 49.60% | |
| Positive | 28 | 73.50% | 0.004 |
| HER2/neu | |||
| Negative | 23 | 77.00% | 0.011 |
| Positive | 27 | 50.70% |
On multivariate analysis, the only factor independently affecting OS was the positive expression of progesterone receptor with hazard ratio (HR): 4.7 (95% CI: 1.3-17.7, p = 0.021). DFS was independently affected by vitamin D deficiency with HR: 2.8 (95% CI: 1.6-7.0, p = 0.022) and advanced stage, i.e. stage II had worse survival compared to stage I with HR 4.8 (95%CI: 1.1-21.7, p = 0.042). On the other hand, stage III compared to stage I had HR of 1.7 (95%CI: 0.3-11.3, p = 0.577).
Discussion
The results of this study demonstrated an association between vitamin D deficiency and poor overall and disease free survival in breast cancer patients. Vitamin D deficiency was positively associated with larger tumors (p < 0.001), higher grade (p = 0.014), advanced stage (p = 0.001), lymph node positivity (p = 0.012) and HER2/neureceptor expression (p = 0.002). On multivariate analysis, DFS was independently affected by vitamin D deficiency and advanced stage.
This relation between vitamin D deficiency and poor survival was previously reported in several studies. In a prospective study including 512 women with early breast cancer, vitamin D was measured recently after diagnosis prior to treatment. The authors reported an association of low vitamin D levels with the risk of distant recurrence and overall survival (Goodwin et al., 2009).
A large prospective cohort study including 1,295 postmenopausal breast cancer patients confirmed an association of low serum 25(OH)D concentrations and poor overall survival (HR: 1.6, 95%CI: 1.0-2.4) and distant disease-free survival (HR: 2.1, 95%CI: 1.3-3.4). This association was restricted to cases when samples were collected before starting chemotherapy (Vrieling et al., 2011). The same authors extended their study to investigate potential effect modification by tumor characteristics and lifestyle factors. They confirmed the negative effect of low post-diagnostic serum 25(OH)D levels on overall mortality and distant disease in stage I-IIIa postmenopausal patients. This association was not strongly modified by lifestyle factors (Vrieling et al., 2014).
In a group of 310 women with luminal-type breast cancer, vitamin D deficiency was associated with increased risk of recurrence. However, this relation was not found in patients with HER2/neu enriched or triple negative cancer subtypes (Kim et al., 2011).
Using serum drawn within 90 days of diagnosis, Tretli et al., (2012) reported better overall survival of BC patients with higher 25(OH)D (≥ 35 ng/mL) compared to levels < 20 ng/mL. These authors also found better survival for colon and lung cancer and lymphoma with higher serum 25(OH)D levels. Similar results were observed in a large cohort study by Hatse and colleagues, including non-metastatic BC patients. Levels <20 ng/mL were associated with larger tumor size at diagnosis in postmenopausal patients only. High serum levels (>30 ng/mL) at diagnosis were significantly associated with improved overall and disease-specific survival (Hatse et al., 2012).
The relation between vitamin D deficiency and poor outcome of BC seems to be critically affected by the timing of measurement of serum 25(OH)D levels. No significant association between 25(OH)D levels and BC recurrence or death was found in a study based on serum obtained approximately 2 years post-diagnosis on average (Jacobs et al., 2011). Another study found no association between post-treatment serum 25(OH)D and cancer-specific mortality after a relatively long follow up (median of 9.2 years) of 48 women with BC. Vitamin D assessment was done 2 years post-enrollment (Villasenor et al., 2013). In line with these findings, Vrieling(2014) in their large study, found that when samples of 25(OH)D were taken 6 months or later after diagnosis, the association becomes no more significant. This may be attributed to the effects of treatment modalities or lifestyle changes owing to the disease itself. This is confirmed by non-significant association in patients with blood samples taken after starting chemotherapy (Vrieling et al., 2011; Vrieling et al., 2014). Significant reduction of 25(OH)D level was reported in 20 patients with early stage breast cancer after nearly 5 months of anthracycline and docetaxel based adjuvant chemotherapy (Santini et al., 2010).
In the current study, vitamin D deficiency was significantly associated with tumor size, grade, stage, nodal metastases and HER2/neureceptor status. As a part of the HEAL (Health, Eating, Activity and Lifestyle) Study, serum concentrations of 25(OH)D was investigated in 790 BC survivors. Authors found that stage of disease independently predicts serum 25(OH)D; localized and regional BC were associated with lower serum 25(OH)D compared to in situ disease (Neuhouser et al., 2008). Palmieri (2006) found that serum 25(OH)D was significantly higher in patients with early-stage breast cancer compared to those with locally advanced disease (Palmieriet al., 2006).A negative correlation was observed by Hatse (2012) between tumor size and lower 25(OH)D levels (Hatse et al., 2012).
A meta-analysis involved 8 studies that investigated the association of circulating 25(OH)D concentrations with recurrence and mortality in breast cancer patients. Low circulating 25(OH)D concentrations were significantly associated with increased overall and disease-specific mortality (Vrieling et al., 2014). This association is further confirmed in another meta-analysis involving five studies with 4,413 breast cancer patients. The authors found that pooled hazard ratios for comparing highest with lowest vitamin D status categories were 0.62 (95% CI: 0.49-0.78) and 0.65 0.58 (0.38-0.84) for overall and disease-specific mortality, respectively (Maalmi et al., 2014). Another meta-analysis of 5 studies reported substantially lower fatality rates in individuals with higher serum concentrations of 25(OH)D (Yao and Ambrosone, 2013).
Several mechanisms have been suggested of the influence of vitamin D on survival. Preclinical research demonstrated the anticancer effects of the active metabolite of vitamin D, 1, 25(OH)2D3, through antiproliferative effects (Ingraham et al., 2008), activation of apoptotic pathways (Mathiasen et al., 1999) and inhibition of angiogenesis (Mantell et al., 2000). In addition, this active metabolite can potentiate the anticancer effects of many cytotoxic and antiproliferative anticancer agents (Deeb et al., 2007).
Therefore, different studies yielded variable results. Several epidemiologic, preclinical, and clinical studies suggested that vitamin D deficiency may be involved in BC initiation, progression and prognosis. Other studies did not show any association. Vitamin D has been suggested to influence BC recurrence and death with diverse results. This may be attributed to timing of measurement of the vitamin D, disease type, stage, menopausal status and hormonal receptor status. In the current study, vitamin D deficiency had a negative effect on overall and disease-free survival. It was positively associated with prognostic factors of the disease including tumor size, stage, grade, nodal status and HER2/neu receptor expression.
References
- Butt Z, Haider SF, Arif S, et al. Breast cancer risk factors:a comparison between pre-menopausal and post-menopausal women. J Pak Med Assoc. 2012;62:120–4. [PubMed] [Google Scholar]
- Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer:potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- Edge SB, Compton CC. The American joint committee on cancer:the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann SurgOncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer:experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Freudenheim JL, Marshall JR, Vena JE, et al. Premenopausal breast cancer risk and intake of vegetables, fruits, and related nutrients. J Natl Cancer Inst. 1996;88:340–8. doi: 10.1093/jnci/88.6.340. [DOI] [PubMed] [Google Scholar]
- Garland CF, Gorham ED, Mohr SB, et al. Vitamin D for cancer prevention:global perspective. Ann Epidemiol. 2009;19:468–83. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Global burden of disease cancer collaboration. Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015:A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, et al. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:3757–63. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- Hammond MEH, Hayes DF, Dowsett M, et al. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134:48–72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- Hatse S, Lambrechts D, Verstuyf A, et al. Vitamin D status at breast cancer diagnosis:correlation with tumor characteristics, disease outcome, and genetic determinants of vitamin D insufficiency. Carcinogenesis. 2012;33:1319–26. doi: 10.1093/carcin/bgs187. [DOI] [PubMed] [Google Scholar]
- Helzlsouer KJ VDPP steering committee. Overview of the cohort consortium vitamin D pooling project of rarer cancers. Am J Epidemiol. 2010;172:4–9. doi: 10.1093/aje/kwq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin. 2008;24:139–49. doi: 10.1185/030079908x253519. [DOI] [PubMed] [Google Scholar]
- Jacobs ET, Kohler LN, Kunihiro AG, et al. Vitamin D and colorectal, breast, and prostate cancers:A review of the epidemiological evidence. J Cancer. 2016;7:232–40. doi: 10.7150/jca.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs ET, Thomson CA, Flatt SW, et al. Vitamin D and breast cancer recurrence in the Women's Healthy Eating and Living (WHEL) Study123. Am J ClinNutr. 2011;93:108–17. doi: 10.3945/ajcn.2010.30009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee YM, Ko BS, et al. Vitamin D deficiency is correlated with poor outcomes in patients with luminal-type breast cancer. Ann SurgOncol. 2011;18:1830–6. doi: 10.1245/s10434-010-1465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulie T, Groff A, Redmer J, et al. Vitamin D:an evidence-based review. J Am Board Fam Med JABFM. 2009;22:698–706. doi: 10.3122/jabfm.2009.06.090037. [DOI] [PubMed] [Google Scholar]
- Lakhani SR, Ellis IO, Schnitt SJ, et al. Pathology and genetics of tumors of breast and female genital organs. 4th ed. Lyon: IARC Press; 2012. Tumors of the breast in world health organization classification of tumors. https://shop.iarc.fr/products/who-iarc-classification-of-tumours-of-the-breast . [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, et al. Vitamin D and calcium supplementation reduces cancer risk:results of a randomized trial. Am J ClinNutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- Lee MM, Lin SS. Dietary fat and breast cancer. Annu Rev Nutr. 2000;20:221–8. doi: 10.1146/annurev.nutr.20.1.221. [DOI] [PubMed] [Google Scholar]
- Maalmi H, Ordóñez-Mena JM, Schöttker B, et al. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients:systematic review and meta-analysis of prospective cohort studies. Eur J Cancer Oxf Engl. 2014;50:1510–21. doi: 10.1016/j.ejca.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Mantell DJ, Owens PE, Bundred NJ, et al. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–20. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- Mathiasen IS, Lademann U, Jäättelä M. Apoptosis induced by vitamin D compounds in hydroxyvitamin breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999;59:4848–56. [PubMed] [Google Scholar]
- Neuhouser ML, Sorensen B, Hollis BW, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr. 2008;88:133–9. doi: 10.1093/ajcn/88.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med Maywood NJ. 2010;235:1034–45. doi: 10.1258/ebm.2010.010014. [DOI] [PubMed] [Google Scholar]
- Palmieri C, MacGregor T, Girgis S, et al. Serum 25- D levels in early and advanced breast cancer. J ClinPathol. 2006;59:1334–6. doi: 10.1136/jcp.2006.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini D, Galluzzo S, Vincenzi B, et al. Longitudinal evaluation of vitamin D plasma levels during anthracycline- and docetaxel-based adjuvant chemotherapy in early-stage breast cancer patients. Ann OncolOff J EurSoc Med Oncol. 2010;21:185–6. doi: 10.1093/annonc/mdp497. [DOI] [PubMed] [Google Scholar]
- Shaukat N, Jaleel F, Moosa FA, et al. Association between Vitamin D deficiency and breast cancer. Pak J Med Sci. 2017;33:645–9. doi: 10.12669/pjms.333.11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretli S, Schwartz GG, Torjesen PA, et al. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma:a population-based study. Cancer Causes Control. 2012;23:363–70. doi: 10.1007/s10552-011-9885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaseñor A, Ballard-Barbash R, Ambs A, et al. Associations of serum 25-hydroxyvitamin D with overall and breast cancer-specific mortality in a multiethnic cohort of breast cancer survivors. Cancer Causes Control. 2013;24:759–67. doi: 10.1007/s10552-013-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling A, Hein R, Abbas S, et al. Serum 25-hydroxyvitamin D and postmenopausal breast cancer survival:a prospective patient cohort study. Breast Cancer Res BCR. 2011;13:R74. doi: 10.1186/bcr2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling A, Seibold P, Johnson TS, et al. Circulating 25-hydroxyvitamin D and postmenopausal breast cancer survival:Influence of tumor characteristics and lifestyle factors? Int J Cancer. 2014;134:2972–83. doi: 10.1002/ijc.28628. [DOI] [PubMed] [Google Scholar]
- Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer:American society of clinical oncology/college of American pathologists clinical practice guideline update. J ClinOncol Off J Am Soc Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes:a pooled analysis from the breast cancer association consortium studies. J Natl Cancer Inst. 2011;103:250–63. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337–41. doi: 10.1016/j.jsbmb.2012.09.010. [DOI] [PubMed] [Google Scholar]


