ABSTRACT
In this study, the microbiota during industrial rearing, processing, and storage of the edible tropical house cricket, Gryllodes sigillatus, was investigated. To this end, we analyzed samples from the cricket feed, obtained before feeding as well as from the cages, and from the crickets during rearing, after harvest, and after processing into frozen, oven-dried, and smoked and oven-dried (smoked/dried) end products. Although the feed contained lower microbial numbers than the crickets, both were dominated by the same species-level operational taxonomic units, as determined by Illumina MiSeq sequencing. They corresponded, among others, to members of Porphyromonadaceae, Fusobacterium, Parabacteroides, and Erwinia. The harvested crickets contained high microbial numbers, but none of the investigated food pathogens Salmonella spp., Listeria monocytogenes, Bacillus cereus, or coagulase-positive staphylococci. However, some possible mycotoxin-producing fungi were isolated from the crickets. A postharvest heat treatment, shortly boiling the crickets, reduced microbial numbers, but an endospore load of 2.4 log CFU/g remained. After processing, an increase in microbial counts was observed for the dried and smoked/dried crickets. Additionally, in the smoked/dried crickets, a high abundance of a Bacillus sp. was observed. Considering the possible occurrence of food-pathogenic species from this genus, it is advised to apply a heat treatment which is sufficient to eliminate spores. Nevertheless, the microbial numbers remained constant over a 6-month storage period, whether frozen (frozen end product) or at ambient temperature (oven-dried and smoked/dried end products).
IMPORTANCE The need for sustainable protein sources has led to the emergence of a new food sector, producing and processing edible insects into foods. However, insight into the microbial quality of this new food and into the microbial dynamics during rearing, processing, and storage of edible insects is still limited. Samples monitored for their microbiota were obtained in this study from an industrial rearing and processing cycle. The results lead first to the identification of process steps which are critical for microbial food safety. Second, they can be used in the construction of a Hazard Analysis and Critical Control Points (HACCP) plan and of a Novel Food dossier, which is required in Europe for edible insects. Finally, they confirm the shelf-life period which was determined by the rearer.
KEYWORDS: food safety, Gryllodes sigillatus, high-throughput sequencing, insect rearing, metagenetics, microbial dynamics, shelf life
INTRODUCTION
Edible insects are increasingly acknowledged as a sustainable alternative animal protein source (1, 2). Consequently, a new food sector is being developed that includes insect rearing companies and insect food producers. Other food-producing sectors have already been subjected to extensive research regarding microbial food safety (3, 4) and can fall back on a clear legislative framework (regulation EC 2073/2005 in Europe [5]). For the insect sector, in contrast, research on food safety and legislation are still in their infancy. It is already known from a few previous studies (6–10) that during an insect rearing process, substantial alterations in terms of microbial quality can occur over time. Furthermore, different edible insect species are being reared for human consumption. They can harbor a completely different microbial community (11, 12), and hence, the microbial qualities of different insect species and production processes should be investigated separately.
It has been suggested that insect feed is an important factor for the development of the insect microbial composition (7, 13–15). Furthermore, the feed may constitute a potential source of hazards of a microbiological origin, such as food pathogens and mycotoxins (7, 16, 17). In addition to the insect feed, the rearing environment and the manual practices of workers may also contribute to the microbiota of edible insects (18). In order to fully understand the relationship between these factors and the microbial community composition, as well as possible food safety hazards, general insight into the microbial dynamics in the insect feed and the insects as such during rearing is required. Furthermore, as already shown in previous research (19–22), postharvest processing of insects has a major impact on the microbial load. Processing techniques, such as blanching, cooking, freezing, oven drying, and freeze-drying, are presently applied for edible insects (2, 8, 19, 23, 24). Usually, companies rearing edible insects produce different end products from the same insect species, including frozen, dried, and seasoned insects. Many of these products are already introduced into the market. Nevertheless, the impact of the processing steps on the microbial quality of the products has not yet been thoroughly assessed. Additionally, given their different production processes, which likely result in different intrinsic properties per product, each product may differ in shelf life. Therefore, research is needed on the microbial stability of these different insect products.
The goal of this study was to investigate the microbial dynamics, including changes in microbial numbers and bacterial community composition, from industrial rearing to processing and storage of the tropical house cricket (Gryllodes sigillatus) reared for human consumption. For this purpose, the egg deposition medium, the feed before administration (here referred to as “feed”), the feed present in the rearing cages and thus in contact with the crickets (here referred to as “substrate”), and the crickets were sampled and analyzed. Additionally, three end products, including crickets that were frozen, oven-dried, and smoked and subsequently oven-dried (smoked/dried), were analyzed after processing and packaging and during their 6-month shelf life, as proposed by the manufacturer (Little Food, Brussels, Belgium). To this end, samples were investigated for their intrinsic parameters, microbial numbers, microbial community composition (bacterial and fungal), and the presence of a selection of foodborne pathogens.
RESULTS
Intrinsic parameters.
Water activity (aw), moisture content, and pH were determined for the egg deposition medium (“peat-peel mix”), the feed (days 0 and 26), the substrate (days 12, 26, and 37), homogenized insects (day 40), and frozen crickets. Due to the small sample size, only water activity and moisture content were determined for the dried crickets and the smoked/dried crickets. The peat-peel mix showed a high aw and moisture content (average, 0.98 and 76.6%, respectively), whereas the pH averaged 4.99 (Table 1). The feed consisted of a dry material with low aw (0.59 to 0.61) and moisture content (10.3%). However, once administered to the crickets in the cage, the water activity and moisture content of the substrate gradually increased to 0.78 and 15.5%, respectively, at day 26. Toward the end of the rearing process (day 37), however, when the substrate was replenished more frequently, the water activity and the moisture content decreased again (P = 0.018 and 0.007, respectively [Table 1]). The values for pH were not statistically different between the feed and substrate, irrespective of the sampling days. After harvest, crickets were high in water activity and moisture content (0.97 and 71.5% on average, respectively) and showed a near-neutral pH of 6.64 on average (Table 1).
TABLE 1.
Intrinsic properties during tropical house cricket rearinga
| Sample | Sample day | Intrinsic property |
||
|---|---|---|---|---|
| pH | aw | Moisture content (%) | ||
| Peat-peel mix | 0 | 4.99 ± 1.09 | 0.98 ± 0.00 | 76.6 ± 3.9 |
| Feed | 0 | 5.51 ± 0.08 a | 0.59 ± 0.02 a | 10.3 ± 0.4 a |
| 26 | 5.59 ± 0.01 a | 0.61 ± 0.01 a | 10.3 ± 0.3 a | |
| Substrate | 12 | 5.57 ± 0.11 a | 0.73 ± 0.02 a | 13.0 ± 1.0 a |
| 26 | 5.41 ± 0.04 a | 0.78 ± 0.04 a | 15.5 ± 1.0 b | |
| 37 | 5.49 ± 0.06 a | 0.61 ± 0.04 b | 9.8 ± 0.2 c | |
| Crickets | 40 | 6.64 ± 0.10 A | 0.97 ± 0.01 A | 71.5 ± 0.7 A |
| Heat-treated cricketsb | 40 | 6.84 ± 0.05 B | 0.98 ± 0.00 A | 73.8 ± 0.4 B |
Data are the mean ± standard deviation values of three replicates. Means per product with the same lowercase letter within the same column do not differ significantly (P > 0.05). Means from different products with the same uppercase letter within the same column do not differ significantly (P > 0.05).
The heat treatment consisted of bringing the crickets to a boil in a kettle with water.
Following heat treatment (bringing to a boil) of the crickets, the mean pH and moisture content significantly increased to 6.84 (P = 0.031) and 73.8% (P = 0.006), respectively. No difference was seen for water activity (Table 1). The frozen crickets did not show any difference in intrinsic parameters from the heat-treated crickets (Table 2). The oven-dried and smoked/dried crickets were significantly lower in aw (P = 0.015 and 0.001, respectively) and moisture content (P = 0.000). During storage, aw (P = 0.024) and moisture content (P = 0.018) of the smoked/dried crickets increased slightly (Table 2).
TABLE 2.
Intrinsic properties during storage of processed tropical house cricketsa
| Product | Storage time (mo) | Intrinsic property |
||
|---|---|---|---|---|
| pH | aw | Moisture content (%) | ||
| Heat-treated cricketsb | 0 | 6.84 ± 0.05 A | 0.98 ± 0.00 A | 73.8 ± 0.4 A |
| Frozen crickets | 0 | 6.85 ± 0.06 aA | 0.98 ± 0.00 aA | 73.6 ± 0.6 aA |
| 3 | 6.95 ± 0.04 a | 0.97 ± 0.01 a | 74.3 ± 1.0 a | |
| 6 | 6.89 ± 0.01 a | 0.98 ± 0.00 a | 75.6 ± 2.0 a | |
| Oven-dried crickets | 0 | ND | 0.35 ± 0.08 aB | 5.1 ± 1.0 aB |
| 3 | ND | 0.36 ± 0.01 a | 4.4 ± 0.4 a | |
| 6 | ND | 0.36 ± 0.01 a | 6.0 ± 0.3 a | |
| Smoked and dried crickets | 0 | ND | 0.24 ± 0.03 aB | 2.2 ± 0.1 abB |
| 3 | ND | 0.30 ± 0.03 ab | 1.9 ± 0.2 a | |
| 6 | ND | 0.34 ± 0.02 b | 5.0 ± 0.9 b | |
Data are the mean ± standard deviation values of three replicates. Means per product with the same lowercase letter within the same column do not differ significantly (P > 0.05). Means from different products with the same uppercase letter within the same column do not differ significantly (P > 0.05). ND, not determined.
The heat treatment consisted of bringing the crickets to a boil in a kettle with water.
Plate counts.
All samples collected during rearing (Fig. 1; also see Table S1 in the supplemental material) were analyzed for their total viable count (TVC) and the numbers of Enterobacteriaceae, lactic acid bacteria (LAB), aerobic bacterial endospores, and fungi. The peat-peel mix showed a high TVC of 8.5 log CFU/g, whereas other counts ranged between 4.3 (LAB) and 6.9 (fungi) log CFU/g (Table 3). The feed, on the other hand, contained lower numbers of microorganisms; the mean TVC ranged from 5.0 to 5.4 log CFU/g and the other counts from 1.8 (LAB) to 4.5 (Enterobacteriaceae and endospores) log CFU/g. The microbial load of the feed did not differ significantly between the samples taken at day 0 and day 26, except for the number of Enterobacteriaceae, which was higher at day 0 (P = 0.017). Once the feed was added to the cages (becoming substrate), the mean TVC increased and ranged from 6.2 to 6.7 log CFU/g, but it did not differ significantly between sampling days 12, 26, and 37. Other average counts for the substrate ranged from 3.2 (fungi) to 5.0 (LAB) log CFU/g. Significant decreases in microbial load were detected from day 12 to day 37 for Enterobacteriaceae (P = 0.018) and LAB (P = 0.023). During the rearing phase, the average TVCs for the crickets ranged from 8.2 to 8.5 log CFU/g. The counts of Enterobacteriaceae ranged from averages of 7.2 to 7.5 log CFU/g, LAB from 6.7 to 7.8 log CFU/g, endospores from 3.5 to 3.8 log CFU/g, and fungi from 5.4 to 6.0 log CFU/g. After heat treatment, all microbial counts were reduced, with those for LAB and fungi being even below the detection limit (1 and 2 log CFU/g, respectively). The reduction was significant for all counts (P = 0.000, 0.018, 0.000, 0.002, and 0.004 for TVC, Enterobacteriaceae, LAB, endospores, and fungi, respectively).
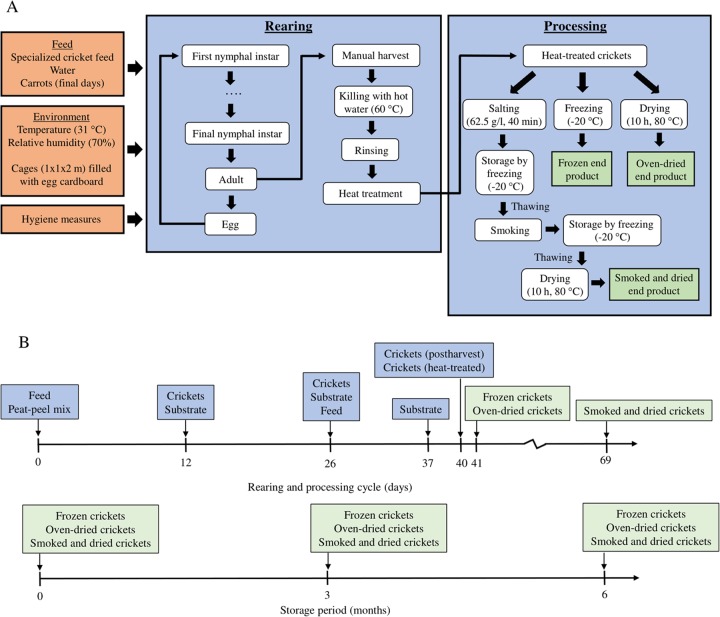
FIG 1.
(A) Schematic representation of the rearing and processing cycle of tropical house crickets. The three final end products are depicted in green. The rearing period, from first instar to harvested adult cricket, took 40 days. In the manual harvesting step, Crickets were harvested by shaking them out of the cardboard boxes into a circular plastic container. The crickets were then killed by submerging them in hot water inside the container. The crickets were then rinsed thoroughly in a colander using running tap water for 5 min per batch. For the heat treatment step, the crickets were submerged in boiling water and until the water boiled again, which took 5 to 10 min. The crickets were salted after heat treatment by submerging them per batch in 4 liters of salted water (62.5 g/liter) for 40 min. Crickets were smoked using a traditional beech wood smoker for 40 min at 80°C. In the drying step, crickets were spread out over a baking tray and dried overnight (10 h at 80°C). (B) Sampling plan throughout rearing, processing, and storage.
TABLE 3.
Microbial counts during tropical house cricket rearinga
| Sample | Sample day | Microbial count (log CFU/g) |
||||
|---|---|---|---|---|---|---|
| Total viable count | Enterobacteriaceae | Lactic acid bacteria | Endospores | Fungi | ||
| Peat-peel mix | 0 | 8.5 ± 0.4 | 6.4 ± 0.7 | 4.3 ± 0.9 | 6.4 ± 0.3 | 6.9 ± 1.0 |
| Feed | 0 | 5.4 ± 0.3 a | 4.5 ± 0.3 a | 1.8 ± 0.2 a | 4.5 ± 0.1 a | 3.6 ± 0.1 a |
| 26 | 5.0 ± 0.3 a | 3.6 ± 0.2 b | 1.8 ± 0.2 a | 4.4 ± 0.3 a | 3.7 ± 0.4 a | |
| Substrate | 12 | 6.3 ± 0.2 a | 4.4 ± 0.1 a | 5.0 ± 0.2 a | 4.3 ± 0.3 a | 3.6 ± 0.4 ab |
| 26 | 6.2 ± 0.3 a | 4.8 ± 0.3 a | 4.7 ± 0.6 a | 4.0 ± 0.4 a | 3.2 ± 0.1 b | |
| 37 | 6.7 ± 0.3 a | 3.6 ± 0.2 b | 3.6 ± 0.5 b | 4.8 ± 0.5 a | 4.0 ± 0.3 a | |
| Crickets | 12 | 8.4 ± 0.1 a | 7.5 ± 0.2 a | 6.7 ± 0.1 a | 3.8 ± 0.2 a | 6.0 ± 0.2 a |
| 26 | 8.2 ± 0.1 a | 7.3 ± 0.5 a | 7.2 ± 0.1 ab | 3.5 ± 0.1 a | 5.4 ± 0.1 b | |
| 40 | 8.5 ± 0.2 aA | 7.2 ± 0.1 aA | 7.8 ± 0.4 bA | 3.7 ± 0.3 aA | 5.6 ± 0.3 abA | |
| Heat-treated cricketsb | 40 | 2.6 ± 0.5 B | <1.5 ± 0.9 B | <1.0 ± 0.0 B | 2.4 ± 0.4 B | <2.0 ± 0.0 B |
Data are the mean ± standard deviation values of three replicates. Means per sample with the same lowercase letter within the same column do not differ significantly (P > 0.05). Means from harvested crickets and heat-treated crickets (day 40) with the same uppercase letter within the same column do not differ significantly (P > 0.05).
The heat treatment consisted of bringing the crickets to a boil in a kettle with water.
Following processing, frozen crickets were stored for 6 months at −25 ± 1°C, dried crickets at 21 ± 1°C, and smoked/dried crickets at 22 ± 2°C. During processing and storage of the crickets (Fig. 1), the TVC and numbers of Enterobacteriaceae and fungi were determined (Table 4). After processing, the TVCs of both dried (P = 0.002) and smoked/dried crickets (P = 0.000) were significantly higher than that of the heat-treated crickets. Additionally, the TVC of the smoked/dried crickets was higher than that of the oven-dried crickets (P = 0.013). Enterobacteriaceae and fungi counts remained below the detection limit, and the amount of endospores increased slightly but not significantly. During the 6-month storage period, all counts remained stable, except the TVC of the smoked/dried crickets, which slightly (but significantly, P = 0.009) decreased over time (Table 4).
TABLE 4.
Microbial counts during storage of processed tropical house cricketsa
| Product | Storage time (mo) | Microbial count (log CFU/g) |
|||
|---|---|---|---|---|---|
| Total viable count | Enterobacteriaceae | Endospores | Fungi | ||
| Heat-treated cricketsb | 0 | 2.6 ± 0.5 A | <1.5 ± 0.9 A | 2.4 ± 0.4 AB | <2.0 ± 0.0 aA |
| Frozen crickets | 0 | 2.4 ± 0.4 aA | <1.0 ± 0.0 aA | 2.0 ± 0.4 aA | <2.0 ± 0.0 aA |
| 3 | 2.5 ± 0.3 a | <1.1 ± 0.1 a | 2.4 ± 0.4 a | <2.1 ± 0.1 a | |
| 6 | 2.2 ± 0.1 a | <1.0 ± 0.0 a | 2.2 ± 0.0 a | <2.0 ± 0.0 a | |
| Oven-dried crickets | 0 | 4.3 ± 0.0 aB | <1.0 ± 0.0 aA | 2.4 ± 0.4 aAB | <2.0 ± 0.0 aA |
| 3 | 3.9 ± 0.8 a | <1.0 ± 0.0 a | 2.3 ± 0.5 a | <2.0 ± 0.0 a | |
| 6 | 3.9 ± 0.8 a | <1.1 ± 0.1 a | 2.5 ± 0.5 a | <2.1 ± 0.1 a | |
| Smoked and dried crickets | 0 | 7.9 ± 0.1 aC | <1.0 ± 0.0 aA | 3.4 ± 0.6 aB | <2.0 ± 0.0 aA |
| 3 | 7.4 ± 0.3 ab | <1.0 ± 0.0 a | 3.9 ± 0.6 a | <2.0 ± 0.0 a | |
| 6 | 7.0 ± 0.2 b | <1.0 ± 0.0 a | 3.2 ± 0.0 a | <2.0 ± 0.0 a | |
Data are the mean ± standard deviation values of three replicates. Means per product with the same lowercase letter within the same column do not differ significantly (P > 0.05). Means from unstored (0 months) products with the same uppercase letter within the same column do not differ significantly (P > 0.05).
The heat treatment consisted of bringing the crickets to a boil in a kettle with water.
Identification of fungal isolates.
Fungi isolated from the peat-peel mix (day 0), the feed (days 0 and 26), the substrate at the end of the rearing period (day 37), and the crickets after harvest (day 40) were identified (Table S2). All isolates obtained from the peat-peel mix corresponded to the genus Trichoderma. For the feed, isolates corresponded to the genera Aspergillus, Hyphopichia, Lichtheimia, and Penicillium. With regard to the substrate, isolates were identified as Aspergillus, Candida, Lichtheimia, Penicillium, and Trichoderma species. The isolates from the crickets corresponded to Aspergillus, Candida, Kodamaea, Lichtheimia, Tetrapisispora, Trichoderma, and Trichosporon species (Table S2).
Pathogen detection.
At the end of the rearing cycle, harvested crickets (day 40) and the substrate (day 37) were assessed for the presence of a number of foodborne pathogenic bacteria. Neither Salmonella spp. nor Listeria monocytogenes were detected in the samples (absent in 25 g). Additionally, Bacillus cereus and coagulase-positive staphylococcal counts were below the detection limit of 100 CFU/g.
Culture-independent analyses.
A selection of samples (Table S1), including samples from the peat-peel mix (day 0), the feed (day 0), the substrate (days 12 and 26), crickets during the rearing phase (days 12 and 26), and crickets after harvest and processing (Fig. 1), were subjected to high-throughput amplicon-based sequencing. Relative abundances and diversity indices were averaged over all DNA extracts of replicate samples. The average coverages, based on the Chao1 estimator, ranged from 96.5% to 99.1%, indicating that the majority of the bacterial community members were recovered (Table 5). Indices for species richness (observed richness and Chao1 [25]) showed that the feed and substrate contained most bacterial species, while the least diversity was observed in the peat-peel mix, although its mean Shannon index (26) was within the range of the other samples (Table 5).
TABLE 5.
Diversity indices for samples subjected to metagenetic analysis in this studya
| Product | Sampling moment | Diversity index |
|||
|---|---|---|---|---|---|
| Observed richness | Chao1b | Coverage (%)c | Shannon indexd | ||
| Peat-peel mix | Start of rearing | 90 ± 5 | 92.70 ± 7.99 | 96.74 ± 3.45 | 3.22 ± 0.20 |
| Feed | Start of rearing | 128 ± 12 | 131.30 ± 7.92 | 97.01 ± 3.30 | 3.45 ± 0.28 |
| Substrate | Rearing day 12 | 127 ± 12 A | 128.28 ± 12.44 A | 99.00 ± 0.56 A | 3.39 ± 0.22 A |
| Rearing day 26 | 130 ± 4 A | 131.75 ± 4.76 A | 98.31 ± 1.24 A | 3.59 ± 0.02 A | |
| Crickets | Rearing day 12 | 115 ± 7 ABC | 116.33 ± 7.14 ABC | 99.08 ± 0.33 A | 3.45 ± 0.02 A |
| Rearing day 26 | 120 ± 1 B | 122.53 ± 0.83 B | 97.94 ± 0.58 A | 3.48 ± 0.06 A | |
| End of rearing | 115 ± 11 ABC | 117.05 ± 8.27 ABCD | 97.75 ± 2.15 A | 3.53 ± 0.01 A | |
| After heat treatment | 101 ± 4 AC | 104.43 ± 4.77 CD | 96.52 ± 1.47 A | 3.18 ± 0.02 B | |
| After freezing | 104 ± 2 A | 106.05 ± 1.41 ACD | 97.83 ± 1.13 A | 3.16 ± 0.03 B | |
| After oven drying | 114 ± 5 AB | 116.35 ± 4.11 AB | 98.19 ± 0.71 A | 3.20 ± 0.18 B | |
| After smoking | 94 ± 1 C | 96.10 ± 0.14 D | 97.82 ± 1.62 A | 2.66 ± 0.05 C | |
Sequences were grouped into OTUs defined by 97% sequence identity at the 16S rRNA gene (V4 region, 250 bp). Data are the mean ± standard deviation values of two analyzed DNA extracts from two replicates per sampling moment. Means per product with the same letter within the same column do not differ significantly (P > 0.05).
Chao1 richness estimator is the total number of OTUs estimated by infinite sampling. A higher number indicates a higher richness (25).
Coverage was determined by using the equation (observed richness/Chao1 estimate) × 100.
Shannon-Wiener diversity index is the index to characterize species diversity based on species richness as well as their relative abundance. A higher value represents more diversity (26).
Identification of the operational taxonomic units (OTUs) (Data Set S3 and Table S4) revealed that the most abundant phyla in the peat-peel mix and feed were Proteobacteria (47.7% ± 11.9% and 39.6% ± 13.0%, respectively) and Bacteroidetes (38.0% ± 13.7% and 43.3% ± 10.2%, respectively). In the feed, the Firmicutes was also among the most abundant phyla (12.6% ± 0.6%). Once the feed had been administered inside the cage, Firmicutes became more abundant (17.7% ± 2.6% at day 12 and 20.2% ± 1.7% at day 26) alongside Bacteroidetes and Proteobacteria (65.8% ± 5.6% and 14.1% ± 2.7%, respectively, at day 12; 47.4% ± 9.9% and 29.2% ± 11.7%, respectively, at day 26). During rearing and processing, Bacteroidetes remained the most abundant phylum in the crickets (ranging from 35.8% ± 0.2% in smoked/dried crickets to 71.2% ± 1.7% in the frozen crickets), followed by Firmicutes (ranging from 5.4% ± 0.3% in the frozen crickets to 56.6% ± 1.9% in the smoked/dried crickets) and Proteobacteria (ranging from 4.9% ± 1.4% in the smoked/dried crickets to 16.0% ± 2.5% in the heat-treated crickets). Other phyla were present in abundances below 10% in any sample of the data set.
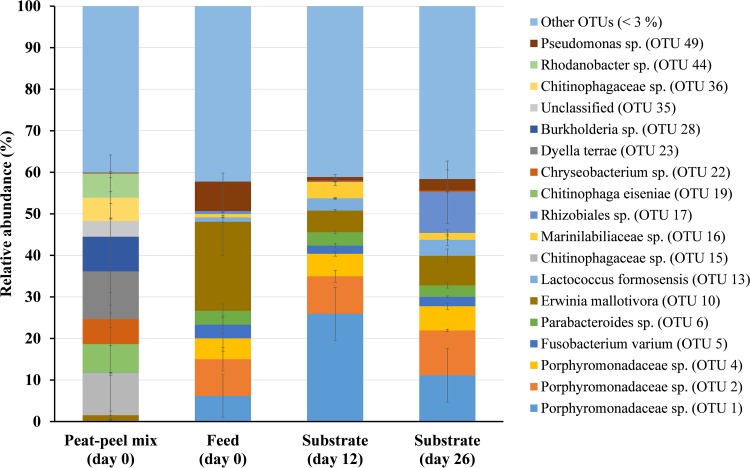
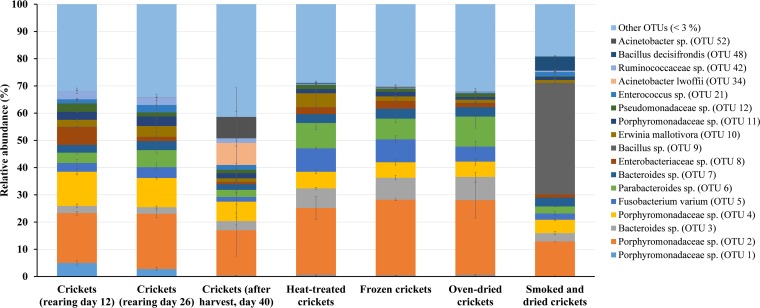
A total of 337 OTUs were identified throughout the data set (Data Set S3). The peat-peel mix (Fig. 2) was dominated (average OTU abundance of >5% in any sample) by OTUs corresponding to members of Chitinophagaceae (OTUs 15, 19, and 36), a Chryseobacterium sp. (OTU 22), a Dyella sp. (OTU 23), a Burkholderia sp. (OTU 28), and a Rhodanobacter sp. (OTU 44). The feed and substrate samples (Fig. 2) were all dominated by the same group of OTUs, consisting of members of the Porphyromonadaceae family (OTUs 1, 2, and 4), an Erwinia sp. (OTU 10), a Rhizobiales sp. (OTU 17), and a Pseudomonas sp. (OTU 49). More than 40% of the sequences recovered belonged to OTUs represented by <3% of the sequences in all samples. Both processed and unprocessed cricket samples (Fig. 3) were abundant in OTUs corresponding to members of Porphyromonadaceae (OTUs 2 and 4) and Enterobacteriaceae (OTU 8), a Bacteroides sp. (OTU 3), a Fusobacterium sp. (OTU 5), a Parabacteroides sp. (OTU 6), and an Erwinia sp. (OTU 10). During rearing, OTU 1 (a Porphyromonadaceae sp.) decreased in average relative abundance from 5% to below 1%. After harvest, crickets showed a high abundance of two Acinetobacter spp. (OTUs 34 and 52), and smoked/dried crickets showed a large abundance (>40%) of a Bacillus species (OTU 9). Also here, a large percentage of OTUs showed a relative abundance of below 3% in any sample.
FIG 2.
Relative abundance (%) of OTUs present in the peat-peel mix, feed and substrate. Data are mean values of two extracts per sample from two samples. Error bars represent the standard deviation. Only OTUs represented by an average relative abundance of more than 3% of sequences in any sample are shown. OTUs with a mean relative abundance of less than 3% are grouped in “Other OTUs (<3%)”.
FIG 3.
Relative abundance (%) of OTUs present in the crickets during the rearing phase and after processing. Data are mean values of two extracts per sample from one (crickets at day 40 and smoked/dried crickets) or two cricket samples. Error bars represent the standard deviation. Only OTUs represented by an average relative abundance of more than 3% of sequences in any sample are shown. OTUs with a mean relative abundance of less than 3% are grouped in “Other OTUs (<3%)”.
DISCUSSION
The goal of the present study was to achieve more insight into the microbial dynamics during rearing, processing, and storage of tropical house crickets sold for human consumption. To this end, crickets during the rearing phase and after processing into three end products, as well as the feed and substrate samples, were assessed for their microbial quality. Additionally, for the three end products, microbial counts were monitored during the shelf life proposed by the manufacturer (Little Food), i.e., 6 months.
Preharvest.
The peat-peel mix contained high numbers of microorganisms, of which the most abundant were typical soilborne bacteria, such as Dyella (OTU 23), Burkholderia (OTU 28), and Rhodanobacter (OTU 44) species and members of Chitinophagaceae (OTU 36) and Rhizobiales (OTU 17) (27, 28). In contrast, none of these OTUs were recovered in substantial abundance in the other samples. This suggests that the hatchlings do not transfer these bacteria to the rearing cages, nor that they retain these bacteria within their gastrointestinal tract. The feed, on the other hand, contained lower microbial numbers, which greatly increased after the feed was placed inside the cage. That effect may be attributed to the increase in water activity and moisture content due to absorption from the environment, thereby facilitating microbial growth and/or cross-contamination from contact with crickets and possible defecation into the substrate. As the crickets grew, the substrate was replenished more frequently toward the end of the rearing cycle to provide them with sufficient resources. This “dilution” may not only explain the decrease in water activity and moisture content toward day 37 in the substrate but also the observed decrease in the number of lactic acid bacteria and Enterobacteriaceae. The microbial numbers of the substrate were consistently lower than those of the crickets for all counts, except for the endospores, suggesting that the crickets are a better matrix for microbial growth than the substrate. This is in contrast to the results obtained for the rearing of lesser mealworms (Alphitobius diaperinus) (8), where the larvae are reared within the substrate, which also contains their excreted feces and exuviae.
Even though the microbial numbers of the feed and substrate are generally lower than those of the crickets, a striking similarity was observed in community composition between the feed, the substrate, and the crickets. Indeed, for example, OTUs corresponding to Porphyromonadaceae (OTUs 1, 2 and 4), a Fusobacterium sp. (OTU 5), a Parabacteroides sp. (OTU 6), and an Erwinia sp. (OTU 10) were abundant in all samples. The OTU most abundant in the feed belonged to the genus Erwinia (OTU 10). Most members of this genus are plant-associated and plant-pathogenic bacteria (28), which could explain their high relative abundances in the plant-based feed. These results suggest that the feed is an important source for the development of the cricket microbiota. Similar results were obtained during a previous study on lesser mealworms, where 50 out of 70 bacterial OTUs identified from the feed were also detected in the larvae (8). In that study, however, the microbial diversity of the larvae decreased during rearing, with 22 OTUs obtained for the harvested larvae. In the present study, the cricket microbial community composition was highly diverse and did not significantly change during rearing, as indicated by the diversity indices as well as by the large portion of OTUs represented by <3% of the sequences. This observation was also reported by Vandeweyer et al. (11), where both the diversity indices and the number of OTUs represented by <3% were higher for crickets than the values observed for mealworms. Crickets thus harbor a bacterial community that is remarkably more complex than that of mealworms, both during and at the end of their rearing period, and with more dominating organisms. Also with regard to community composition, similarities were observed between the crickets in the present study and those analyzed by Vandeweyer et al. (11). Indeed, those authors also report the presence of OTUs corresponding to the families Enterobacteriaceae and Pseudomonadaceae and the genera Bacteroides, Parabacteroides, Erwinia, and Fusobacterium in fresh crickets. Altogether, it is reasonable to assume that Bacteroides spp. and Parabacteroides spp., among other Porphyromonadaceae spp., are typical members of the endogenous intestinal bacterial community of crickets. Also, a Fusobacterium sp. has already been observed to be a member of the tropical house cricket microbiome (11). Noteworthy is the presence of Acinetobacter spp. in our data set, e.g., OTUs 34 and 52 in the sample from harvested crickets. Acinetobacter species are widely distributed in nature and commonly occur in soil and water but also in insect guts and plant-related environments. Furthermore, the genus Acinetobacter contains multiple nosocomial opportunistic pathogens, of which A. baumannii is the most well-known (29). Interestingly, Acinetobacter species were previously found in carrots (30) and their wash water (31), which may explain their appearance in the harvested crickets, since exclusively carrots were administered during the final days before harvest.
With regard to the microbial quality of fresh harvested crickets, the plate count numbers obtained in our study were comparable to those obtained by Vandeweyer et al. (32) for tropical house crickets and house crickets (Acheta domesticus). Since the beginning of the rearing process, most microbial counts remained stable within the crickets, except for the LAB, which showed a significant increase in numbers over time. This significant rise may be explained by the good growth of (a few) LAB species which may be well adapted to the cricket gut environment. The retrieved Enterococcus sp. (OTU 21) in this study is a possible candidate for this hypothesis, because the abundance of this OTU in the crickets during the rearing phase rose since day 12. Moreover, Enterococcus species have been frequently detected in other fresh edible cricket samples (11).
None of the four investigated foodborne pathogens were recovered from the crickets after harvest or from the substrate, suggesting they were not present in the rearing cycle under study. To our knowledge, L. monocytogenes has not yet been detected in edible insects for human consumption (8, 32–35). However, Salmonella spp., B. cereus, and Staphylococcus aureus have been reported in products, including rhinoceros beetles (Oryctes monoceros), grasshoppers, mealworms (Tenebrio molitor), and/or house crickets (A. domesticus) (34–37). Of note, it remains to be investigated whether a pathogen possibly present in the insect feed may contaminate the insect (38, 39) and thus pose a hazard for food safety of the end product. Although no food pathogens were detected, some possible mycotoxin-forming fungi, more specifically Aspergillus spp. and Penicillium spp., were recovered from the feed, substrate, and/or crickets. Future research should focus on the presence of these fungal genera in insects and their potential to produce mycotoxins. Mycotoxins can be very heat resistant (40) and, if present, will likely not be reduced by heat treatment and further processing and will be able to cause mycotoxicosis. Fungal infections (mycoses) caused by, e.g., Aspergillus or Candida spp., via consumption of the crickets, on the other hand, are highly unlikely, as the heat treatment was shown to reduce fungi to below the detection limit (<2 log CFU/g).
Postharvest.
The application of heat caused all counts to drop significantly. However, a substantial number of endospores remained, as was expected based on previous research on (lesser) mealworms and crickets (8, 19, 21). The unexpected increase of bacterial counts after processing the crickets into oven-dried and smoked/dried end products can likely be explained by several factors, including cross-contamination through the equipment and installations used, postcontamination through human interaction while removing legs and during packaging, and/or, for smoked/dried crickets, the possibility of microbial growth during subsequent processing steps, including freezing and thawing cycles. To reduce risks for such contamination, it is advised to incorporate good hygiene and manufacturing practices, such as the wearing of gloves and proper cleaning and disinfection of equipment. During the 6-month storage of the products, only the water activity and the moisture content of the smoked/dried crickets changed significantly, which indicates that the packaging technique used (glass tube with cork stop) allows moisture to enter the product. The aw value, however, never rose above 0.60 to allow microbial growth (41) and therefore explains the stable microbial quality during the proposed shelf life.
Heat-treated crickets had a bacterial community composition similar to that of the crickets during the rearing phase. However, it should be noted that the heat treatment, although reducing microbial numbers significantly, possibly did not break down all bacterial DNA, which can explain the comparable relative abundances of the recovered OTUs. Many genera observed in this study were also encountered in previous research on processed crickets performed by Garofalo et al. (12). Highly remarkable, however, is the appearance of a strongly abundant Bacillus species (OTU 9, 40.9%), a typical spore-forming bacterium, in the smoked/dried crickets. The succession of processing steps to obtain the smoked/dried crickets may have triggered subsequent cycles of spore formation and germination, while the relative abundance of the background microbiota was reduced. The high abundance of this OTU also explains the significantly lower bacterial diversity in the smoked/dried crickets. As the genus Bacillus contains the food pathogen B. cereus, special attention should be paid to the heat treatment, even though B. cereus was not detected using the classical enumeration method (ISO 7932:2004 [42]) performed on the harvested crickets. It is advised to apply a heat treatment which is sufficient to eliminate endospores and to decrease the amount of freeze-thaw cycles in the production process of such insect products to a minimum.
Conclusions.
In this study, the microbial dynamics during rearing, processing, and storage of tropical house crickets were investigated. The most abundant bacterial community members identified from the cricket feed (e.g., Porphyromonadaceae spp., a Bacteroides sp., a Parabacteroides sp., an Erwinia sp., and a Fusobacterium sp.) were also recovered from cricket samples, suggesting the feed to be an important source for the cricket microbiota. High microbial numbers of crickets during the rearing phase were significantly reduced by a heat treatment. Neither Salmonella spp., Listeria monocytogenes, coagulase-positive staphylococci, nor Bacillus cereus were recovered from the crickets, but some mycotoxin-producing fungal genera were isolated. Further research on the possible presence of mycotoxins in insects is therefore advised. After processing into dried and smoked/dried end products, an increase in total microbial count could be detected as a result of postcontamination, but the microbial community composition remained comparable to that of the live crickets. However, a high abundance of a Bacillus sp. was introduced in the smoked/dried crickets. It is advised to apply a heat treatment sufficient to eliminate endospores and to minimize the number of freeze-thawing cycles during processing. During their 6-month shelf life, the microbial quality of the cricket products, as indicated by their microbial numbers, remained stable. The shelf life recommended by the manufacturer (Little Food) is therefore acceptable from a microbiological point of view.
MATERIALS AND METHODS
Industrial rearing cycle.
A complete rearing cycle in a Belgian company rearing crickets for human consumption (Little Food, Brussels, Belgium) was monitored. An overview of the whole industrial cricket production process, including postharvest treatments, is given in Fig. 1A. Eggs were laid by adult crickets in the final 1 to 2 days before harvest into a mixture of peat soil and coconut peel (peat-peel mix) in small (17.5 by 13 by 5 cm) plastic containers. These plastic containers were then placed in a larger container on top of a pile of egg cardboard where the freshly emerged nymphs could reside. The nymphs were removed from the container and placed in a larger cage (approximately 2 by 1 by 1 m), consisting of a wooden skeleton and Perspex walls, which was open on the upper side. Inside the cage, egg cartons were piled up to create a dark habitat with crevices. All cages were situated in a ventilated room at an average temperature of 31°C and an average relative humidity of 70%. Artificial light was present on average 8 h/day except during weekends. A specialized cricket feed (main components were wheat bran, linseed flakes, and sunflower seed flakes) was added on a cardboard plate on top of the egg cartons, and water was presented separately in a plastic dispenser. New feed was added one to three times a week in quantities ranging from 1 to 2 kg, with both frequency and quantity increasing as the crickets aged. The water bowl was refilled when empty. Two days before harvest, only carrots were provided as feed and water source and, according to the rearing company, to improve reproduction and taste. Additionally, a small plastic container with peat and coconut peel (see above) was placed inside the cage for oviposition. After 40 days, the crickets were harvested, and the cages were cleaned with a brush and, if necessary, with water containing disinfectant. Subsequently, the crickets were killed by submersion in hot water (60°C) and rinsed (5 min) with regular tap water. Afterwards, they were given a heat treatment by placing them in a kettle with boiling water and keeping them submerged until the water boiled again (after 5 to 10 min). Finally, heat-treated crickets were further processed into three end products. Crickets were either frozen to −20°C (frozen crickets), oven-dried overnight at 80°C (dried crickets), or smoked. The smoking process involved a combination of salting (submerging in brine of 62.5 g NaCl/liter for 40 min), freezing to −20°C (crickets were stored in the freezer until they were smoked), thawing, smoking (traditional beech wood smoker for 40 min at 80°C), and finally oven-drying overnight at 80°C (smoked/dried crickets).
Sample collection.
During the rearing cycle, samples were taken from the peat-peel mix, the feed before addition, the feed in the cage (substrate), and the crickets every 2 weeks during the rearing cycle (Fig. 1B). At every sampling time point, three replicates were obtained per sample (i.e., 3-fold sampling). For substrates and crickets, each replicate was collected from a separate cage. At the end of the cycle, crickets were sampled after harvest and after heat treatment, again in triplicate. After processing, nine packed samples of all three end products (frozen, dried, and smoked/dried crickets) were obtained as well.
Storage conditions.
All samples taken during and at the end of the rearing cycle (fresh samples) as well as the heat-treated samples were kept at 3°C for a maximum of 24 h (cricket samples) or 48 h (feed samples) until analyses. Of the end products, three packages were analyzed immediately after sampling, while the remaining samples were saved for long-term storage and evaluated in 3-fold after 3 and 6 months of storage (Fig. 1B). Frozen cricket samples were stored in sealed plastic bags at −25°C. Dried and smoked/dried cricket samples were stored in individual glass tubes with a cork stop (which is the packaging used by the company to commercialize the crickets) at ambient temperature. During the entire storage period, temperatures were monitored using data loggers (Escort iLog internal sensor; VWR International, Leuven, Belgium).
Intrinsic parameters.
All selected cricket samples (Table S1) were pulverized prior to analyses, as described by Stoops et al. (43). Samples of the frozen end products were thawed for 4 h at 3°C before pulverization. Peat-peel mix, feed, and substrate samples were analyzed without preparation. Water activity was measured using a water activity meter (LabMaster aw; Novasina, Lachen, Switzerland) until the aw and temperature (25°C) were stable for 15 and 5 min, respectively. The moisture content was determined by calculating the difference in weight of 3 g (dried samples) or 5 g (other samples) of the initial sample before and after oven drying for 17 h at 105°C. The pH was measured directly in the (homogenized) samples using a digital pH meter (Portamess 911; Knick, Berlin, Germany, with SI analytics electrode; Mainz, Germany).
Microbiological plate counts.
For the selected (pulverized) samples (Table S1), plate counts were performed according to the ISO standards for microbial analyses of food and feed as compiled by Dijk et al. (44). TVCs were determined on plate count agar (PCA; Biokar Diagnostics, Beauvais, France) after incubation at 30°C for 72 h. The number of LAB was determined on de Man-Rogosa-Sharpe (MRS) agar (Biokar Diagnostics), with the addition of sorbic acid (0.14%, causing the pH to drop to 5.20) to prevent fungal growth, after incubation for 72 h at 30°C. Enterobacteriaceae were determined on violet red bile glucose (VRBG) medium (Biokar Diagnostics) after incubation at 37°C for 24 h. Aerobic bacterial endospores were determined by subjecting the 10−1 dilution to a heat shock treatment (10 min at 80°C), followed by a 10-fold serial dilution, plating onto PCA, and incubation at 37°C for 48 h. Fungi were determined on dichloran rose bengal chloramphenicol (DRBC) medium (Biokar Diagnostics) after incubation at 25°C for 6 days. The relative humidity of the atmosphere during incubation was 54%.
Identification of fungal isolates.
For the feed (day 0), the peat-peel mix (day 0), the substrate (day 37), and the crickets (day 40), a selection of fungal colonies with distinct morphology was picked from the DRBC medium for further identification (if possible, 10 colonies per sample type). Subsequently, isolates were grown on potato dextrose agar (PDA; Biokar Diagnostics) and incubated at 25°C. After 2 to 7 days of incubation (depending on the growth), genomic DNA was extracted from purified strains using the phenol-chloroform DNA extraction procedure described by Lievens et al. (45). Identifications were performed by amplifying and sequencing the internal transcribed spacer (ITS) region (ITS1-5.8S ribosomal DNA [rDNA]-ITS2) as described previously (45). The obtained sequences were compared with the nucleotide database in GenBank (46) (excluding unclassified and environmental entries), and isolates were assigned to the highest taxonomic rank possible.
Pathogen detection.
For three replicate cricket and substrate samples at the end of the rearing phase (day 40 and day 37, respectively), the presence of four food pathogens was assessed. The presence of Salmonella was assessed according to ISO 6579-1:2017 (47) (absence in 25 g) and the presence of L. monocytogenes according to AFNOR BRD 07/4-09/98 (48) (absence in 25 g). Furthermore, detection of B. cereus was performed according to ISO 7932:2004 (42) (plate count) and the prevalence of coagulase-positive staphylococci according to AFNOR 3M 01/9-04/03 B (49) (plate count).
16S rRNA gene amplicon sequencing.
The bacterial community composition of the selected samples (Table S1) was determined using Illumina MiSeq sequencing of partial 16S rRNA gene amplicons (V4 region, 250 bp). To this end, two replicates of each sample were pulverized as described for the intrinsic property and plate count analyses. Subsequently, DNA extraction, PCR amplification (primer design shown in Table S5), library preparation, sequencing, sequence processing, and diversity analyses were performed as described by Wynants et al. (8). For each pulverized replicate, genomic DNA was extracted in duplicate, resulting in a total of 4 DNA extracts per sample. Downstream diversity analyses used data rarefied to 1,700 sequences per DNA extract. For the harvested (fresh) and the smoked/dried crickets, only two DNA extractions (of one replicate) delivered useful sequences; the others were not retained for data analysis. Sequences were clustered into operational taxonomic units (OTUs) based on a 97% similarity cutoff as proxies for species. The taxonomic origin of each OTU was determined to the genus level with the SINTAX algorithm implemented in USEARCH (50) based on the Silva Living Tree Project (LTP) version 123 database. Taxonomic assignments were considered reliable when bootstrap confidence values exceeded 0.80 (Data Set S3). In case the genus could not be determined reliably (bootstrap value, <0.80) based on the Silva database, OTU representative sequences were compared to the nucleotide database in GenBank (excluding uncultured/environmental entries; Table S4). Chao1 and Shannon-Wiener diversity indices were calculated using the R package Phyloseq (version 1.19.0) (51).
Statistical analyses.
Differences in the intrinsic parameters, microbial counts, and diversity parameters (OTU richness, Chao1, coverage, and Shannon-Wiener indices) during rearing, processing, and storage of the crickets were analyzed by one-way analysis of variance (ANOVA), followed by Tukey's post hoc test. In case of unequal variances, Welch's ANOVA with a Games-Howell post hoc test was used. All tests were performed with SPSS Statistics 23 (IBM, New York, NY, USA) and considered significant at a P value below 0.05.
Accession number(s).
The obtained sequences from the identified fungal isolates have been deposited in GenBank under the accession numbers MG655272 to MG655305. The sequences obtained from the Illumina MiSeq platform were deposited in the Sequence Read Archive (SAMN08032682 to SAMN08032721) under BioProject accession PRJNA418072. Additionally, representative sequences per OTU have been submitted to GenBank under accession numbers MG558004 to MG558332.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Flanders Innovation & Entrepreneurship (VLAIO, project 141129) and the Belgian Federal Public Service Health, Food Chain Safety and Environment (project EDINCO, RT 15/9).
We declare no conflicts of interest in the collaboration with the insect-rearing company Little Food.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00255-18.
REFERENCES
- 1.Oonincx DGAB, de Boer IJM. 2012. Environmental impact of the production of mealworms as a protein source for humans—a life cycle assessment. PLoS One 7:e51145. doi: 10.1371/journal.pone.0051145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Huis A, Van Itterbeeck J, Klunder HC, Mertens E, Halloran A, Muir G, Vantomme P. 2013. Edible insects: future prospects for food and feed security. Food and Agricultural Organization of the United Nations, Rome, Italy. [Google Scholar]
- 3.Adams MR, Moss MO, McClure P. 2015. Food microbiology, 4th ed The Royal Society of Chemistry, Cambridge, United Kingdom. [Google Scholar]
- 4.Oyarzabal OA, Backert S. 2012. Microbial food safety. Springer Science+Business Media, New York, NY. [Google Scholar]
- 5.Commission of the European Communities. 2005. Commission regulation (EC) 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. European Union, Brussels, Belgium: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:338:0001:0026:EN:PDF. [Google Scholar]
- 6.Li L, Xie B, Dong C, Wang M, Liu H. 2016. Can closed artificial ecosystem have an impact on insect microbial community? A case study of yellow mealworm (Tenebrio molitor L.). Ecol Eng 86:183–189. doi: 10.1016/j.ecoleng.2015.09.015. [DOI] [Google Scholar]
- 7.EFSA Scientific Committee. 2015. Risk profile related to production and consumption of insects as food and feed. EFSA J 13:4257. doi: 10.2903/j.efsa.2015.4257. [DOI] [Google Scholar]
- 8.Wynants E, Crauwels S, Verreth C, Gianotten N, Lievens B, Claes J, Van Campenhout L. 2018. Microbial dynamics during production of lesser mealworms (Alphitobius diaperinus) for human consumption at industrial scale. Food Microbiol 70:181–191. doi: 10.1016/j.fm.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Varotto Boccazzi I, Ottoboni M, Martin E, Comandatore F, Vallone L, Spranghers T, Eeckhout M, Mereghetti V, Pinotti L, Epis S. 2017. A survey of the mycobiota associated with larvae of the black soldier fly (Hermetia illucens) reared for feed production. PLoS One 12:e0182533. doi: 10.1371/journal.pone.0182533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng L, Crippen TL, Singh B, Tarone AM, Dowd S, Yu Z, Wood TK, Tomberlin JK. 2013. A survey of bacterial diversity from successive life stages of black soldier fly (Diptera: Stratiomyidae) by using 16S rDNA pyrosequencing. J Med Entomol 50:647–658. doi: 10.1603/ME12199. [DOI] [PubMed] [Google Scholar]
- 11.Vandeweyer D, Crauwels S, Lievens B, Van Campenhout L. 2017. Metagenetic analysis of the bacterial communities of edible insects from diverse production cycles at industrial rearing companies. Int J Food Microbiol 261:11–18. doi: 10.1016/j.ijfoodmicro.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo C, Osimani A, Milanović V, Taccari M, Cardinali F, Aquilanti L, Riolo P, Ruschioni S, Isidoro N, Clementi F. 2017. The microbiota of marketed processed edible insects as revealed by high-throughput sequencing. Food Microbiol 62:15–22. doi: 10.1016/j.fm.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Dillon RJ, Dillon VM. 2004. The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 14.Engel P, Moran NA. 2013. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol Rev 37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 15.Jeon H, Park S, Choi J, Jeong G, Lee SB, Choi Y, Lee SJ. 2011. The intestinal bacterial community in the food waste-reducing larvae of Hermetia illucens. Curr Microbiol 62:1390–1399. doi: 10.1007/s00284-011-9874-8. [DOI] [PubMed] [Google Scholar]
- 16.Charlton AJ, Dickinson M, Wakefield ME, Fitches E, Kenis M, Han R, Zhu F, Kone N, Grant M, Devic E, Bruggeman G, Prior R, Smith R. 2015. Exploring the chemical safety of fly larvae as a source of protein for animal feed. J Insects Food Feed 1:7–16. doi: 10.3920/JIFF2014.0020. [DOI] [Google Scholar]
- 17.Pinotti L, Ottoboni M, Giromini C, Dell'Orto V, Cheli F. 2016. Mycotoxin contamination in the EU feed supply chain: a focus on cereal byproducts. Toxins (Basel) 8:45. doi: 10.3390/toxins8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider JC. 2009. Principles and procedures for rearing high quality insects. Mississippi State University, Starkville, MS. [Google Scholar]
- 19.Vandeweyer D, Lenaerts S, Callens A, Van Campenhout L. 2017. Effect of blanching followed by refrigerated storage or industrial microwave drying on the microbial load of yellow mealworm larvae (Tenebrio molitor). Food Control 71:311–314. doi: 10.1016/j.foodcont.2016.07.011. [DOI] [Google Scholar]
- 20.Stoops J, Vandeweyer D, Crauwels S, Verreth C, Boeckx H, Van Der Borght M, Claes J, Lievens B, Van Campenhout L. 2017. Minced meat-like products from mealworm larvae (Tenebrio molitor and Alphitobius diaperinus): microbial dynamics during production and storage. Innov Food Sci Emerg Technol 41:1–9. doi: 10.1016/j.ifset.2017.02.001. [DOI] [Google Scholar]
- 21.Klunder HC, Wolkers-Rooijackers J, Korpela JM, Nout MJR. 2012. Microbiological aspects of processing and storage of edible insects. Food Control 26:628–631. doi: 10.1016/j.foodcont.2012.02.013. [DOI] [Google Scholar]
- 22.Rumpold BA, Fröhling A, Reineke K, Knorr D, Boguslawski S, Ehlbeck J, Schlüter OK. 2014. Comparison of volumetric and surface decontamination techniques for innovative processing of mealworm larvae (Tenebrio molitor). Innov Food Sci Emerg Technol 26:232–241. doi: 10.1016/j.ifset.2014.09.002. [DOI] [Google Scholar]
- 23.Fombong FT, Van Der Borght M, Vanden Broeck J. 2017. Influence of freeze-drying and oven-drying post blanching on the nutrient composition of the edible insect Ruspolia differens. Insects 8:102–116. doi: 10.3390/insects8030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanboonsong Y, Jamjanya T, Durst PB. 2013. Six-legged livestock: edible insect farming, collecting and marketing in Thailand. Food and Agriculture Organization of the United Nations, Regional Office for Asia and the Pacific, Bangkok, Thailand. [Google Scholar]
- 25.Chao A. 1984. Non-parametric estimation of the number of classes in a population. Scand J Stat 11:265–270. [Google Scholar]
- 26.Shannon CE. 1948. A mathematical theory of communication. Bell Syst Tech J 27:379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x. [DOI] [Google Scholar]
- 27.Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB. 2010. Bergey's manual of systematic bacteriology, vol 4, 2nd ed Springer Science + Business Media, New York, NY. [Google Scholar]
- 28.Brenner DJ, Krieg NR, Staley JT, Garrity GM, Boone DR, De Vos P, Goodfellow M, Rainey FA, Schleifer K-H. 2005. Bergey's manual of systematic bacteriology, vol 2, parts A, B and C, 2nd ed Springer Science + Business Media, New York, NY. [Google Scholar]
- 29.Van Assche A, Álvarez-Pérez S, de Breij A, De Brabanter J, Willems KA, Dijkshoorn L, Lievens B. 2017. Phylogenetic signal in phenotypic traits related to carbon source assimilation and chemical sensitivity in Acinetobacter species. Appl Microbiol Biotechnol 101:367–379. doi: 10.1007/s00253-016-7866-0. [DOI] [PubMed] [Google Scholar]
- 30.Dahiru M, Enabulele OI. 2015. Incidence of Acinetobacter in fresh carrot (Daucus carota subsp. sativus). Int J Nutr Food Eng 9:1192–1195. [Google Scholar]
- 31.Hausdorf L, Fröhling A, Schlüter O, Klocke M. 2011. Analysis of the bacterial community within carrot wash water. Can J Microbiol 57:447–452. doi: 10.1139/w11-013. [DOI] [PubMed] [Google Scholar]
- 32.Vandeweyer D, Crauwels S, Lievens B, Van Campenhout L. 2017. Microbial counts of mealworm larvae (Tenebrio molitor) and crickets (Acheta domesticus and Gryllodes sigillatus) from different rearing companies and different production batches. Int J Food Microbiol 242:13–18. doi: 10.1016/j.ijfoodmicro.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Giaccone V. 2005. Hygiene and health features of “minilivestock,” p 579–598. In Paoletti MG. (ed), Ecological implications of minilivestock: potential of insects, rodents, frogs and snails. Science Publishers, Inc, Enfield, NH. [Google Scholar]
- 34.Grabowski NT, Klein G. 2016. Microbiology of processed edible insect products—results of a preliminary survey. Int J Food Microbiol 243:103–107. doi: 10.1016/j.ijfoodmicro.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Osimani A, Garofalo C, Milanović V, Taccari M, Cardinali F, Aquilanti L, Pasquini M, Mozzon M, Raffaelli N, Ruschioni S, Riolo P, Isidoro N, Clementi F. 2017. Insight into the proximate composition and microbial diversity of edible insects marketed in the European Union. Eur Food Res Technol 243:1157–1171. doi: 10.1007/s00217-016-2828-4. [DOI] [Google Scholar]
- 36.Banjo AD, Lawal OA, Adeyemi AI. 2006. The microbial fauna associated with the larvae of Oryctes monocerus. J Appl Sci Res 2:837–843. [Google Scholar]
- 37.Ali A, Mohamadou BA, Siadou C, Aoudou Y, Tchiegang C. 2010. Physico-chemical properties and safety of grasshoppers, important contributors to food security in the far north region of Cameroon. Res J Anim Sci 4:108–111. doi: 10.3923/rjnasci.2010.108.111. [DOI] [Google Scholar]
- 38.Zheng L, Crippen TL, Sheffield CL, Poole TL, Yu Z, Tomberlin JK. 2012. Evaluation of Salmonella movement through the gut of the lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae). Vector Borne Zoonotic Dis 12:287–292. doi: 10.1089/vbz.2011.0613. [DOI] [PubMed] [Google Scholar]
- 39.Templeton JM, De Jong AJ, Blackall PJ, Miflin JK. 2006. Survival of Campylobacter spp. in darkling beetles (Alphitobius diaperinus) and their larvae in Australia. Appl Environ Microbiol 72:7909–7911. doi: 10.1128/AEM.01471-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magan N, Olsen M. 2004. Mycotoxins in food: detection and control, 1st ed CRC Press, Boca Raton, FL. [Google Scholar]
- 41.Jay JM, Loessner MJ, Golden DA. 2005. Modern food microbiology, 7th ed Springer Science + Business Media, New York, NY. [Google Scholar]
- 42.International Organization for Standardization. 2004. ISO 7932:2004 Microbiology and food and animal feeding stuffs—horizontal method for the enumeration of presumptive Bacillus cereus—colony-count technique at 30°C. International Organization for Standardization, Geneva, Switzerland: https://www.iso.org/standard/38219.html. [Google Scholar]
- 43.Stoops J, Crauwels S, Waud M, Claes J, Lievens B, Van Campenhout L. 2016. Microbial community assessment of mealworm larvae (Tenebrio molitor) and grasshoppers (Locusta migratoria migratorioides) sold for human consumption. Food Microbiol 53:122–127. doi: 10.1016/j.fm.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Dijk R, van den Berg D, Beumer R, de Boer E, Dijkstra A, Mout L, Stegeman H, Uyttendaele M, in 't Veld S. 2015. Microbiologie van voedingsmiddelen: methoden, principes en criteria, 5th ed MYbusinessmedia, Capelle aan den Ijssel, The Netherlands. [Google Scholar]
- 45.Lievens B, Brouwer M, Vanachter ACRC, Lévesque CA, Cammue BPA, Thomma BPHJ. 2003. Design and development of a DNA array for rapid detection and identification of multiple tomato vascular wilt pathogens. FEMS Microbiol Lett 223:113–122. doi: 10.1016/S0378-1097(03)00352-5. [DOI] [PubMed] [Google Scholar]
- 46.Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. 2013. GenBank. Nucleic Acids Res 41:D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.International Organization for Standardization. 2017. ISO 6579-1:2017 Microbiology of the food chain—horizontal method for the detection, enumeration and serotyping of Salmonella—part I: detection of Salmonella spp. International Organization for Standardization, Geneva, Switzerland: https://www.iso.org/standard/56712.html. [Google Scholar]
- 48.Association Française de Normalisation. 1998. AFNOR BRD 07/4-09/98 RAPID'L.mono (Detection). Association Française de Normalisation, La Plain Saint-Denis, France: https://nf-validation.afnor.org/wp-content/uploads/sites/2/2014/03/BRD-07-04-09-98_en.pdf. [Google Scholar]
- 49.Association Française de Normalisation. 2003. AFNOR 3M 01/9-04/03 B. 3M Petrifilm Staph Express Count System. Association Française de Normalisation, La Plain Saint-Denis, France: https://nf-validation.afnor.org//en/wp-content/uploads/sites/2/2014/03/3M-01-09-04-03-B_en.pdf. [Google Scholar]
- 50.Edgar RC. 2016. SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv. doi: 10.1101/074161. [DOI]
- 51.R Development Core Team. 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.