ABSTRACT
Fifteen currently marketed intravaginal protection products (11 types of tampon and 4 types of menstrual cup) were tested by the modified tampon sac method to determine their effect on Staphylococcus aureus growth and toxic shock syndrome toxin 1 (TSST-1) production. Most tampons reduced S. aureus growth and TSST-1 production, with differences based on brand and composition, and the level of S. aureus growth was higher in destructured than in unaltered tampons. We observed higher levels of S. aureus growth and toxin production in menstrual cups than in tampons, potentially due to the additional air introduced into the bag by cups, with differences based on cup composition and size.
IMPORTANCE Menstrual toxic shock syndrome is a rare but severe disease. It occurs in healthy women vaginally colonized by Staphylococcus aureus producing toxic shock syndrome toxin 1 using intravaginal protection, such as tampons or menstrual cups. Intravaginal protection induces TSS by the collection of catamenial products, which act as a growth medium for S. aureus. Previous studies evaluated the impact of tampon composition on S. aureus producing toxic shock syndrome toxin 1, but they are not recent and did not include menstrual cups. This study demonstrates that highly reproducible results for S. aureus growth and TSST-1 production can be obtained by using a simple protocol that reproduces the physiological conditions of tampon and cup usage as closely as possible, providing recommendations for tampon or cup use to both manufacturers and consumers. Notably, our results do not show that menstrual cups are safer than tampons and suggest that they require similar precautions.
KEYWORDS: tampon, menstrual cup, Staphylococcus aureus, toxic shock syndrome toxin 1, biofilm, elastomer, fiber
INTRODUCTION
Toxic shock syndrome (TSS) is a rare but severe disease characterized by fever, hypotension, a skin rash with subsequent desquamation, and multiple organ dysfunctions (1). It can occur in healthy menstruating women using intravaginal protection, such as tampons or menstrual cups, and colonized by Staphylococcus aureus producing toxic shock syndrome toxin 1 (TSST-1) (2–5). Intravaginal protection-induced TSS is linked to the potential abilities of the colonizing S. aureus strain to use the catamenial products collected in the tampon or cup as a growth medium and proliferate (6). S. aureus produces TSST-1 when it reaches a threshold concentration and then gains access to the bloodstream and induces systemic illness (7, 8).
S. aureus tightly regulates TSST-1 production by several systems, such as the Agr quorum-sensing system. Its production is additionally affected by physical and chemical factors, such as oxygen, carbon dioxide and divalent cation concentrations, pH, and temperature, by other two-component sensor systems, such as SaeRS and SrrAB (9–12).
Menstrual TSS was initially described in women using tampons made of high-absorbency fibers composed of carboxymethylcellulose and polyester, which favored S. aureus growth and TSST-1 production (13). Although carboxymethylcellulose and polyester fibers are no longer used, the disease continues to occur in menstruating women using currently marketed tampons made of cotton, viscose, and rayon (14), and a case of menstrual TSS in a woman using a silicone menstrual cup was also described (5).
Previous studies investigated whether tampons made of cotton or rayon affect S. aureus growth and TSST-1 production using bacterial cultivation with or without the addition of tampons and found that none of them amplified TSST-1 production (15–20). However, those reports are several decades old, and the experimental conditions were generally quite different from those of typical tampon use, sometimes including sterilization of the tampons before the experiment, a volume of broth medium exceeding both the tampon absorption capacity and normal menstrual flow volume, longer incubation times, or incubation without oxygen limitation in accordance with vaginal gas conditions. Tampon compositions may have changed over time, for example, with the introduction of viscose. Most importantly, tampon use continues to be associated with menstrual TSS, and a case of menstrual TSS associated with a silicone menstrual cup has been described, calling the effects of menstrual cups on S. aureus growth and TSST-1 production into question (5, 14).
The aim of this study was to reevaluate the impact of currently marketed tampons and menstrual cups on S. aureus growth and TSST-1 production under experimental conditions close to those of typical tampon and cup usage, without tampon sterilization and with appropriate amounts of broth medium and oxygen access.
RESULTS
Impact of tampons and cups on S. aureus growth.
A calibrated suspension of S. aureus was cultivated during 8 h in brain heart infusion (BHI) broth at 37°C inside sterile plastic bags in the presence or absence of a tampon or cup to determine whether tampons and cups affect S. aureus growth (Tables 1 and 2). The bacterial solution was collected for bacterial quantification using both a flow cytometer and plate spreading followed by enumeration with an automatic colony counter at the end of the incubation. No aerobic growth was observed in BHI broth after 8 h of incubation with any tampon or menstrual cup in the absence of S. aureus inoculation.
TABLE 1.
Physicochemical characteristics of the tampons and their effects on S. aureus growth and TSST-1 productiona
| Product | Composition | Mean tampon absorbency (ml) ± SD |
S. aureus |
TSST-1 |
||
|---|---|---|---|---|---|---|
| Mean bacterial count (CFU/ml) ± SD | P value | Mean concn (ng/ml) ± SD | P value | |||
| Medium alone (positive control) | 2.5 × 109 ± 0.2 × 109 | 73.9 ± 8.5 | ||||
| Tampons | ||||||
| Tampax compak | ||||||
| Regular | Cotton and rayon | 26.2 ± 1.2 | 3.2 × 107 ± 2 × 107 | <0.001 | 0.1 ± 0 | <0.001 |
| Super plus | Cotton and rayon | 40.6 ± 0.3 | 7.9 × 106 ± 3 × 106 | <0.001 | 1.4 ± 0.3 | <0.001 |
| Natracare | ||||||
| Regular | Cotton | 28.2 ± 1.2 | 8.2 × 108 ± 3 × 108 | <0.001 | 17.9 ± 3.1 | <0.001 |
| Super plus | Cotton | 36.1 ± 1.1 | 7.4 × 108 ± 5 × 108 | <0.001 | 70.5 ± 7.5 | NS |
| Nett Procomfort | ||||||
| Nuit super | Viscose | 25.3 ± 0.3 | 1.7 × 108 ± 0.7 × 108 | <0.001 | 1.3 ± 0.7 | <0.001 |
| Normal | Viscose | 21.9 ± 0.2 | 1.9 × 108 ± 1 × 108 | <0.001 | 0.7 ± 0.2 | <0.001 |
| Super plus | Viscose | 34.4 ± 0.5 | 1.0 × 108 ± 0.4 × 108 | <0.001 | 0.7 ± 0.2 | <0.001 |
| o.b. ProComfort | ||||||
| Light days | Cotton and viscose | 18.1 ± 1.2 | 2.1 × 108 ± 0.6 × 108 | <0.001 | 0.8 ± 0.1 | <0.001 |
| Super | Cotton and viscose | 30.5 ± 1.5 | 1.1 × 108 ± 0.6 × 108 | <0.001 | 2.1 ± 1.0 | <0.001 |
| Normal | Cotton and viscose | 25.2 ± 1.2 | 3.2 × 108 ± 1 × 108 | <0.001 | 2.5 ± 1.3 | <0.001 |
| Super plus | Cotton and viscose | 35.7 ± 0.5 | 1.1 × 108 ± 0.6 × 108 | <0.001 | 1.8 ± 0.8 | <0.001 |
Values are means ± standard deviations (n = 3 to 8). Student's t tests were used to compare bacterial CFU and TSST-1 concentrations in the presence of tampons to the values for the control. NS, not significant.
TABLE 2.
Characteristics of menstrual cups and their effects on S. aureus growth and TSST-1 productiona
| Product | Composition | Diam/ht (cm) |
S. aureus |
TSST-1 |
||
|---|---|---|---|---|---|---|
| Mean bacterial count (CFU/ml) ± SD | P value | Mean concn (ng/ml) ± SD | P value | |||
| Medium alone (positive control) | 2.5 × 109 ± 0.2 × 109 | 73.9 ± 8.5 | ||||
| Cups | ||||||
| be'Cup | ||||||
| Size 1 | Silicone | 4/4.5 | 1.1 × 109 ± 0.3 × 109 | 0.001 | 50.6 ± 8.5 | 0.03 |
| Size 2 | Silicone | 4.5/5 | 4.4 × 109 ± 1.1 × 109 | 0.001 | 87.3 ± 4.1 | 0.001 |
| Me Luna | ||||||
| Size S | TPE | 3.8/4.5 | 5.7 × 108 ± 4 × 108 | <0.001 | 87.3 ± 4.1 | 0.043 |
| Size M | TPE | 4.5/5 | 7.6 × 108 ± 2 × 108 | 0.001 | 108.7 ± 23.2 | 0.03 |
TPE, thermoplastic elastomers. Values are means ± standard deviations (n = 3 to 8). Student's t tests were used to compare bacterial CFU and TSST-1 concentrations in the presence of cups to the values for the control.
Tampons and cups affect S. aureus growth, as shown in Tables 1 and 2. The level of S. aureus growth was lower than that for the positive control in the presence of tampons, with final bacterial concentrations ranging from 7.9 × 106 ± 3 × 106 CFU/ml to 8.2 × 108 ± 3 × 108 CFU/ml, versus 2.5 × 109 ± 0.2 × 109 CFU/ml with the positive control. S. aureus growth was not statistically correlated with tampon absorbance, but S. aureus behaviors varied significantly as a function of the tampon composition. The level of S. aureus growth was lower with tampons composed of a mix of rayon and cotton than with those composed of viscose with or without the addition of cotton or cotton alone (P < 0.001). Levels of S. aureus growth were equivalent for tampons made of viscose or a mix of viscose and cotton and lower than those for tampons made of cotton alone (P < 0.001).
In the presence of menstrual cups, the level of S. aureus growth was close to that of the positive control but statistically different, with final bacterial concentrations ranging from 5.7 × 108 ± 4 × 108 CFU/ml to 4.4 × 109 ± 1.1 × 109 CFU/ml (P < 0.05). The level of S. aureus growth was significantly lower with cups made from thermoplastic elastomers (TPE) than with those made from silicone (P < 0.001).
Impact of tampons and cups on TSST-1 production by S. aureus.
The level of TSST-1 was also quantified in S. aureus growth supernatant in the absence or presence of tampons or cups (Tables 1 and 2). The level of TSST-1 production ranged from 0.1 to 70.5 ± 7.5 ng/ml in the presence of tampons, while the level of production of the control was 73.9 ± 8.5 ng/ml. The level of TSST-1 production was significantly lower in the presence of tampons than for the control (P < 0.001), except for one tampon made of cotton (Natracare super plus). Levels of TSST-1 production in the presence of tampons made of viscose alone and a mix of cotton and rayon were similar and were lower than those in the presence of tampons made with a mix of cotton and viscose (P < 0.001). The levels of production of TSST-1 with these 3 tampon compositions were also significantly lower than that in the presence of tampons made of cotton alone (P < 0.001). We observed a strong correlation between TSST-1 production and S. aureus growth (P < 0.001).
In contrast to the levels shown with tampons, the levels of TSST-1 production were increased in the presence of 3 of the 4 cups tested (87.3 ± 4.1 to 108.7 ± 23.2 ng/ml versus 73.9 ± 8.5 ng/ml), both TPE cups (P < 0.05) and 1 of the 2 silicon cups. The level of TSST-1 production with the other silicone cup (be'Cup size 1, 50.6 ± 8.5 ng/ml) was lower than that seen in the control (P = 0.03). The level of TSST-1 increased with the size of the cup, and the level of production of TSST-1 significantly increased with S. aureus growth (P = 0.001).
The search for molecules interfering with S. aureus in tampons and menstrual cups.
To understand how tampon composition and menstrual cups modify S. aureus growth and TSST-1 production, we first examined whether tampons or cups released molecules that could interfere with S. aureus. Tampons and cups were incubated in BHI broth for 8 h at 37°C before removal, and preincubated BHI broth with a tampon or cup (BHI-T) was used as the template for this experiment. We compared S. aureus growth and TSST-1 production in BHI broth and BHI-T broth and found no difference in S. aureus growth and TSST-1 production with the use of any tampon or menstrual cup during preincubation (data not shown). These results suggest that tampons and cups do not release molecules that significantly interfere with S. aureus.
Does tampon structure modify S. aureus growth and TSST-1 production?
Since Tierno and Hanna suggested that the structure of tampons may impact S. aureus growth and TSST-1 production (18), we examined the impact of tampon structure on S. aureus growth and TSST-1 production after determining that tampons and cups do not release any inhibiting molecules. Tampons composed of rayon and cotton (Tampax compak super plus), viscose and cotton (o.b. ProComfort super plus), viscose alone (Nett Procomfort normal), and cotton alone (Natracare regular) were deconstructed with a sterile scalpel in sterile plates. Deconstructed and unaltered tampons were placed into plastic bags and incubated with S. aureus, as described below. S. aureus growth was again significantly inhibited by the presence of each of the four unaltered tampons (structured), regardless of the composition (P < 0.001), as well as by the deconstructed tampons (P < 0.001) (Fig. 1). Interestingly, the level of S. aureus growth was significantly higher in deconstructed than in unaltered tampons if they were composed of cotton and rayon (3-fold; P = 0.04) or cotton and viscose (16-fold; P = 0.01) but not in tampons made of cotton or viscose alone.
FIG 1.
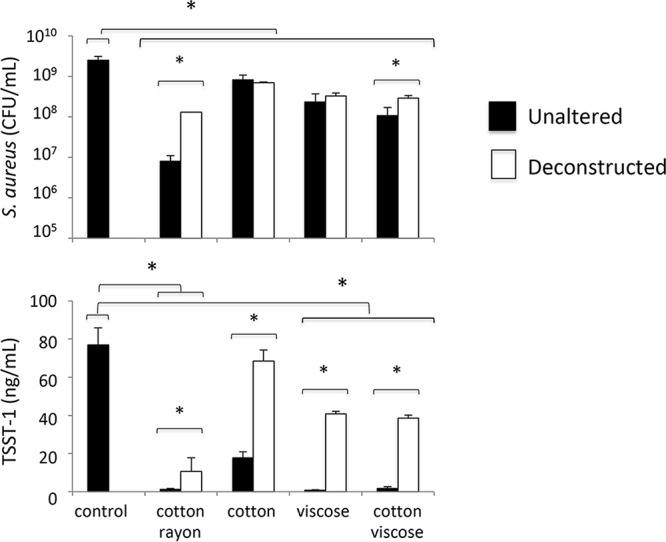
Effects of tampon fiber composition and their structure on S. aureus growth and TSST-1 production. S. aureus was incubated in BHI broth in sterile bags in the presence or absence of unaltered tampons or tampons deconstructed by dilacerations for 8 h at 37°C with shaking before quantification of S. aureus and TSST-1. Results shown are means of data from 3 experiments ± standard deviations. Student's t tests were used to compare bacterial CFU and TSST-1 concentrations in the presence of deconstructed tampons to those in the presence of unaltered tampons (*, P < 0.05).
Similar results were observed for TSST-1 production. The levels of TSST-1 production were higher in deconstructed than in unaltered tampons (P < 0.001) and were 7-fold higher in mixed cotton-and-rayon tampons, 4-fold higher in cotton tampons, 54-fold higher in viscose tampons, and 21-fold higher in mixed cotton-and-viscose tampons.
Tampon composition modifies S. aureus growth and TSST-1 production.
We observed that S. aureus growth and toxin production were inhibited in the presence of tampon fibers in the deconstructed samples, varying according to the nature of the fibers (Fig. 1). The level of S. aureus inhibition was highest in the presence of mixed cotton-and-rayon fibers, followed by mixed cotton and viscose, viscose alone, and cotton alone.
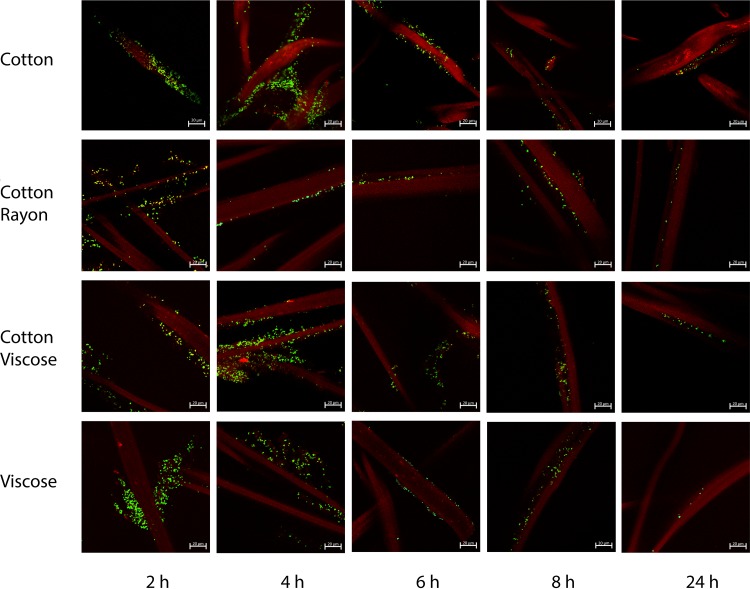
We investigated the interactions of green fluorescent protein (GFP)-labeled S. aureus cells with tampon fibers using confocal microscopy (Fig. 2). We selected 4 types of tampon composed of different fibers: mixed rayon and cotton (Tampax compak super plus), mixed viscose and cotton (o.b. ProComfort super plus), cotton alone (Natracare super plus), and viscose alone (Nett Procomfort super plus). S. aureus colonized the tampon fibers to various degrees, depending on the incubation time and the nature of the fibers. A higher level of colonization of tampon fibers was seen with short incubation times (2 and 4 h) than with longer incubations (6, 8, and 24 h). However, heterogeneous adhesion between S. aureus and fibers was seen, and no strong differences in colonization levels by S. aureus were observed between the fibers of the different tampons (Fig. 2).
FIG 2.
Effects of tampon fiber composition and incubation time on S. aureus colonization. The fibers of tampons composed of cotton alone (Natracare super plus), mixed rayon and cotton (Tampax compak super plus), mixed viscose and cotton (o.b. ProComfort super plus), and viscose alone (Nett Procomfort super plus) were incubated for 2, 4, 6, 8, or 24 h at 37°C without agitation in the presence of GFP-labeled S. aureus strain LUG2902. Fibers were then washed, mounted under coverslides, and observed by confocal microscopy, as described in Materials and Methods. Three-dimensional (3D) projections were reconstructed by using the ZENV2.3 software package from z-stacks with 1-μm serial optical sections. Tampon fibers are shown in red, and S. aureus cells are labeled green.
Does cup composition modify S. aureus growth and TSST-1 production?
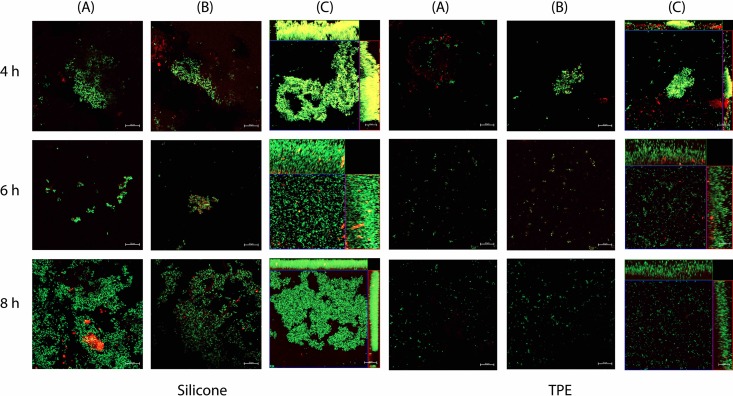
We quantified biofilm formation to understand how S. aureus growth and TSST-1 production were affected by cup composition. Silicone and TPE menstrual cups (be'Cup size 2 [silicone] and Me Luna size S [TPE]) were inserted vertically into sterile bags and used as S. aureus growth recipients for 8 h at 37°C under static conditions. We then quantified bacteria in the growth medium (i.e., planktonic cells) and in the biofilm formed at the surface of the cup after disruption by sonication (Fig. 3). Levels of S. aureus growth and TSST-1 production were significantly lower under static conditions than with shaking (Table 2) (P ≤ 0.002) for the control and the two cups. Under static conditions, the total numbers of S. aureus bacteria were not significantly different between either of the two cups and the control. Interestingly, the number of S. aureus bacteria in a biofilm was higher in the silicone be'Cup size 2 cup than in the TPE Me Luna cup. Microscopy analysis of the interaction of GFP-labeled S. aureus with sections of cups showed that the S. aureus biofilm was more developed at 4, 6, and 8 h with the cup made of silicone than with those made of TPE, with more fluorescent bacteria on the surface and a thicker biofilm (Fig. 4).
FIG 3.
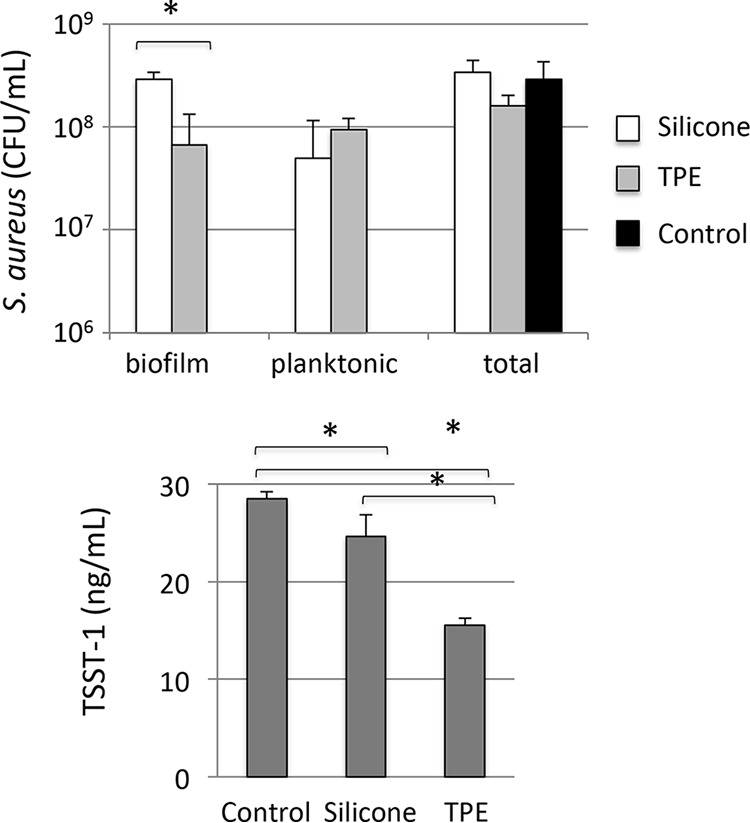
Biofilm formation and TSST-1 production by S. aureus differ between cups. S. aureus was incubated in BHI broth in sterile bags in the presence of be'Cup size 2 (silicone) and Me Luna size S (TPE) menstrual cups for 8 h at 37°C without shaking, before the separation of biofilm and planktonic S. aureus cells. Cell numbers and levels of TSST-1 production in both biofilm and planktonic compartments were quantified, and the total number of bacteria represents the sum of results from biofilm and planktonic compartments. Results shown are means of data from 3 experiments ± standard deviations. Student's t tests were used to compare bacterial CFU in the presence of TPE cups to those in the presence of silicone cups and to compare TSST-1 concentrations in the presence of cups to the values for the control (*, P < 0.05).
FIG 4.
Biofilm formation by S. aureus on sections of cups made of silicone and TPE. Sections of 1 by 1.5 cm of be'Cup size 1, made of silicone, and Me Luna size S, made of TPE, were incubated in the presence of GFP-labeled S. aureus LUG2902 without agitation. After 4, 6, and 8 h, sections of cups were recovered, washed, mounted under coverslides, and observed by confocal microscopy, as described in Materials and Methods. 3D projections were reconstructed by using the ZENV2.3 software package from z-stacks with 1-μm serial optical sections. S. aureus cells are labeled green. Panels A and B correspond to two-dimensional (2D) observations at different locations, while panels C correspond to the 3D projections from z-stacks.
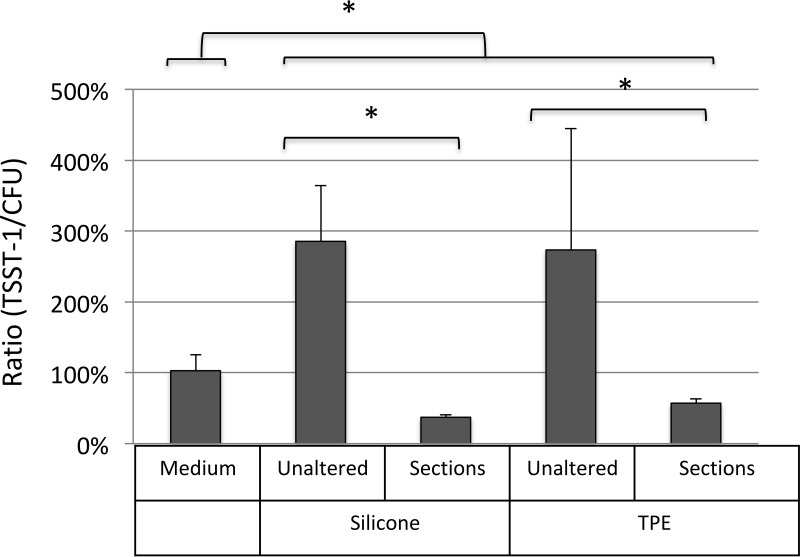
Under static conditions, the levels of TSST-1 production in the presence of both the be'Cup and Me Luna menstrual cups were significantly lower than that for the control (P = 0.045 and P < 0.001, respectively) (Fig. 3). The level of TSST-1 was also significantly lower in the presence of the Me Luna (TPE) cup than in the presence of be'Cup (silicone) (P = 0.002) (Fig. 3). Since the shapes of the Me Luna and the be'Cup menstrual cups were not identical, it remained unclear if differences in the volumes of air or in the compositions of these menstrual cups were responsible for the variation in TSST-1 production. Thus, be'Cup size 1 made of silicone and Me Luna size S made of TPE were cut into sections of 1 by 1.5 cm before experiments using the sac method with shaking (Fig. 5). Interestingly, bacterial growth was significantly affected by the alteration of the two models of cup. The level of S. aureus growth was higher with sections of cups than with native cups (1.9-fold with be'Cup and 1.5-fold with Me Luna). We suspect that the presence of sections increased the shaking of the medium and favored S. aureus growth. Since toxin production depended on the bacterial concentration, the ratio of toxin/CFU of bacteria was used to compare the effects of cup alterations. The ratios of toxin/CFU were similar with the two models of cup with unaltered cups and sections of cups. Moreover, when the cup was cut into sections, the toxin/CFU ratio decreased dramatically compared to that with the unaltered cup. Our results suggested that aeration, more than the composition of the cup, influences toxin production.
FIG 5.
Differential impacts of the shape and composition of menstrual cups on TSST-1 production. S. aureus was incubated in BHI broth in sterile bags in the presence of unaltered cups or sections of 1 by 1.5 cm of be'Cup size 1, made of silicone, and Me Luna size S, made of TPE. be'Cup size 1 (silicone) and Me Luna size S (TPE) menstrual cups were incubated for 8 h at 37°C with shaking, before the separation of biofilm and planktonic S. aureus cells. Bacterial numbers and TSST-1 production in planktonic compartments were quantified as described in the text. Results are expressed as ratios of TSST-1 (nanograms per milliliter)/bacteria (CFU per milliliter) and are the means of data from at least 3 experiments ± standard deviations. Student's t tests were used to compare ratios between the control (medium) and unaltered cups or sections of menstrual cups made of silicone or TPE and between unaltered cups or sections of cups made of silicone and those made of TPE (*, P < 0.05).
DISCUSSION
Menstrual TSS is a rare but severe disease that occurs in women using intravaginal protection, such as tampons or menstrual cups, who are vaginally colonized by S. aureus producing TSST-1 (2–5). Intravaginal protection promotes menstrual TSS indirectly by the collection of catamenial products, which can act as a growth medium for S. aureus present in the vagina under favorable temperature, atmospheric (oxygen), and acidic pH neutralization conditions (6, 9, 10). The historical association between one tampon brand with a unique chemical composition and menstrual TSS and in vitro experiments have highlighted the direct impact that tampons can have on S. aureus proliferation and TSST-1 production (13). The aim of this study was to reexamine the impact of currently marketed tampons and menstrual cups on S. aureus growth and TSST-1 production in vitro.
We designed novel experimental conditions inspired by the tampon sac method described previously by Reiser et al. (21), including nonsterilized tampons, sterile hermetic bags manually deflated to limit the air introduced with the product, and a volume of broth medium limited to 15 ml that could be absorbed entirely by each tampon and allow for an 8-h incubation (the manufacturers recommend against using the products for more than 8 h). Under these experimental conditions, designed to be simple while reproducing as much of the physiological conditions of tampon and cup use as possible, we obtained highly reproducible results for S. aureus growth and TSST-1 production. We believe that our method meets the standards required for such an investigation.
Using this procedure, we found that TSST-1 production correlated with S. aureus growth, all tampons tested inhibited S. aureus growth, and most of them reduced TSST-1 production. Only one cotton tampon did not influence TSST-1 production, and none of them enhanced S. aureus growth and TSST-1 production, as seen previously using carboxymethylcellulose and polyester tampons (6, 20, 22). Our results were in agreement with data from previous reports (15–20), and we observed various impacts of the tampon brand and model on S. aureus behaviors. Schematically, the addition of tampons composed of cotton (Natracare) to S. aureus in broth medium has little impact on bacterial growth and TSST-1 production, while the addition of viscose (Nett), mixed cotton-and-rayon (Tampax), or mixed cotton-and-viscose (o.b.) tampons inhibited S. aureus growth and TSST-1 production more effectively. Our results did not support the hypothesis of Tierno and Hanna suggesting that tampons composed exclusively of cotton could be intrinsically safer than those made of mixed cotton and rayon or viscose or tampons composed exclusively of viscose, as previously observed (16, 18).
The observed differences between tampon brands could not be explained by contaminants or additive molecules released from the tampons that could interfere with S. aureus during the experiments. Confocal analysis revealed little interaction between S. aureus and cotton tampon fibers, with clusters of staphylococci being seen in contact with fibers during early time points (2 to 4 h), which later disappeared. This observation suggests that S. aureus is not able to produce a stable biofilm in contact with the four types of fibers tested (cotton, viscose, or mixed cotton and rayon or cotton and viscose). Interestingly, the differences observed between tampons persist but dramatically decrease when the tampons are deconstructed. This suggests that in addition to the nature of the fiber, with the possible presence of additive products not dissociable from them (with the exception of carboxymethylcellulose), the structure of the tampon and, possibly, the fiber density have major impacts on S. aureus growth and TSST-1 production. The space between the fibers in the tampon may contribute to the intake of air in the vagina and could represent the major site of S. aureus growth and toxin production, as previously suggested (15, 23). Long tampon carriage may also alter the structure in a manner favoring S. aureus growth and TSST-1 production, advocating for a short time of use and frequent changing.
We also examined the impact of menstrual cups on S. aureus growth and TSST-1 production using the same experimental procedure and observed higher levels of S. aureus growth and toxin production with cups than with tampons. We suspect that this difference may be explained by the experimental procedure, specifically that a higher volume of air was inserted into the bag with cups than with tampons due to their shape, which is known to favor S. aureus growth and TSST-1 production (9, 10).
This was confirmed by the significant increase in S. aureus growth and TSST-1 production observed with larger models of the cups, regardless of the brand or composition. Interestingly, when incubation was performed with shaking, the level of S. aureus growth was higher in the presence of the silicone be'Cup cup than in the presence of the TPE Me Luna cup, but the opposite result was found for TSST-1 production. When incubation was performed without shaking, similar total numbers of S. aureus bacteria were observed for silicone or TPE cups and the control. However, the levels of biofilm and TSST-1 production were higher with the silicone be'Cup cup than with the TPE Me Luna cup. The oxygen permeability of silicone compared to TPE may explain this difference (24). This was confirmed for biofilm formation by confocal microscopy using sections of cups. Additionally, to differentiate the respective roles of the shape and composition of menstrual cups, both of which differ between the two brands, we repeated sac method experiments with sections cut from the different cups. With these sections, we were able to totally deflate the plastic bag filled with sections of cups and did not observe differences in TSST-1 production between cups anymore. Our results suggest that aeration, linked to the shape and volume of the cup, influences toxin production more than does the composition of the cup. While our results with the cups were obtained with in vitro experiments, they suggest some indications for cup use. Our results show that the menstrual cup is a risk factor for menstrual toxic shock syndrome. When possible, the use of a small menstrual cup should be recommended. Air inserted into the vagina along with the cup may favor S. aureus growth and TSST-1 production in the catamenial products collected in the cup. When the volume of menstrual fluid exceeds that of the cup receptacle, TSST-1 produced in the fluid is in contact with the vaginal mucosa, allowing toxin transcytosis into the blood and the development of menstrual toxic shock syndrome. Time-based advice on cup emptying and removal should be modulated with the volume of menstrual loss (25).
Additionally, a significant amount of biofilm of S. aureus remained after 8 h and 3 washes with water, regardless of the cup model or composition. Manual instructions for the menstrual cup indicate that the cup could be removed, emptied, and rinsed with tap water before being reinserted, but our results suggest that women may reinsert a contaminated cup when following this advice. A protocol including a second cup that allows for cup sterilization by boiling between uses should be recommended.
In conclusion, we showed that none of the cotton, viscose, or mixed cotton-and-rayon or cotton-and-viscose tampons enhance in vitro S. aureus growth and toxin production, using a new method for bacterial cultivation in the presence of intravaginal protection that reproduces physiological conditions. Some of them even decrease S. aureus growth and toxin production by a mechanism that seems unrelated to the release of molecules from the tampon. We observed slight increases of S. aureus growth and toxin production with menstrual cups, due to the introduction of a higher volume of air than that occurring with tampons in our in vitro system. The use of a small cup should be advised to limit this effect. Additionally, S. aureus forms a compact biofilm in contact with the cup, which is resistant to simple washes with water. Boiling of the menstrual cup between uses of the cup should be recommended. Both intravaginal devices appear to be risk factors for the development of menstrual toxic shock syndrome, and precautions should be advised.
MATERIALS AND METHODS
In accordance with French legislation, written informed patient consent was not required for the research use of the clinical isolates (French South-East ethics committee [reference L13-156]).
Strains.
S. aureus strain ST20140321 was isolated from the tampon of a patient with menstrual TSS in 2014. The strain was genotyped by using diagnostic DNA microarrays and identibac S. aureus genotyping (Alere), as described previously (26). It is classified as an agr-3, methicillin-sensitive, clonal complex 30, TSST-1-producing strain. The isolate is deposited and available at the Centre National de Reference des Staphylocoque, Institut des Agents Infectieux, Centre de Biologie Nord, Hôpital de la Croix-Rousse, Lyon, France.
We used the pACL1484 plasmid (Table 3) for the construction of the GFP-ST20140321 S. aureus strain (LUG2902). pACL1484 is a pSK236-derived shuttle plasmid containing the gene encoding GFPuvr, a variant of GFP from Aequorea victoria optimized for maximal fluorescence when excited by standard UV light (360 to 400 nm), a derivative of the GFPuv-encoding gene (27). The PCR primers used to clone the S. aureus 16S rRNA gene promoter in pALC1484 are listed in Table 3. Chromosomal DNA from S. aureus strain SH1000 was used as the PCR template, and amplified regions were ligated into the EcoRI and XbaI sites of pALC1484. The plasmid was then introduced into S. aureus RN4220 by electroporation (28), and chloramphenicol-resistant colonies were selected before the plasmid was transferred into S. aureus strain SH1000 (29) and into clinical S. aureus strain ST20140321 via standard electroporation or transduction techniques (30).
TABLE 3.
Plasmids and primers used for GFP labeling of S. aureus strain LUG2902
| Plasmid or primer | Description or sequencea | Source |
|---|---|---|
| Plasmids | ||
| pACL1484 | Promoterless gfpuvr shuttle vector | A. Cheung |
| pLUG1283 | 16S rRNA gene promoter cloned into EcoRI-XbaI site of pACL1484 | This study |
| Primers | ||
| P16s-for-EcoRI | CGGAATTCAGCGTAAGGAATTACACTTT | This study |
| P16s-rev-XbaI | GCTCTAGATGATGTTTGATTAGCTCATAAATAC | This study |
Restriction sites are underlined.
Tampons and menstrual cups.
Eleven types of tampons manufactured by Tampax, Nett, o.b., and Natracare and four types of menstrual cups manufactured by be'Cup and Me Luna were purchased from supermarkets. Tampax compak regular and super plus are made of rayon and cotton; Natracare regular and super plus are made of cotton; Nett Procomfort Nuit super, normal, and super plus are made of viscose; and o.b. ProComfort light days, super, normal, and super plus are made of cotton and viscose. Tampon absorbencies were determined by measuring weight gain after 8 h of incubation in 100 ml of sterile water and are summarized in Table 1. Applicators and plastic wrappers were removed with sterile gloves before the experiment. The be'Cup (size 1 and size 2) menstrual cups were made of silicone, and the Me Luna cups were made of TPE (classic model, sizes S and M). They were pretreated by sequential ebullition for 3 and 7 min, respectively, as advised by the manufacturers.
Experimental procedure.
We used the tampon sac method described previously by Reiser et al., with several modifications (21). Tampons and menstrual cups were inserted into 532-ml sterile plastic bags (Whirl Pak) by using sterile gloves, followed by the addition of 15 ml of either BHI broth (Difco) alone or a BHI solution with 105 CFU/ml of S. aureus strain LUG2902. Excess air introduced during the insertion of the tampon or cup into the bags was removed by manual deflation, and the bags were then hermetically sealed. The bags were incubated vertically at 37°C with shaking (200 rpm) for 8 h. The BHI solution inoculated with 105 CFU/ml S. aureus ST20140321 was also incubated under the same conditions without tampons or menstrual cups as a positive control. Four milliliters of the solution was sampled from the plastic bag at the end of the incubation. Tampons were kneaded within the bag for 5 s, and the fluid was expressed by compression of the tampon and collected for bacterial and toxin quantification, as described below.
BHI broth was also incubated with each tampon for 8 h at 37°C with shaking (200 rpm) to determine whether the tampons or cups released molecules. Tampons were then kneaded within the bag for 5 s, and BHI broth was recovered from the tampons by compression. Preincubated BHI broth, denominated BHI-T, was sterilized by filtration (0.22 μm; Micropore) and conserved at 4°C before being used as the culture medium for S. aureus LUG2902 in microplates. A total of 180 μl of BHI-T broth from each tampon was inoculated with 20 μl of LUG2902 (107 CFU/ml). Plates were incubated for 8 h at 37°C in a Spark 10M system (Tecan), and absorbances were observed every 10 min at an λ of 600 nm. The amounts of S. aureus and TSST-1 were quantified as described below.
To examine the impact of specific fibers, tampons were deconstructed under sterile conditions by laceration with a sterile scalpel before use, as described below.
To examine the impact of the composition of menstrual cups, cups were cut into sections of 1 by 1.5 cm under sterile condition with a sterile scalpel before use, as described below.
Each cup was inserted in the vertical position into a sterile plastic bag to examine biofilm formation. Fifteen milliliters of either BHI broth or BHI broth with 105 CFU/ml of S. aureus ST20140321 was put into the cavities of the cups, excess air was removed by manual deflation, and the bags were hermetically sealed. The plastic bags were then incubated vertically at 37°C without shaking for 8 h, and the bacterial solution was harvested for bacterial and toxin quantification at the end of the incubation. Cups were washed 3 times with 300 ml phosphate-buffered saline (PBS) (Difco) before sonication in 150 ml of PBS for 10 min at 100% using the BactoSonic instrument (Bandelin Electronics), and the number of S. aureus bacteria released by sonication was quantified, as described below.
S. aureus and TSST-1 quantification.
The number of bacteria in diverse solutions was estimated by flow cytometry using an Accuri C6 flow cytometer (BD Biosciences) according to the manufacturer's recommendations. The concentration was confirmed by the inoculation of a Trypticase soy agar (TSA; Difco) plate using the easySpiral automatic plater (Interscience), and the exact number of CFU was determined with the Scan1200 automatic colony counter (Interscience) after 24 h of incubation at 37°C in an aerobic atmosphere.
The level of TSST-1 in the supernatant was quantified by a specific enzyme-linked immunosorbent assay (ELISA; Toxin Technology), as recommended by the manufacturer.
Observation of S. aureus colonization of tampon fibers and menstrual cups by confocal microscopy.
Tampons of each brand were deconstructed by laceration with a sterile scalpel. A piece of approximately 0.5 cm3 of each fiber was incubated inside the wells of a 24-well plate (Falcon) in the presence of 1 ml of a standardized cell suspension of 107 CFU/ml of S. aureus LUG2902 in BHI broth. After 2, 4, 6, 8, and 24 h of incubation without agitation, the fibers were washed 5 times with 2 ml of PBS and mounted onto glass slides in the presence of Aqua-Poly/Mount mounting medium (Polysciences Inc., Warrington, PA).
Sections of 1 by 1.5 cm of be'Cup size 1 made of silicone and Me Luna size S made of TPE were mechanically attached to the bottom of wells of a 24-well plate (Falcon) before being incubated with 3 ml of a standardized suspension of S. aureus LUG2902 (107 CFU/ml) in BHI broth. After 2, 4, 6, and 8 h of incubation without agitation, pieces of cups were recovered, washed 5 times with 5 ml of PBS, and mounted onto glass slides in the presence of Aqua-Poly/Mount mounting medium.
Images were acquired with an LSM 800 confocal microscope (Zeiss, Oberkochen, Germany) using a 40× oil objective. Fibers (pseudocolored red) were observed by using 405 nm as the excitation wavelength and 400 to 570 nm as the emission wavelengths, and GFP-labeled S. aureus bacteria (pseudocolored green) were visualized by using 488 nm as the excitation wavelength and 504 to 555 nm as the emission wavelengths. z-stacks were acquired by using 1-μm serial optical sections, and images were merged by using the ZENV2.3 software package.
Statistical analysis.
The data were analyzed by using SPSS version 12.0. Bacterial CFU and TSST-1 concentrations between groups were compared by Student's t test. The Spearman correlation was used to evaluate the relationship between continuous variables such as TSST-1 levels, S. aureus CFU, and tampon absorbency volumes.
ACKNOWLEDGMENTS
We are grateful to the members of the Centre National de Référence des Staphylocoques and Isaline Jacquemond for fruitful comments and technical help.
This work was supported by the Labex Ecofect (ANR-11-LABX-0048) of the Université de Lyon within the program Investissements d'Avenir (ANR-11IDEX-0007) operated by the French National Research Agency (ANR) and the FINOVI Foundation.
REFERENCES
- 1.Stevens D. 1996. The toxic shock syndromes. Infect Dis Clin North Am 10:727–746. doi: 10.1016/S0891-5520(05)70324-X. [DOI] [PubMed] [Google Scholar]
- 2.Shands K, Schmid G, Dan B, Blum D, Guidotti R, Hargrett N, Anderson R, Hill D, Broome C, Band J, Fraser D. 1980. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med 303:1436–1442. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- 3.Bergdoll M, Crass B, Reiser R, Robbins R, Davis J. 1981. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet i:1017–1021. [DOI] [PubMed] [Google Scholar]
- 4.Schlievert P, Shands K, Dan B, Schmid G, Nishimura R. 1981. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis 143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell M, Bisch S, Arntfield S, Hosseini-Moghaddam S. 2015. A confirmed case of toxic shock syndrome associated with the use of a menstrual cup. Can J Infect Dis Med Microbiol 26:218–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melish M, Murata S, Fukunaga C, Frogner K, McKissick C. 1989. Vaginal tampon model for toxic shock syndrome. Rev Infect Dis 1(Suppl 1):S238–S246. doi: 10.1093/clinids/11.Supplement_1.S238. [DOI] [PubMed] [Google Scholar]
- 7.Sarafian S, Morse S. 1987. Environmental factors affecting toxic shock syndrome toxin-1 (TSST-1) synthesis. J Med Microbiol 24:75–78. doi: 10.1099/00222615-24-1-75. [DOI] [PubMed] [Google Scholar]
- 8.Davis C, Kremer M, Schlievert P, Squier C. 2003. Penetration of toxic shock syndrome toxin-1 across porcine vaginal mucosa ex vivo: permeability characteristics, toxin distribution, and tissue damage. Am J Obstet Gynecol 189:1785–1791. doi: 10.1016/S0002-9378(03)00873-1. [DOI] [PubMed] [Google Scholar]
- 9.Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick R. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet 202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 10.Todd J, Todd B, Franco-Buff A, Smith C, Lawellin D. 1987. Influence of focal growth conditions on the pathogenesis of toxic shock syndrome. J Infect Dis 155:673–681. doi: 10.1093/infdis/155.4.673. [DOI] [PubMed] [Google Scholar]
- 11.Baroja M, Herfst C, Kasper K, Xu S, Gillett D, Li J, Reid G, McCormick J. 2016. The SaeRS two-component system is a direct and dominant transcriptional activator of toxic shock syndrome toxin 1 in Staphylococcus aureus. J Bacteriol 198:2732–2742. doi: 10.1128/JB.00425-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pragman A, Ji Y, Schlievert P. 2007. Repression of Staphylococcus aureus SrrAB using inducible antisense srrA alters growth and virulence factor transcript levels. Biochemistry 46:314–321. doi: 10.1021/bi0603266. [DOI] [PubMed] [Google Scholar]
- 13.Kehrberg M, Latham R, Haslam B, Hightower A, Tanner M, Jacobson J, Barbour A, Noble V, Smith C. 1981. Risk factors for staphylococcal toxic-shock syndrome. Am J Epidemiol 114:873–879. doi: 10.1093/oxfordjournals.aje.a113257. [DOI] [PubMed] [Google Scholar]
- 14.DeVries A, Lesher L, Schlievert P, Rogers T, Villaume L, Danila R, Lynfield R. 2011. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One 6:e22997. doi: 10.1371/journal.pone.0022997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins R, Reiser R, Hehl G, Bergdoll M. 1987. Production of toxic shock syndrome toxin 1 by Staphylococcus aureus as determined by tampon disk-membrane-agar method. J Clin Microbiol 25:1446–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiser R, Hinzman S, Bergdoll M. 1987. Production of toxic shock syndrome toxin 1 by Staphylococcus aureus restricted to endogenous air in tampons. J Clin Microbiol 25:1450–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischetti V, Chapman F, Kakani R, James J, Grun E, Zabriskie J. 1989. Role of air in growth and production of toxic shock syndrome toxin 1 by Staphylococcus aureus in experimental cotton and rayon tampons. Rev Infect Dis 11(Suppl 1):S176–S181. doi: 10.1093/clinids/11.Supplement_1.S176. [DOI] [PubMed] [Google Scholar]
- 18.Tierno P, Hanna B. 1994. Propensity of tampons and barrier contraceptives to amplify Staphylococcus aureus toxic shock syndrome toxin-I. Infect Dis Obstet Gynecol 2:140–145. doi: 10.1155/S1064744994000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlievert P. 1995. Comparison of cotton and cotton/rayon tampons for effect on production of toxic shock syndrome toxin. J Infect Dis 172:1112–1114. doi: 10.1093/infdis/172.4.1112. [DOI] [PubMed] [Google Scholar]
- 20.Parsonnet J, Modern P, Giacobbe K. 1996. Effect of tampon composition on production of toxic shock syndrome toxin-1 by Staphylococcus aureus in vitro. J Infect Dis 173:98–103. doi: 10.1093/infdis/173.1.98. [DOI] [PubMed] [Google Scholar]
- 21.Reiser R, Denzin L, Bergdoll M. 1988. Effects of blood and different media on the production of toxic shock syndrome toxin 1 by Staphylococcus aureus in the tampon sac method. J Clin Microbiol 26:2672–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong A, Downs S. 1989. Investigation by improved syringe method of effect of tampons on production in vitro of toxic shock syndrome toxin 1 by Staphylococcus aureus. J Clin Microbiol 27:2482–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlievert P, Nemeth K, Davis C, Peterson M, Jones B. 2010. Staphylococcus aureus exotoxins are present in vivo in tampons. Clin Vaccine Immunol 17:722–727. doi: 10.1128/CVI.00483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massey LK. 2003. Permeability properties of plastics and elastomers. A guide to packaging and barrier materials, 2nd ed William Andrew Publishing, Norwich, NY. [Google Scholar]
- 25.Fraser I, Warner P, Marantos P. 2001. Estimating menstrual blood loss in women with normal and excessive menstrual fluid volume. Obstet Gynecol 8:806–814. doi: 10.1097/00006250-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Monecke S, Luedicke C, Slickers P, Ehricht R. 2009. Molecular epidemiology of Staphylococcus aureus in asymptomatic carriers. Eur J Clin Microbiol Infect Dis 28:1159–1165. doi: 10.1007/s10096-009-0752-2. [DOI] [PubMed] [Google Scholar]
- 27.Wolz C, Pöhlmann-Dietze P, Steinhuber A, Chien Y, Manna A, van Wamel W, Cheung A. 2000. Agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol Microbiol 36:230–243. doi: 10.1046/j.1365-2958.2000.01853.x. [DOI] [PubMed] [Google Scholar]
- 28.Kreiswirth B, Löfdahl S, Betley M, O'Reilly M, Schlievert P, Bergdoll M, Novick R. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 29.Horsburgh M, Aish J, White I, Shaw L, Lithgow J, Foster S. 2002. SigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schenk S, Laddaga R. 1992. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett 73:133–138. doi: 10.1111/j.1574-6968.1992.tb05302.x. [DOI] [PubMed] [Google Scholar]