ABSTRACT
Staphylococcus aureus is the main pathogen that causes skin and skin structure infections and is able to survive and persist in keratinocytes of the epidermis. Since the evolution of multidrug-resistant bacteria, the use of phages and their lysins has presented a promising alternative approach to treatment. In this study, a cell wall hydrolase (also called lysin) derived from Staphylococcus phage JD007 (JDlys) was identified. JDlys showed strong lytic activity against methicillin-resistant Staphylococcus aureus (MRSA) strains from different sources and of different multilocus sequence typing (MLST) types. Furthermore, a fusion protein consisting of a cell-penetrating peptide derived from the trans-activating transcription (Tat) factor fused to JDlys (CPPTat-JDlys) was used to kill MRSA bacteria causing intracellular infections. CPPTat-JDlys, in which the fusion of CPPTat to JDlys had almost no effect on the bacteriolytic activity of JDlys, was able to effectively eliminate intracellular MRSA bacteria and alleviate the inflammatory response and cell damage caused by MRSA. Specifically, CPPTat-JDlys was able to combat MRSA-induced murine skin infections and, consequently, expedite the healing of cutaneous abscesses. These data suggest that the novel antimicrobial CPP-JDlys may be a worthwhile candidate as a treatment for skin and skin structure infections caused by MRSA.
IMPORTANCE S. aureus is the main cause of skin and skin structure infections due to its ability to invade and survive in the epithelial barrier. Due to the overuse of antibiotics in humans and animals, S. aureus has shown a high capacity for acquiring and accumulating mechanisms of resistance to antibiotics. Moreover, most antibiotics are usually limited in their ability to overcome the intracellular persistence of bacteria causing skin and skin structure infections. So, it is critical to seek a novel antimicrobial agent to eradicate intracellular S. aureus. In this study, a cell-penetrating peptide fused to lysin (CPP-JDlys) was engineered. Our results show that CPP-JDlys can enter keratinocytes and effectively eliminate intracellular MRSA. Meanwhile, experiments with mice revealed that CPP-JDlys efficiently inhibits the proliferation of MRSA in murine skin and thus shortens the course of wound healing. Our results indicate that the CPP-fused lysin has potential for use for the treatment of skin infections caused by MRSA.
KEYWORDS: Staphylococcus aureus, lysin, cell-penetrating peptide, keratinocytes, skin infection
INTRODUCTION
Healthy skin provides a unique ecological environment and immunological barrier against pathogenic microorganisms, especially Staphylococcus aureus, which is the main cause of skin and skin structure infections (SSSIs) (1, 2). Keratinocytes are the major cell type in the epidermis and actively participate in and orchestrate the innate immune response of the skin (3). Recent studies have illuminated that, like immune cells, keratinocytes are initial sensors for skin and important producers of antimicrobial peptides and proinflammatory cytokines at the preliminary stage of an S. aureus infection (4–6). However, evidence indicates that S. aureus bacteria have the ability to survive in keratinocytes, where they might escape the immune response, leading to persistent infection in the skin (7, 8). It is known that the efficacy of the majority of antimicrobial agents is restricted by the cutaneous intracellular accumulation of S. aureus because most antibiotics hardly enter eukaryotic cells freely and micromolecule antimicrobial peptides disrupt the balance of the microbiota colonizing the skin since they possess a broad spectrum of activity (9, 10). Moreover, the overuse of antibiotics in humans and animals has increased the selection of resistant S. aureus strains. As a result, a mass of S. aureus strains (e.g., methicillin-resistant S. aureus [MRSA]) has been declared to be resistant to various antimicrobials (11).
As a potential alternative or complement to current antimicrobial agents (i.e., antibiotics), phage therapy has been drawing more and more attention as a strategy to treat MRSA infections (12, 13). The potent bactericidal effect of phages is mainly attributed to their genome-encoded cell wall hydrolase, also called lysin, which exhibits a lethal effect by forming holes in the cell wall through peptidoglycan digestion (14). Evidence has shown that lysins exhibit a high level of antibacterial activity, and they have proven their effectiveness for the treatment of infections caused by bacteria resistant to conventional chemical antibiotics in diverse animal models (15–17). A number of studies have revealed the enormous potential of the use of phage lysins, which even surpasses that of intact phage, as therapeutics for MRSA infections (16, 18).
Despite the immense potential of lysins for eradicating infections caused by drug-resistant strains, until now, only a few clinical trials of lysins have been carried out in humans. The main reason for this is that when they are administered systemically, the lysins trigger an immune response, leading to the production of antibodies, which can drastically reduce the effectiveness of the antimicrobial treatment (14, 19). Moreover, lysins are rarely relied on to get rid of intracellular bacteria because they do not possess the ability to penetrate eukaryotic cells (14, 20). So far, many studies have disclosed that cell-penetrating peptides (CPPs) are short peptides that are able to penetrate biological membranes and drive the bioactive cargo into the cells by internalization (21–23). Specifically, many types of drugs have been transported into cells using CPPs, including antisense oligonucleotides, small-molecule pharmaceuticals, and therapeutic proteins (24). Given this, in this study a lysin from phage JD007 (JDlys) fused to CPP (CPP-JDlys) was constructed to facilitate JDlys entry into keratinocytes, and its ability to inhibit the proliferation of S. aureus in murine skin and, consequently, shorten the course of wound healing was demonstrated.
RESULTS
Characterization of staphylococcal lysin derived from phage JD007.
A previous study demonstrated that phage JD007 exhibits strong antibacterial activity against various types of S. aureus strains (25). In this study, a lysin was identified from the whole-genome sequence of phage JD007 (JDlys). Cloning, prokaryotic expression, and purification of JDlys were carried out as detailed in Materials and Methods. The purified JDlys was approximately 57 kDa, according to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (see Fig. S1 in the supplemental material). To examine the bacteriolytic activity of JDlys against Staphylococcus, a MIC assay was performed. As shown in Table 1, JDlys was able to inhibit the growth of Staphylococcus strains from different sources and of different multilocus sequence typing (MLST) types.
TABLE 1.
The various strains used in this study and lytic activity of JDlys and CPP-JDlys
| Species | Strain | MLST type | MIC (μg/ml) |
Sourcea | |||
|---|---|---|---|---|---|---|---|
| Jdlys | CPPTat-JDlys | CPPAnt-JDlys | CPPTP10-JDlys | ||||
| Methicillin-resistant Staphylococcus aureus | USA300 | STb 8 | 20 | 40 | 160 | 160 | III |
| MS5 | ST 5 | 10 | 10 | 160 | 160 | I | |
| MS6 | ST 5 | 20 | 40 | 320 | 320 | I | |
| MS8 | ST 9 | 20 | 20 | —c | 320 | I | |
| MS10 | ST 239 | 80 | 80 | — | — | I | |
| MS11 | ST 9 | 20 | 40 | 320 | 320 | I | |
| MS15 | ST 5 | 20 | 40 | 320 | 160 | I | |
| MS16 | ST 9 | 20 | 40 | 320 | 320 | I | |
| MS17 | ST 398 | 80 | 80 | — | — | I | |
| MS19 | ST 9 | 10 | 20 | 320 | 320 | I | |
| MS20 | ST 398 | 20 | 20 | — | 160 | I | |
| MS21 | ST 398 | 20 | 40 | — | — | I | |
| Methicillin-susceptible Staphylococcus aureus | ATCC 25923 | ST 243 | 20 | 40 | 160 | 160 | III |
| ATCC 29213 | ST 5 | 40 | 40 | 320 | 320 | III | |
| S1 | ST 398 | 80 | 80 | — | — | I | |
| S2 | ST 239 | 20 | 20 | 160 | 160 | I | |
| S4 | ST 239 | 20 | 20 | 320 | 160 | I | |
| S5 | ST 398 | 10 | 20 | 160 | 160 | I | |
| SH-5 | ST 9 | 20 | 20 | 320 | 160 | II | |
| SH-6 | ST 9 | 40 | 40 | — | 320 | II | |
| Staphylococcus sciuri | Sci-3 | 40 | 40 | 320 | 320 | II | |
| Staphylococcus saprophyticus | Sap-1 | 40 | 80 | — | — | II | |
| Staphylococcus caseolyticus | Cas-1 | 80 | 80 | 320 | 320 | II | |
I, clinically isolated pathogenic strains; II, strains isolated from milk samples of dairy cows with mastitis; III, purchased from the American Type Culture Collection.
ST, sequence type.
—, no antimicrobial activity.
Construction and bactericidal activity of the CPP-JDlys fusion protein.
In order to enable the JDlys entering eukaryotic cells to combat intracellular S. aureus, three kinds of CPPs were fused to either the amino terminus or the carboxy terminus of JDlys (Table 2). The results of analysis of the bactericidal effect showed that CPPTat-JDlys (where CPPTat, the CPP derived from the trans-activating transcription [Tat] factor of human immunodeficiency virus type 1, was fused to the amino terminus of JDlys) presented the same antibacterial spectrum as JDlys, although CPPTat-JDlys showed reduced a bactericidal effect against a small proportion of strains compared with that of JDlys (Table 1). However, CPPAnt-JDlys (where CPPAnt, the CPP derived from the Antennapedia homeodomain of Drosophila, was fused to the amino terminus of JDlys) and CPPTP10-JDlys (where CPPTP10, the CPP derived from the shorter analog of transportan, was fused to the amino terminus of JDlys) exhibited significantly reduced bactericidal effects against a large proportion of strains. Unfortunately, JDlys completely lost its bactericidal effect after it was fused to any of the three kinds of CPPs at the carboxy terminus (data not shown).
TABLE 2.
Amino acid sequences of CPPs
Intracellular killing activity of CPP-JDlys.
Previous research found that certain S. aureus strains exhibit an ability to adhere to and invade eukaryotic cells (26). Here, our research showed that MRSA strain USA300 had a relatively strong ability to adhere to and invade HaCaT keratinocytes (Fig. S2). To investigate the activities of the various CPP-JDlys constructs against intracellular MRSA strains, HaCaT keratinocytes were infected with USA300 and subsequently treated with JDlys and the three CPP-JDlys constructs (where the CPPs were fused to the amino terminus of JDlys). CPPTat-JDlys exhibited a greater ability to kill intracellular MRSA strains than JDlys, indicating that CPPTat could deliver JDlys into keratinocytes so that it could exert its activity against intracellular bacteria (Fig. 1 and S3). However, neither CPPAnt-JDlys nor CPPTp10-JDlys could eliminate intracellular MRSA. Previous evidence showed that chloramphenicol (Cm) has the ability to kill intracellular S. aureus (27). However, the result showed that 80 μg/ml (2× MIC) of CPPTat-JDlys had a bactericidal effect in keratinocytes similar to that of 100 μg/ml (8× MIC) of chloramphenicol (Fig. S3).
FIG 1.
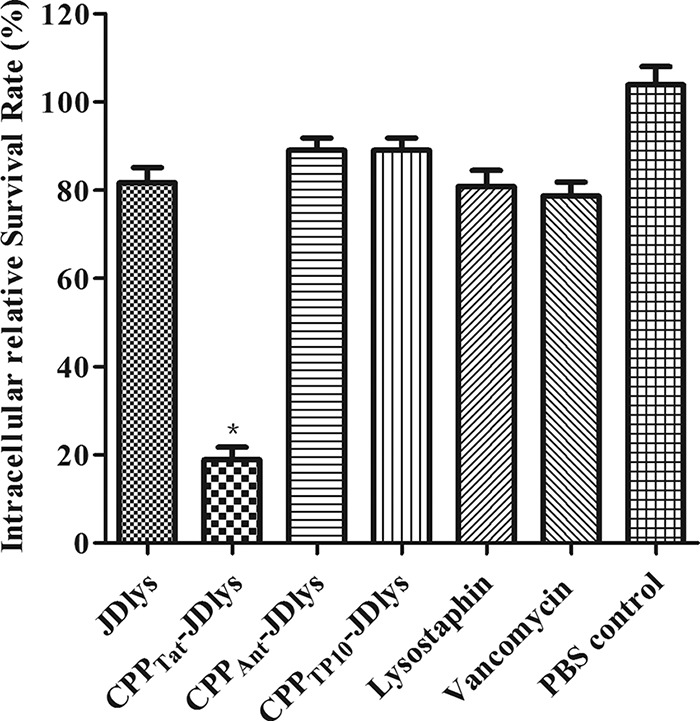
Elimination of intracellular MRSA in HaCaT keratinocytes. Keratinocytes were infected, treated with gentamicin to kill the extracellular S. aureus, and then treated with purified JDlys, CPP-JDlys, commercial lysostaphin, or vancomycin. Infected cells were treated with PBS as a control. *, the intracellular bacterial numbers after treatment with CPPTat-JDlys were significantly lower (P < 0.05) than those after the other treatments. The data shown are means ± SEMs from at least three independent experiments.
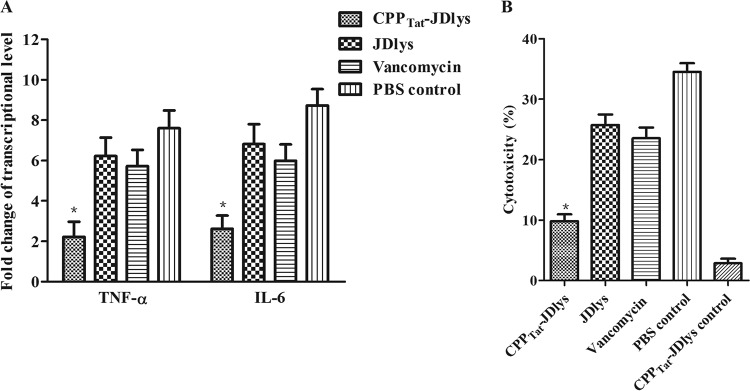
The levels of proinflammatory cytokines and cytotoxicity can be used to assess the severity of the effect of an infection with an intracellular pathogen on eukaryotic cells. Our study found that treatment with CPPTat-JDlys decreased the amounts of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and lactate dehydrogenase (LDH) released compared with the amounts released after treatment with JDlys and vancomycin. This demonstrates that treatment with CPPTat-JDlys can alleviate the inflammation and reduce the cytotoxicity caused by intracellular S. aureus (Fig. 2A and B).
FIG 2.
Intracellular MRSA-induced cytokine expression and cytotoxicity in HaCaT keratinocytes. Keratinocytes were infected, treated with gentamicin to kill the extracellular S. aureus, and then treated with purified CPPTat-JDlys, JDlys proteins, or vancomycin. Infected cells were treated with PBS as a control. (A) The mRNA levels of TNF-α and IL-6 were measured by real-time PCR at 3 h after the treatments mentioned above. The levels of TNF-α and IL-6 mRNA were normalized to the levels of β-actin mRNA and then were expressed as the n-fold increases with respect to the levels in uninfected cells. (B) Effect of bacterial cell-mediated cytotoxicity measured by LDH cytotoxicity assays. Infected cells were treated with PBS as a control. Uninfected cells were treated with CPPTat-JDlys as a CPPTat-JDlys control. *, the cytokine levels or cytotoxicity for samples treated with CPPTat-JDlys were significantly lower (P < 0.05) than those for samples treated with JDlys, vancomycin, and the PBS control. The data shown are means ± SEMs from at least three independent experiments.
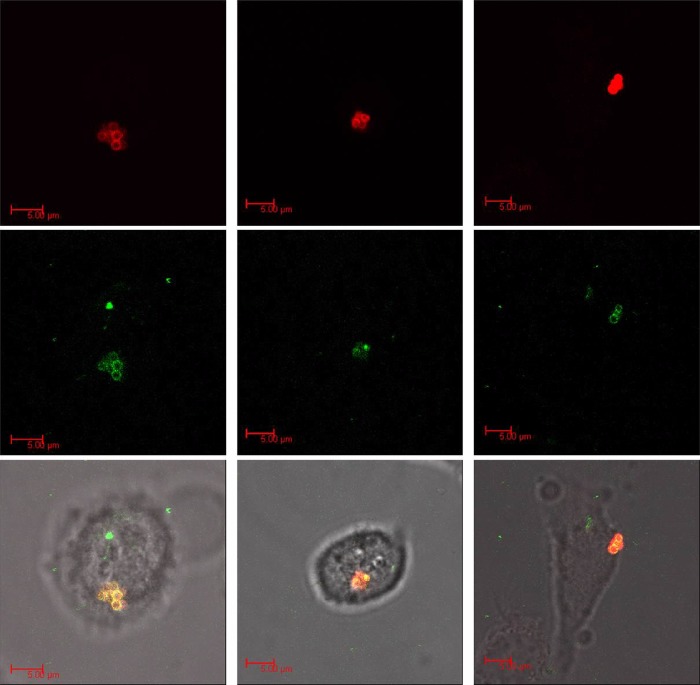
To determine whether CPPTat-JDlys transferred across the keratinocyte membrane, keratinocytes were exposed to fluorescently labeled CPPTat-JDlys (green) and fluorescently labeled MRSA strain USA300 (red) and monitored in real time by confocal microscopy. USA300 and the CPPTat-JDlys were identified to be intracellularly colocalized (yellow) in a single z plane within the keratinocytes (Fig. 3).
FIG 3.
Confocal microscopy images of infected HaCaT keratinocytes treated with CPP-JDlys. Keratinocytes were exposed to fluorescently labeled CPPTat-JDlys (green; middle row) and fluorescently labeled MRSA strain USA300 (red; top row) and monitored in real time by confocal microscopy. S. aureus bacteria were colocalized with CPPTat-JDlys (yellow) in the combined panels in the bottom row. Images of the three columns are representative of three independent experiments.
Activity of CPPTat-JDlys in a mouse model of cutaneous abscesses.
To validate the bactericidal efficacy of CPPTat-JDlys against local infections, mice were subcutaneously infected with MRSA and treated with proteins over the course of 3 days. The efficacy of CPPTat-JDlys was measured by the use of multiple parameters, as described below.
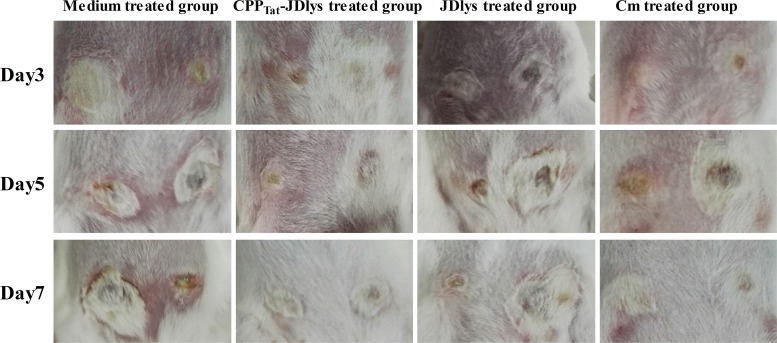
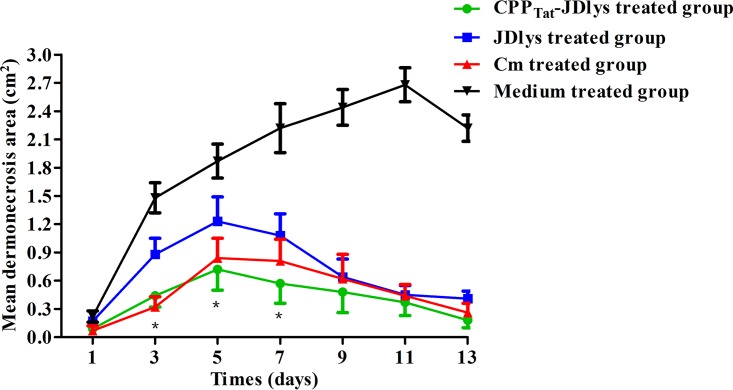
Over 13 days, CPPTat-JDlys treatment significantly restricted the area of the abscess caused by USA300 compared with that in control mice treated with medium only (Fig. 4 and 5). The maximum area of the abscess at day 5 after treatment with CPPTat-JDlys was about 0.72 cm2, which was significantly smaller than that in the medium-treated controls (about 2.68 cm2 at day 11) (Fig. 5). Furthermore, the CPPTat-JDlys-treated group exhibited a relatively smaller area of the abscess at days 3, 5, and 7 (about 0.44 cm2, 0.72 cm2, and 0.57 cm2, respectively) than the JDlys-treated group (about 0.88 cm2, 1.23 cm2, and 1.08 cm2, respectively). As a critical assessment of CPPTat-JDlys's efficacy, the number of CFU per abscess was determined in the mice. The result showed that the number of bacterial CFU from the abscesses was obviously lower for CPPTat-JDlys-treated mice than for JDlys-treated mice (almost 10-fold lower) and medium-treated mice (more than 100-fold lower) at every time point (Fig. 6). The substantial reductions in the number of CFU associated with CPPTat-JDlys were congruent with the observed reductions in the area of the abscess (Fig. 5).
FIG 4.
Restriction of the dermonecrotic area and the severity of dermonecrosis at days 3, 5, and 7 in the mouse model. Images of the dermonecrotic areas in the medium control-, CPPTat-JDlys-, JDlys-, and Cm-treated mice are shown and are representative of those from three experiments. Note the smaller areas of dermonecrosis and the faster healing of lesions in CPPTat-JDlys-treated mice than in the control mice, in which the lesions remained exudative.
FIG 5.
Progression of mean dermonecrotic area over 13 days in a mouse model of infection. The areas of the abscesses in each mouse flank were measured during the period of treatments. *, time points when the dermonecrotic areas treated with the medium buffer or JDlys were significantly greater (P < 0.05) than those treated with CPPTat-JDlys. The data shown are means ± SEMs from at least three independent experiments.
FIG 6.
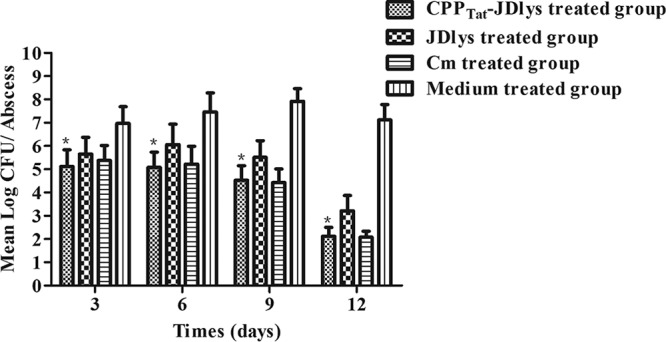
CPP-JDlys-mediated reduction in abscess burden, given as the number of CFU. Three mice were humanely sacrificed at preselected time points. Each lesion (two lesions per mouse) was aseptically excised and homogenized. *, time points when the burden (number of CFU) in lesions treated by CPPTat-JDlys was significantly lower (P < 0.05) than that in lesions treated with the medium buffer or JDlys. The data shown are means ± SEMs from at least three independent experiments.
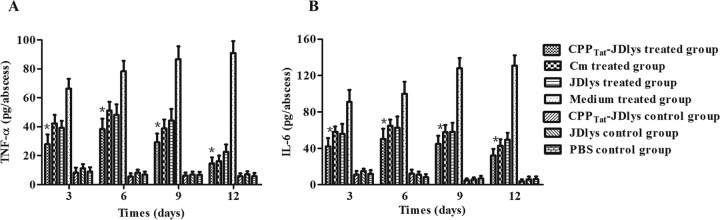
The strength and efficiency of the host immune response depend on the level of proinflammatory cytokines, including TNF-α and IL-6, which are indicators of the severity of skin infections (28, 29). In this study, the levels of proinflammatory cytokines (TNF-α and IL-6) in the abscess tissues were measured by the use of cytokine enzyme-linked immunosorbent assay (ELISA) kits. The magnitudes of the TNF-α and IL-6 responses in the CPPTat-JDlys-treated mice were significantly greater in the early stages (before day 6) of treatment but decreased rapidly over time (Fig. 7). Conversely, for the medium-treated mice, the levels of cytokines increased gradually over time, indicating that CPPTat-JDlys effectively inhibited the inflammation due to MRSA on the skin surface. Interestingly, the levels of proinflammatory cytokines in the Cm-treated mice were higher than those in the CPPTat-JDlys-treated mice at every time point (Fig. 7). However, there was no difference in the abscess size or the number of bacterial CFU between the two treated groups (Fig. 4 to 6). Moreover, both groups of uninfected mice treated with JDlys and CPPTat-JDlys exhibited proinflammatory cytokine levels similar to those in the PBS-treated control group.
FIG 7.
Systemic immune responses to CPPTat-JDlys in the mouse model. Supernatants from lesion homogenates were collected at different time points after treatment and assayed for TNF-α (A) and IL-6 (B) by ELISA. Three mice (two lesions per mouse) per group were sacrificed at each time point. Uninfected mice treated with JDlys, CPPTat-JDlys, and PBS were used to represent the CPPTat-JDlys, JDlys, and PBS control groups, respectively. Infected mice treated with PBS served as the medium-treated group. *, time points when the cytokine levels in lesions treated with CPPTat-JDlys were significantly lower (P < 0.05) than those in lesions treated with the medium buffer. The data shown are means ± SEMs from at least three independent experiments.
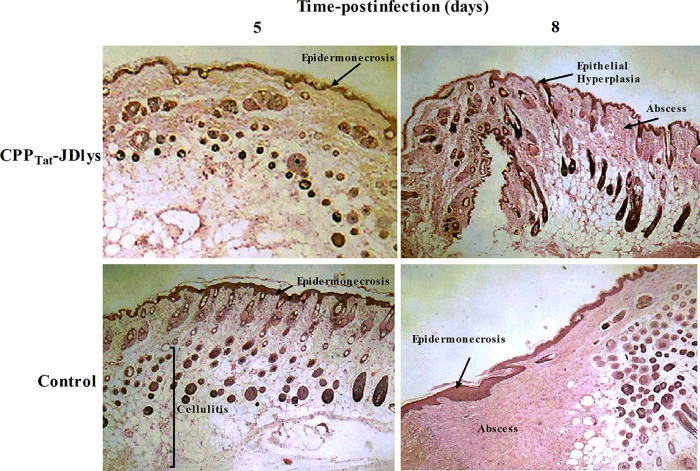
Histopathological assays of the abscess tissues were carried out at 5 and 8 days after treatment with CPPTat-JDlys. Skin lesions from untreated (medium-treated) mice exhibited extensive epidermal and soft tissue necrosis after 5 days. As time went on, an undiminished infection with a large abscess and inflammatory cell infiltration emerged in these mice (Fig. 8), whereas CPPTat-JDlys-treated mice showed relatively moderate epidermal necrosis and cellulitis after 5 days. After 8 days, the epidermis was regenerating rapidly and the wound was healing gradually (Fig. 8).
FIG 8.
CPPTat-JDlys reduced MRSA-induced cutaneous abscesses and promoted the healing of skin lesions. BALB/c mice were infected with strain USA300 and treated with CPPTat-JDlys. Infected skin was collected from the animals at 5 and 8 days postinfection and processed for H&E staining and microscopic examination. Magnifications, ×10.
DISCUSSION
S. aureus is the major cause of SSSIs and has the ability to invade and survive in distinctly differentiated keratinocytes (1, 3). However, the intracellular persistence of the bacteria in SSSIs contributes to the difficulty of antibiotic treatment, especially in MRSA infections (30, 31). So, it is critical to seek a novel antimicrobial agent that has the excellent properties of high selectivity and specificity to eradicate intracellular S. aureus.
Lysins have been broadly investigated as potential antimicrobial agents in recent years (15, 32). However, when used alone and/or without modification, it is difficult for lysins to kill intracellular pathogens (14). In order to address this concern, three kinds of CPPs which could deliver the cargo into eukaryotic cells were fused with JDlys (21–23). However, when the CPPs were fused at the carboxy terminus of JDlys, the ability of the three kinds of fusion proteins to lyse S. aureus in vitro was completely inactivated. Numerous researchers have demonstrated that the cell wall binding domain of lysins (usually in the carboxy terminus of lysins) plays a pivotal role in the ability of lysins to recognize their substrates (bacterial cell wall peptidoglycan) (14). In addition, the lysin might lose bactericidal activity once the binding domain is destroyed (32, 33). Therefore, it is presumed that CPPs fused to the carboxy terminus of lysins might influence the activity of JDlys by interfering with the cell wall binding domain in the carboxy terminus. Fortunately, JDlys fused with the three kinds of CPPs at the amino terminus retained bactericidal activity. Further, we confirmed that CPPTat, which fused to the amino terminus of JDlys, could deliver the cargo (JDlys) into keratinocytes, which is in accordance with the findings of Johnson et al. (21). Meanwhile, our results indicated that CPPTat-JDlys is able to eliminate intracellular bacteria effectively and alleviate the MRSA-induced inflammatory response and cell damage (Fig. 1 and 2). However, neither CPPAnt-JDlys nor CPPTP10-JDlys was able to eliminate intracellular MRSA effectively. The possible reason may be that the cell-penetrating ability of CPPAnt and CPPTP10 was inhibited by the advanced structure of the JDlys protein. Further studies are needed to evaluate this possibility.
As a main constituent in the epidermis, keratinocytes can provide a shelter for pathogens to evade immune defenses, and thus, pathogens, especially S. aureus, inflict persistent damage to the skin (34). Previously, we revealed that CPPTat-JDlys is capable of penetrating the membrane of keratinocytes to eliminate intracellular bacteria. Wang et al. revealed that a cell-penetrating peptide (which has an amino acid sequence similar to that of CPPTat) could deliver the drug through the skin and thus overcome the barrier function of the skin (35). So, we suspect that CPPTat helped JDlys gain access to deeper tissue layers and prevent the skin damage caused by MRSA, although subcutaneous therapy is a complicated process involving integrated tissue consisting of numerous cell types besides keratinocytes. Fortunately, our study confirmed that treatment with CPPTat-JDlys could significantly reduce the number of MRSA bacteria in cutaneous abscesses and shorten the course of wound healing compared with that required with JDlys treatment (Fig. 4 to 8). Furthermore, lysins as proteolytic enzymes, like other proteins introduced into mammalian organisms, are quickly cleared from the systemic circulation (36). So, continuous administration of CPP-JDlys (over a 3-day duration) was performed in order to maintain the enzymatic activity of the lysin, which then worked steadily in the cutaneous abscesses in the mice.
Meanwhile, the adverse effects of lysins in vivo have also been studied. Adverse effects are associated with bactericidal agents, including the release of large amounts of pathogen-associated molecular patterns that are recognized by Toll-like receptors and the induction of proinflammatory cytokines in mammals (37). However, our studies showed that no significant differences in the expression of proinflammatory cytokines were seen between uninfected mice treated with JDlys or CPP-JDlys and mice treated with PBS as a control over 12 days (Fig. 7). In addition, uninfected mice treated with either JDlys or CPP-JDlys did not show abscess formation (data not shown). Thus, it is suggested that there is no observable adverse effect of therapy with JDlys or CPP-JDlys. Moreover, it has been reported that lysin can stimulate the host to produce antibodies to influence its bactericidal activity in vivo (14). Unlike antibiotics or micromolecule antibacterial peptides, which are small and nonimmunogenic, lysins are peptides that stimulate the immune response, regardless of the way in which they are administered into the body, leading to the formation of antibodies that can reduce the activity of the lysin in vivo (14). However, Zhang et al. suggested that lysin antibodies do not affect the bactericidal efficacy of lysin in vitro or in vivo because the affinity of binding of the cell wall to lysin might be higher than the affinity of binding of antibodies to lysin (16). Our results also found that CPP-JDlys could maintain high levels of activity and continuously inhibit the survival of MRSA strains in vivo.
Collectively, our findings suggest that the fusion protein CPP-JDlys is a novel antimicrobial agent active against intracellular and skin infections caused by MRSA. So far, lysin still cannot replace antibiotics for clinical treatment (e.g., because of the narrow antibacterial spectrum [14]). However, the proper transformation of lysin could contribute to the better control of antibiotic-resistant bacteria as well as the development of a wider range of applications for antimicrobials.
MATERIALS AND METHODS
Ethics statement.
The animal experiments were carried out according to animal welfare standards and approved by the Ethical Committee for Animal Experiments of Shanghai Jiao Tong University, Shanghai, China. All animal experiments complied with the guidelines of the Animal Welfare Council of China.
Bacterial strains, bacteriophage, and growth conditions.
The bacteria used in this study are listed in Table 1. Staphylococcus strains were cultured aerobically in tryptic soy broth (TSB) or brain heart infusion (BHI) broth in a shaker (180 rpm) at 37°C. The bacterial burden, given as the number of CFU of Staphylococcus, was measured in BHI agar supplemented with 2% (vol/vol) fetal bovine serum (FBS; Gibco, Invitrogen Corp., Carlsbad, CA) under static conditions at 37°C. Escherichia coli strains, used for gene cloning and producing recombinant proteins, were cultured aerobically in Luria-Bertani (LB) broth with shaking (180 rpm) at 37°C. The JD007 phage was kindly provided by ZeLin Cui (Shanghai General Hospital, Shanghai, China) (25). Purification and genomic DNA extraction were performed as described previously (13).
Cloning, expression, and purification of JDlys and CPP-JDlys.
The region encoding the lysin of phage JD007 (JDlys) was PCR amplified with primers 5′ BamHI_JDlys (CGCGGATCCATGGCTAAGACTCAAGCA, where the BamHI site is underlined) and 3′ XhoI_JDlys (CCGCTCGAGCTACTTGAATACTCCCCA, where the XhoI site is underlined), using the JD007 phage genome (GenBank accession number JX878671.1) as a template for JDlys. The PCR product was digested with BamHI/XhoI and cloned into the pET28a(+) vector. CPPs and linker sequences (Table 2) were identified by reverse translation into nucleotide sequences (with an E. coli codon usage bias), followed by commercial synthesis (Sangon, Biotech Co., Ltd., Shanghai, China). The individual CPP sequences were inserted at the BamHI site (at the amino terminus of the JDlys sequence) or XhoI site (at the carboxy terminus of the JDlys sequence) of the pET28a(+) vector. Furthermore, green fluorescent protein (GFP; GenBank accession number LC336974.1) was synthesized (Sangon) and inserted at the BamHI site (at the amino terminus of the CPPTat-JDlys sequence) of the pET28a(+) vector. Constituted plasmids were expressed in E. coli BL21(DE3) grown to an optical density at 600 nm (OD600) of 0.6 at 37°C, induced with 1 mM isopropyl-β-d-thiogalactoside (IPTG), and expressed for 12 h at 18°C. Cells were disrupted by sonication and purified by use of an Ni-Sepharose 6 Fast Flow resin gravity column (GE Healthcare BioSciences, Pittsburgh, PA, USA), as described previously (38). Moreover, for proteins used in cellular and murine experiments, endotoxins were removed by treatment with phosphate-buffered saline (PBS) containing a 0.1% Triton X-114 (Sigma-Aldrich, St. Louis, MO) elution of proteins on Ni-nitrilotriacetic acid columns (39).
Bactericidal activity of JDlys and CPP-JDlys.
The MICs of JDlys or CPP-JDlys for multiple Staphylococcus strains were determined as described by Wiegand et al. with minor modifications (40). Briefly, Staphylococcus strains were freshly cultured to early exponential phase (OD600, 0.2 to 0.4). The MIC was monitored by blending 100 μl of the bacterial suspension (diluted to 5 × 106 cells/ml) with 100 μl of the enzyme at different final concentrations (10, 20, 40, 80, 160, 320, and 640 μg/ml) in a 96-well microtiter plate. The plates were incubated for 20 h at 37°C and read with a 96-well plate reader (determination of the absorbance at 600 nm).
Determination of ability of bacteria to adhere to and invade HaCaT keratinocytes.
HaCaT keratinocytes were grown in RPMI 1640 medium supplied with 10% FBS at 37°C in 5% CO2. For the adhesion assay, keratinocytes were seeded into 12-well plates at a concentration of approximately 105 cells per well. On the next day, prepared bacterial strains were added to the keratinocytes at a multiplicity of infection (MOI) of 10. The plates were incubated for 60 min at 37°C in 5% CO2. The keratinocytes were washed three times with PBS to remove nonadhered bacterial cells. After that, the keratinocytes were lysed with PBS containing 0.02% Triton X-100, and the adhered bacteria were quantified by serial dilution plating. The adhesion rate was calculated as follows: number of CFU of adhered bacteria/number of CFU of the initial number of bacteria.
For the invasion assay, keratinocytes were seeded into 12-well plates at a concentration of approximately 105 cells per well. On the next day, prepared bacterial strains were added to the keratinocytes at an MOI of 10. The plates were incubated for 60 min at 37°C in 5% CO2. After that, the keratinocytes were washed three times with PBS, the medium was replaced with 1% FBS–RPMI 1640 medium containing 50 μg/ml of gentamicin to kill the extracellular S. aureus bacteria, and the keratinocytes were incubated for 60 min. After that, the keratinocytes were washed three times with PBS and then incubated for 15 min at 37°C in PBS with 0.02% Triton X-100 to lyse the keratinocytes and release the intracellular invading bacteria. The invasion rate was calculated as follows: number of CFU of invading bacteria/number of CFU of the initial number of bacteria.
CPP-JDlys-mediated intracellular S. aureus eradication assay.
MRSA strain USA300 was cultured in BHI broth to exponential phase (about 1 × 109 CFU/ml) in a shaker at 37°C, harvested by centrifugation at 5,000 × g for 5 min, washed 3 times in PBS, and resuspended in fresh, serum-free RPMI 1640 medium without antibiotics. The keratinocyte monolayers were grown in 12-well tissue culture plates and infected with S. aureus cells (106 bacteria/well) at an MOI of 10 for 1 h at 37°C. Extracellular bacteria were removed by washing the keratinocytes 3 times with PBS, the medium was replaced with 1% FBS–RPMI 1640 medium containing 50 μg/ml of gentamicin to kill the extracellular S. aureus bacteria, and the keratinocytes were incubated for 1 h. Subsequently, the cells were washed 3 times with PBS to remove the gentamicin. This time point was defined as time zero. Equal volumes of purified JDlys (40 μg/ml [2× MIC]), CPPTat-JDlys (40, 80, 160, and 320 μg/ml [1×, 2×, 4×, and 8× MIC, respectively]), CPPAnt-JDlys (320 μg/ml [2× MIC]), and CPPTP10-JDlys (320 μg/ml [2× MIC]) proteins, commercial lysostaphin (40 μg/ml [2× MIC]; Sigma-Aldrich), vancomycin (5 μg/ml [2× MIC]; Sigma-Aldrich), chloramphenicol (Cm; 12.5, 25, 50, and 100 μg/ml [1×, 2×, 4×, and 8× MIC, respectively]), and PBS were added to cells that had been cleaned of gentamicin for 3 h. Cells were cultured throughout all experiments at 37°C in 5% CO2. After that, infected cells were washed 3 times with PBS and then incubated for 15 min at 37°C with 0.02% Triton X-100 to lyse the keratinocytes and release the intracellular bacteria. Various dilutions of the lysates were plated on BHI agar plates and incubated overnight at 37°C. The colonies were counted to determine the number of intracellular bacteria. To analyze the number of surviving bacteria compared to the initial number of intracellular bacteria (time zero), the relative number of CFU (rCFU) was calculated as follows: number of CFU at 3 h/number of CFU at time zero (41).
Confocal microscopy of HaCaT keratinocytes.
HaCaT keratinocytes were seeded onto uncoated 50-mm, glass-bottom dishes (MatTek Corporation, Ashland, MA) in RPMI 1640 medium supplemented with 5% (vol/vol) FBS at 37°C in 5% CO2. After 12 h of culture, when the keratinocytes had about 90% coverage, the S. aureus USA300 cells were added and then incubated at 37°C for 1 h. After that, samples were washed 3 times with PBS, the medium was replaced with 1% FBS–RPMI 1640 medium containing 50 μg/ml of gentamicin to kill the extracellular S. aureus bacteria, and the keratinocytes were incubated for 1 h. Samples were then incubated with monoclonal mouse anti-S. aureus protein A (1:200; catalog number ab37644; Abcam, Cambridge, UK) for 30 min at 37°C. After that, samples were rinsed and incubated with goat anti-mouse IgG conjugated to tetramethyl rhodamine isocyanate (1:600; catalog number ab6786; Abcam) for 30 min at 37°C. After the washes, samples were exposed to 80 μg/ml of recombinant CPP-JDlys fused with GFP for 30 min. The keratinocytes were washed 3 times with PBS and then viewed using a confocal microscope (a Leica DM6000 CFS confocal fluorescence imaging microscope on the Leica TCS-SP5 platform) at ×60 magnification. All fluorescence signals were collected on a photomultiplier after passage through appropriate filter sets.
LDH cytotoxicity assay.
The cytotoxicity of the keratinocytes was detected by measuring the lactate dehydrogenase (LDH) activity present in the culture supernatant. A CytoTox 96 nonradioactive cytotoxicity assay (Promega, Madison, WI) was used according to the manufacturer's instructions. Briefly, keratinocytes were sampled at 3 h after treatment with JDlys, CPP-JDlys, and vancomycin as described above. Maximum release was achieved by lysing the monolayers with Triton X-100 at a final concentration of 1% (vol/vol). The activity of the enzymes was determined using a microplate reader (determination of the absorbance at 492 nm). The LDH released was designated that released spontaneously. Cytotoxicity was calculated as follows: percent cytotoxicity = (amount of LDH released in the test − amount released spontaneously)/(maximal amount released − amount released spontaneously).
Cytokine assays.
To measure the cytokine levels of the infected keratinocytes, keratinocytes were sampled at 3 h after treatment with JDlys, CPP-JDlys, and vancomycin as described above. Total RNA was isolated from the supernatants of the keratinocytes using an AllPrep RNA microkit (Qiagen, West Sussex, UK). cDNA synthesis was performed using a PrimeScript RT reagent kit (TaKaRa, Dalian, China) according to the manufacturer's instructions. The mRNA levels were measured using a two-step relative quantitative reverse transcription-PCR (qRT-PCR). The β-actin housekeeping gene was amplified as an internal control. The sequences of the primers for tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and β-actin are listed in Table 3. Gene expression was normalized to the expression of the housekeeping gene β-actin. Real-time PCR was carried out using a SYBR Premix Ex Taq kit (TaKaRa) and a CFX Connect RT-PCR system (Bio-Rad, Hercules, CA, USA). The comparative cycle threshold (2−ΔΔCT) method was used to analyze the mRNA levels.
TABLE 3.
Primers used for qRT-PCR
| Primer | Sequence (5′–3′) | Function |
|---|---|---|
| IL-6-F | AGCCTCAATGACGACCTA | A fragment for human IL-6 gene |
| IL-6-R | ATCTTTGTTGGAGGGTGA | |
| TNF-α-F | AGCCGCATCGCCGTCTCCTA | A fragment for human TNF-α gene |
| TNF-α-R | CAGCGCTGAGTCGGTCACCC | |
| β-actin-F | CCACGAAACTACCTTCAACTCC | A fragment for human β-actin gene |
| β-actin-R | GTGATCTCCTTCTGCATCCTGT |
Murine model of cutaneous abscesses.
BALB/c female mice aged 6 weeks were used for the subcutaneous abscess model as previously described by Ding et al. with minor modifications (42). In brief, mice were anesthetized, their flanks were shaved and sterilized, and the mice were randomized into appropriate study groups (20 mice in each group). Next, 50 μl of MRSA strain USA300 (5 × 107 CFU/flank) mixed with an equal volume of autoclaved Cytodex-1 beads (131 to 220 μm; Sigma-Aldrich) in PBS was injected subcutaneously. At 24 h postinfection, purified JDlys (50 μg/flank), CPP-JDlys (100 μg/flank), or chloramphenicol (100 μg/flank) was injected subcutaneously near the abscesses over the course of 3 days. CPP-JDlys alone (i.e., no bacteria) and medium (PBS buffer) were injected subcutaneously, and these mice served as control groups.
Abscess evaluation after therapy. (i) Abscess area.
The areas of the abscesses in each mouse flank were measured during the period of treatment with JDlys, CPP-JDlys, Cm, and PBS buffer. To do so, the mice were anesthetized and the lesion site length and width were measured to quantify the abscess area (given in square centimeters).
(ii) Bacterial burden as number of CFU.
The mice were humanely sacrificed at preselected time points after the treatments with JDlys, CPP-JDlys, Cm, and PBS buffer. Each flank was aseptically excised and homogenized. The suspension of abscesses was serially diluted in sterile PBS for quantitative culturing onto a BHI agar plate. All cultures were incubated (37°C) for 24 h, and the resulting colonies were enumerated as the number of CFU per abscess.
(iii) Cytokine quantification.
Commercial enzyme-linked immunosorbent assay (ELISA) kits (Abcam) were used to examine the levels of the proinflammatory cytokines (i.e., TNF-α and IL-6) that were released from the abscess postinfection or after the treatments with CPP-JDlys, JDlys, Cm, and PBS buffer. The abscesses were excised, weighed, and homogenized as mentioned above. The cytokine levels were measured according to the manufacturer's instructions.
(iv) Histopathology analysis.
The mice treated with CPP-JDlys or PBS were humanely sacrificed at 5 and 8 days postinfection. Abscess tissues were gently removed and immediately placed in 4% formalin. Formalin-fixed tissues were processed and stained with hematoxylin and eosin (H&E) and toluidine blue using a routine staining procedure and were subsequently analyzed using microscopy.
Statistical analyses.
In all experiments, data points were plotted using GraphPad Prism (v.7.01) software (GraphPad Software, Inc., San Diego, CA). Data are presented as mean values ± standard errors of the means (SEMs). Related experiments were performed using the nonparametric Mann-Whitney U test. A P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (grants 31672524, 31772744, and 31372500), the Key Project of Shanghai Municipal Agricultural Commission (grant 2016-6-2-1), the Agri-X Fund from Shanghai Jiao Tong University, the Science and Technology Commission of Shanghai Municipality (grants 16391903400 and 17391901400), and a grant from the Special Fund for Public Welfare Industry of the Chinese Ministry of Agriculture (201303041).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00380-18.
REFERENCES
- 1.Schittek B. 2011. The antimicrobial skin barrier in patients with atopic dermatitis. Curr Probl Dermatol 41:54–67. doi: 10.1159/000323296. [DOI] [PubMed] [Google Scholar]
- 2.Shahbazian JH, Hahn PD, Ludwig S, Ferguson J, Baron P, Christ A, Spicer K, Tolomeo P, Torrie AM, Bilker WB, Cluzet VC, Hu B, Julian K, Nachamkin I, Rankin SC, Morris DO, Lautenbach E, Davis MF. 22 September 2017. Multidrug and mupirocin resistance in environmental methicillin-resistant Staphylococcus aureus (MRSA) collected from the homes of people diagnosed with a community-onset (CO-) MRSA infection. Appl Environ Microbiol. doi: 10.1128/AEM.01369-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitschar K, Wolz C, Krismer B, Peschel A, Schittek B. 2017. Keratinocytes as sensors and central players in the immune defense against Staphylococcus aureus in the skin. J Dermatol Sci 87:215–220. doi: 10.1016/j.jdermsci.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki T, Kawai T. 2014. Toll-like receptor signaling pathways. Front Immunol 5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skabytska Y, Kaesler S, Volz T, Biedermann T. 2016. The role of innate immune signaling in the pathogenesis of atopic dermatitis and consequences for treatments. Semin Immunopathol 38:29–43. doi: 10.1007/s00281-015-0544-y. [DOI] [PubMed] [Google Scholar]
- 6.Di Meglio P, Perera GK, Nestle FO. 2011. The multitasking organ: recent insights into skin immune function. Immunity 35:857–869. doi: 10.1016/j.immuni.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Soong G, Paulino F, Wachtel S, Parker D, Wickersham M, Zhang D, Brown A, Lauren C, Dowd M, West E, Horst B, Planet P, Prince A. 2015. Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. mBio 6:e00289-15. doi: 10.1128/mBio.00289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srisuwan S, Voravuthikunchai SP. 2017. Rhodomyrtus tomentosa leaf extract inhibits methicillin-resistant Staphylococcus aureus adhesion, invasion, and intracellular survival in human HaCaT keratinocytes. Microb Drug Resist 23:1002–1012. doi: 10.1089/mdr.2016.0284. [DOI] [PubMed] [Google Scholar]
- 9.Brinch KS, Sandberg A, Baudoux P, Van Bambeke F, Tulkens PM, Frimodt-Moller N, Hoiby N, Kristensen HH. 2009. Plectasin shows intracellular activity against Staphylococcus aureus in human THP-1 monocytes and in a mouse peritonitis model. Antimicrob Agents Chemother 53:4801–4808. doi: 10.1128/AAC.00685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Bambeke F, Carryn S, Seral C, Chanteux H, Tyteca D, Mingeot-Leclercq MP, Tulkens PM. 2004. Cellular pharmacokinetics and pharmacodynamics of the glycopeptide antibiotic oritavancin (LY333328) in a model of J774 mouse macrophages. Antimicrob Agents Chemother 48:2853–2860. doi: 10.1128/AAC.48.8.2853-2860.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera AM, Boucher HW. 2011. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc 86:1230–1243. doi: 10.4065/mcp.2011.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borysowski J, Lobocka M, Miedzybrodzki R, Weber-Dabrowska B, Gorski A. 2011. Potential of bacteriophages and their lysins in the treatment of MRSA: current status and future perspectives. BioDrugs 25:347–355. doi: 10.2165/11595610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Zheng P, Ji W, Fu Q, Wang H, Yan Y, Sun J. 2016. SLPW: a virulent bacteriophage targeting methicillin-resistant Staphylococcus aureus in vitro and in vivo. Front Microbiol 7:934. doi: 10.3389/fmicb.2016.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rios AC, Moutinho CG, Pinto FC, Del Fiol FS, Jozala A, Chaud MV, Vila MM, Teixeira JA, Balcao VM. 2016. Alternatives to overcoming bacterial resistances: state-of-the-art. Microbiol Res 191:51–80. doi: 10.1016/j.micres.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Bi Y, Shang X, Wang M, Linden SB, Li Y, Li Y, Nelson DC, Wei H. 2016. Antibiofilm activities of a novel chimeolysin against Streptococcus mutans under physiological and cariogenic conditions. Antimicrob Agents Chemother 60:7436–7443. doi: 10.1128/AAC.01872-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Li D, Li X, Hu L, Cheng M, Xia F, Gong P, Wang B, Ge J, Zhang H, Cai R, Wang Y, Sun C, Feng X, Lei L, Han W, Gu J. 2016. LysGH15 kills Staphylococcus aureus without being affected by the humoral immune response or inducing inflammation. Sci Rep 6:29344. doi: 10.1038/srep29344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attai H, Rimbey J, Smith GP, Brown PJB. 2017. Expression of a peptidoglycan hydrolase from lytic bacteriophages Atu_ph02 and Atu_ph03 triggers lysis of Agrobacterium tumefaciens. Appl Environ Microbiol 83:e01498-17. doi: 10.1128/AEM.01498-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh PK, Donovan DM, Kumar A. 2014. Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob Agents Chemother 58:4621–4629. doi: 10.1128/AAC.00126-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischetti VA. 2010. Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int J Med Microbiol 300:357–362. doi: 10.1016/j.ijmm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sulakvelidze A, Alavidze Z, Morris JG Jr. 2001. Bacteriophage therapy. Antimicrob Agents Chemother 45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson JL, Lowell BC, Ryabinina OP, Lloyd RS, McCullough AK. 2011. TAT-mediated delivery of a DNA repair enzyme to skin cells rapidly initiates repair of UV-induced DNA damage. J Investig Dermatol 131:753–761. doi: 10.1038/jid.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Jiang K, Tai L, Liu Y, Wei G, Lu W, Pan W. 2016. Facile noninvasive retinal gene delivery enabled by penetratin. ACS Appl Mater Interfaces 8:19256–19267. doi: 10.1021/acsami.6b04551. [DOI] [PubMed] [Google Scholar]
- 23.Di Pisa M, Chassaing G, Swiecicki JM. 2015. Translocation mechanism(s) of cell-penetrating peptides: biophysical studies using artificial membrane bilayers. Biochemistry 54:194–207. doi: 10.1021/bi501392n. [DOI] [PubMed] [Google Scholar]
- 24.Copolovici DM, Langel K, Eriste E, Langel U. 2014. Cell-penetrating peptides: design, synthesis, and applications. ACS Nano 8:1972–1994. doi: 10.1021/nn4057269. [DOI] [PubMed] [Google Scholar]
- 25.Cui Z, Feng T, Gu F, Li Q, Dong K, Zhang Y, Zhu Y, Han L, Qin J, Guo X. 2017. Characterization and complete genome of the virulent Myoviridae phage JD007 active against a variety of Staphylococcus aureus isolates from different hospitals in Shanghai, China. Virol J 14:26. doi: 10.1186/s12985-017-0701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha B, Herrmann M. 2005. Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb Haemost 94:266–277. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CY, Yang SC, Sung CT, Weng YH, Fang JY. 2017. Anti-MRSA malleable liposomes carrying chloramphenicol for ameliorating hair follicle targeting. Int J Nanomedicine (Lond) 12:8227–8238. doi: 10.2147/IJN.S147226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller LS, Cho JS. 2011. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed MF, Abdelkhalek A, Seleem MN. 2016. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci Rep 6:29707. doi: 10.1038/srep29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poovelikunnel T, Gethin G, Humphreys H. 2015. Mupirocin resistance: clinical implications and potential alternatives for the eradication of MRSA. J Antimicrob Chemother 70:2681–2692. doi: 10.1093/jac/dkv169. [DOI] [PubMed] [Google Scholar]
- 31.Carryn S, Chanteux H, Seral C, Mingeot-Leclercq MP, Van Bambeke F, Tulkens PM. 2003. Intracellular pharmacodynamics of antibiotics. Infect Dis Clin North Am 17:615–634. doi: 10.1016/S0891-5520(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 32.Gu J, Feng Y, Feng X, Sun C, Lei L, Ding W, Niu F, Jiao L, Yang M, Li Y, Liu X, Song J, Cui Z, Han D, Du C, Yang Y, Ouyang S, Liu ZJ, Han W. 2014. Structural and biochemical characterization reveals LysGH15 as an unprecedented “EF-hand-like” calcium-binding phage lysin. PLoS Pathog 10:e1004109. doi: 10.1371/journal.ppat.1004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermoso JA, Garcia JL, Garcia P. 2007. Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr Opin Microbiol 10:461–472. doi: 10.1016/j.mib.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Edwards AM, Potter U, Meenan NA, Potts JR, Massey RC. 2011. Staphylococcus aureus keratinocyte invasion is dependent upon multiple high-affinity fibronectin-binding repeats within FnBPA. PLoS One 6:e18899. doi: 10.1371/journal.pone.0018899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Wang S, Shi P. 2016. Transcriptional transactivator peptide modified lidocaine-loaded nanoparticulate drug delivery system for topical anesthetic therapy. Drug Deliv 23:3193–3199. doi: 10.3109/10717544.2016.1160459. [DOI] [PubMed] [Google Scholar]
- 36.Loeffler JM, Djurkovic S, Fischetti VA. 2003. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect Immun 71:6199–6204. doi: 10.1128/IAI.71.11.6199-6204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelesidis T. 2014. The interplay between daptomycin and the immune system. Front Immunol 5:52. doi: 10.3389/fimmu.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Zhang C, Wang H, Yan YX, Sun J. 2016. A novel prophage lysin Ply5218 with extended lytic activity and stability against Streptococcus suis infection. FEMS Microbiol Lett 363:fnw186. doi: 10.1093/femsle/fnw186. [DOI] [PubMed] [Google Scholar]
- 39.Reichelt P, Schwarz C, Donzeau M. 2006. Single step protocol to purify recombinant proteins with low endotoxin contents. Protein Expr Purif 46:483–488. doi: 10.1016/j.pep.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 40.Wiegand I, Hilpert K, Hancock RE. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Guo C, Xu Y, Liu G, Lu C, Liu Y. 2014. Two novel functions of hyaluronidase from Streptococcus agalactiae are enhanced intracellular survival and inhibition of proinflammatory cytokine expression. Infect Immun 82:2615–2625. doi: 10.1128/IAI.00022-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding Y, Fu Y, Lee JC, Hooper DC. 2012. Staphylococcus aureus NorD, a putative efflux pump coregulated with the Opp1 oligopeptide permease, contributes selectively to fitness in vivo. J Bacteriol 194:6586–6593. doi: 10.1128/JB.01414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.