ABSTRACT
Carpets and other soft surfaces have been associated with prolonged and reoccurring human norovirus (HuNoV) outbreaks. Environmental hygiene programs are important to prevent and control HuNoV outbreaks. Despite our knowledge of HuNoV transmission via soft surfaces, no commercially available disinfectants have been evaluated on carpets. Our aim was to adapt a current standardized method for virucidal testing by assessing two disinfection technologies, silver dihydrogen citrate (SDC) and steam vapor, against one HuNoV surrogate, feline calicivirus (FCV), on wool and nylon carpets. First, we evaluated the effect of both technologies on the appearance of carpet. Next, we evaluated the efficacy of SDC in suspension and the efficacy of SDC and steam vapor against FCV on a glass surface, each with and without serum. Lastly, we tested both technologies on two types of carpet, wool and nylon. Both carpets exhibited no obvious color changes; however, SDC treatments left a residue while steam vapor left minor abrasions to fibers. SDC in suspension and on glass reduced FCV by 4.65 log10 and >4.66 log10 PFU, respectively, but demonstrated reduced efficacy in the presence of serum. However, SDC was only efficacious against FCV on nylon (3.62-log10 PFU reduction) and not wool (1.82-log10 PFU reduction). Steam vapor reduced FCV by >4.93 log10 PFU on glass in 10 s and >3.68 log10 PFU on wool and nylon carpet carriers in 90 s. There was a limited reduction of FCV RNA under both treatments compared to that of infectivity assays, but RNA reductions were higher in samples that contained serum.
IMPORTANCE Human noroviruses (HuNoV) account for ca. 20% of all diarrheal cases worldwide. Disease symptoms may include diarrhea and vomit, with both known to contribute to transmission. The prevention and control of HuNoV are difficult because they are environmentally resilient and resistant to many disinfectants. Several field studies have linked both hard and soft surfaces to HuNoV outbreaks. However, many disinfectants efficacious against HuNoV surrogates are recommended for hard surfaces, but no commercially available products have demonstrated efficacy against these surrogates on soft surfaces. Our research objectives were to evaluate liquid and steam-based technologies in suspension and on hard surface carriers in addition to adapting and testing a protocol for assessing the virucidal effects of disinfection technologies on carpet carriers. These results will inform both the government and industry regarding a standard method for evaluating the virucidal effects of disinfectants on carpet while demonstrating their efficacy relative to suspension and hard-surface tests.
KEYWORDS: human norovirus, feline calicivirus, disinfection, sanitization, hard surface, soft surface, carpet
INTRODUCTION
Human noroviruses (HuNoV) are the leading causes of acute gastroenteritis worldwide as well as the most common cause of foodborne disease in the United States. Worldwide, its economic burden is estimated to be $4.2 billion in health care costs alone, illustrating its significance as a public health problem (1, 2). Transmission occurs through the fecal-oral or vomitus-oral route via person-to-person contact, food, water, or environmental surfaces (1). Person-to-person contact and food are estimated to be the predominate mode of transmission (3). However, the survival of HuNoV on environmental surfaces and their subsequent transmission via fomites may play important roles in their outbreaks.
The environmental transmission of HuNoV is estimated to be low (3). Even so, several epidemiological investigations and laboratory-based studies suggest the environment plays an important role in transmission, as both hard and soft surfaces may initiate and prolong HuNoV outbreaks (4). For example, a hotel in the United Kingdom experienced a 5-month-long HuNoV outbreak (5). Outbreak investigators suggested that in addition to HuNoV environmental stability, the ineffective decontamination of soft surfaces contributed to the prolonged outbreak.
Focusing on environmental sanitation is a recommended strategy to prevent and control HuNoV outbreaks. The challenge to effective environmental sanitation is that HuNoV can be shed in high titers from infected individuals. This, coupled with its environmental stability, low infectious dose, and resistance to many commonly used disinfectants, e.g., phenolic and quaternary ammonium compounds, makes HuNoV outbreaks difficult to control. The current recommendation for HuNoV cleanup includes using 1,000 to 5,000 ppm bleach or an Environmental Protection Agency (EPA)-registered disinfectant (6). However, these disinfectants were validated for hard surfaces but not soft surfaces. Moreover, their use may damage the appearance of soft surfaces, such as carpet and soft furnishings. Furthermore, carpet and other soft furnishings can absorb toxic active ingredients of disinfectants, causing irritation of the skin, eyes, and respiratory tract (7). Fogging with certain chemistries, e.g., ozone and H2O2, has demonstrated efficacy against some enteric viruses but is impractical in some settings, e.g., long-term-care facilities and residential homes, due to the temporary removal of residents and the cost (8). Altogether, the shortcomings of current decontamination procedures present a gap in evidence-based control strategies for disinfecting soft surfaces contaminated with viruses, suggesting the need to evaluate safe and practical technologies for use on soft surfaces.
To our knowledge, only one previous study has investigated the use of disinfection technologies, all of which are liquid-based, against viruses on carpets (9). Investigators found that a glutaraldehyde-based solution could achieve EPA efficacy standards for disinfection on soft surfaces against a HuNoV surrogate, feline calicivirus (FCV) (3.0-log10 reduction). However, glutaraldehyde has been linked to negative health effects, such as skin rashes and respiratory irritation (10). Other chemistries tested were salt and quaternary ammonium-based products, which have shown little efficacy against HuNoV (9). Transitional metal ions, such as silver, have demonstrated broad-range efficacy against microorganisms. Silver, as an antimicrobial, is also known to be more stable when generated in the presence of citric acid to form silver dihydrogen citrate (SDC). The benefits of SDC include a lower toxicity compared to other chemistries, i.e., glutaraldehyde, and that it may be gentler on delicate soft surfaces. Previous studies with SDC have demonstrated its efficacy against HuNoV, but these studies lack infectious data required by the EPA to be registered (11, 12).
The Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Hazard Administration (OSHA) have both recommended steam cleaning for carpets after a suspected HuNoV contamination event (13). Steam has been shown to be efficacious against non-spore-forming bacteria on hard surfaces, but there is scant literature of its efficacy against viruses. A steam vapor system with thermo-accelerated nano-crystal sanitation (TANCS) technology (Advanced Vapor Technologies, Seattle, WA) is a promising tool for disinfecting virally contaminated soft surfaces. Previous work with MS2 phage and FCV on hard surfaces demonstrated >6-log and >4-log reductions in less than 5 and 10 s, respectively (14, 15). However, the efficacy of steam or moist heat against viruses contaminating soft surfaces has not been evaluated under controlled conditions (8). This is due, in part, to a lack of standardized test methods for the quantitative assessment of disinfectants intended for carpets contaminated with viruses.
The gap in knowledge regarding the efficacy of soft-surface disinfection technologies and the lack of standardized test methods for carpets warrant further exploration. To our knowledge, no published studies have investigated either SDC or steam vapor against infectious HuNoV surrogates on carpet. The aim of this study was to adapt a standardized method for virucidal efficacy testing on carpets by assessing these two disinfection technologies against FCV on wool and nylon carpets. The specific objectives were to assess SDC (i) in suspension, (ii) with steam vapor on a hard surface with current American Society for Testing and Materials (ASTM) International standards, and (iii) on carpets by adapting a current ASTM International standard (16).
RESULTS
Cytotoxicity and neutralization of SDC.
Neutralizers 1 and 2 were evaluated on the basis of their abilities to neutralize and prevent the cytotoxicity of SDC. SDC showed no apparent cytotoxicity toward Crandell-Rees feline kidney (CRFK) cells after 1 h of incubation at the 1:10 and 1:20 dilutions in both neutralizers during phase 1 testing. Furthermore, during phase 2, the validation of neutralization, both neutralizers achieved >80% recovery of FCV compared to that of the controls. However, during phase 3, the assessment of neutralized SDC's interference with infectivity, neutralizer 1 exhibited 100% cytopathic effects at both 1:10 and 1:20 dilutions, whereas neutralizer 2 yielded >84% recovery of FCV with 1:10 and 1:20 dilutions of SDC, with no apparent cytotoxicity. As a result, neutralizer 2 was used in the following studies.
Efficacy of SDC in suspension.
Table 1 shows the efficacy of SDC against infectious FCV with and without 5% serum at contact times ranging from 1 to 30 min in suspension. With the addition of serum, infectious FCV was reduced by 4.29 log10 PFU within 1 min and continued to be inactivated up to 4.65 log10 PFU after 30 min. Conversely, SDC treatments of FCV with no serum were reduced by 4.51 log10 PFU within 1 min, but no additional inactivation was observed after 5 min. By comparison, between 1 and 10 min, serum significantly reduced SDC's ability to inactivate FCV compared to that of treatments that were serum free. Likewise, contact time significantly affected SDC in the presence of serum but not for treatments without serum. However, overall, there was no significant difference observed between serum treatments after a 30-min contact time.
TABLE 1.
Virucidal efficacy of silver dihydrogen citrate against FCV in suspension measured by plaque assay and qRT-PCR
| Contact time (min) | Reductiona |
|||
|---|---|---|---|---|
| Plaque assay (log10 PFU) |
qRT-PCR (log10 copies) |
|||
| With 5% serumb | Control without 5% serumc | With 5% serumb | Control without 5% serumc | |
| 1 | 4.29 ± 0.12 AA | 4.51 ± 0.09 AB | 1.93 ± 0.07 AA | 1.84 ± 0.19 AA |
| 5 | 4.37 ± 0.06 ABA | 4.69 ± 0.28 AB | 1.85 ± 0.05 AA | 1.72 ± 0.17 AB |
| 10 | 4.41 ± 0.08 BA | 4.67 ± 0.01 AB | 1.89 ± 0.04 AA | 1.84 ± 0.10 AA |
| 30 | 4.65 ± 0.11 CA | 4.65 ± 0.05 AA | 1.88 ± 0.07 AA | 1.78 ± 0.08 AA |
The data are expressed as means ± standard deviations (SDs) from 9 replicates in 3 independent experiments. Values with different nonsuperscript letters in the same column are significantly different (P < 0.05), whereas values with different superscript letters in the same row for each detection method are significantly different.
FCV stocks concentrated via ultrafiltration were diluted in complete Dulbecco's modified Eagle's medium with 5% low-endotoxin (<10 endotoxin units [EU]/ml) fetal bovine serum.
FCV stocks concentrated via ultrafiltration were diluted in 1× phosphate-buffered saline.
Table 1 shows the efficacy of SDC against FCV evaluated via reverse transcription-quantitative PCR (qRT-PCR). The reductions in FCV RNA ranged from 1.85 log10 to 1.93 log10 copies and from 1.72 log10 to 1.84 log10 copies for treatments with and without serum, respectively. Contact time did not significantly affect the FCV's RNA, regardless of serum presence. However, there was a significant difference observed among samples treated with and without serum after SDC exposure for 5 min, albeit the difference was only 0.12 log10 copies. Similarly, other serum-free treatments exhibited lower copy reductions than treatments with serum, but they were not significant.
Efficacy of SDC and dry steam on glass carriers.
Table 2 shows the efficacy of SDC against FCV on a glass surface with and without serum at contact times ranging from 1 to 30 min. On glass, the initial levels of FCV recovered from controls during SDC testing for samples with and without serum were 5.68 ± 0.24 log10 PFU and 4.50 ± 0.04 log10 PFU, respectively. SDC reduced FCV by >4.66 log10 and >3.46 log10 PFU within 30 and 10 min with and without serum, respectively. The inactivation of FCV by SDC was significantly affected by time. A higher log reduction was observed in the presence of serum than in serum-free treatments. However, due to desiccation, there was significant difference (1.18 log10 PFU) among recovered control samples from serum and serum-free carriers at zero time prior to SDC exposure. SDC qRT-PCR results showed there was a maximum reduction of 1.01 log10 copies over 30 min. As in the suspension tests, serum-free samples exhibited a lower reduction in RNA than serum-treated samples.
TABLE 2.
Virucidal efficacy of silver dihydrogen citrate against FCV on glass measured by plaque assay and qRT-PCR
| Contact time (min) | Reductiona |
|||
|---|---|---|---|---|
| Plaque assay (log10 PFU) |
qRT-PCR (log10 copies) |
|||
| With 5% serumb | Control without 5% serumc | With 5% serumb | Control without 5% serumc | |
| 1 | 1.17 ± 0.30 AA | 0.77 ± 0.41 AA | 0.12 ± 0.25 AA | −0.85 ± 0.09 AB |
| 5 | 3.71 ± 0.35 BA | 2.49 ± 0.08 BB | 0.53 ± 0.07 ABA | −0.58 ± 0.26 AB |
| 10 | 3.84 ± 0.45 B | >3.46 A | 0.45 ± 0.06 BA | −0.66 ± 0.10 AB |
| 30 | >4.66 C | >3.46 A | 1.01 ± 0.28 CA | −0.45 ± 0.38 AB |
The data are expressed as means ± SDs from 9 replicates in 3 independent experiments. Values with different nonsuperscript letters in the same column are significantly different (P < 0.05), whereas values with different superscript letters in the same row for each detection method are significantly different.
FCV stocks concentrated via ultrafiltration were diluted in complete Dulbecco's modified Eagle's medium with 5% low-endotoxin (<10 EU/ml) fetal bovine serum. Recovered control was 5.68 log10 ± 0.24 log10 PFU.
FCV stocks concentrated via ultrafiltration were diluted in 1× phosphate-buffered saline. Recovered control was 4.50 log10 ± 0.04 log10 PFU.
Table 3 shows the efficacy of steam vapor with TANCS against FCV on a glass surface, with and without serum, at contact times ranging from 10 to 90 s. On glass, the initial levels of FCV recovered from controls during steam vapor testing for samples with and without serum were 5.99 ± 0.20 log10 PFU and 5.17 ± 0.16 log10 PFU, respectively. Steam vapor reduced infectious FCV by >4.93 log10 and >4.11 log10 PFU within 10 s with and without serum, respectively. These values represent the method's limit of detection for each treatment. As such, no further inactivation of FCV was detected beyond the 10-s treatment. There was no observed time effect between 10 and 90 s. The treatments with serum produced a significantly higher reduction than that in serum-free samples. However, this was due to a difference in recoverable FCV from controls after drying. An analysis via qRT-PCR showed that steam vapor could reduce FCV's RNA between 1.92 log10 and 2.31 log10 copies. Unlike samples treated with serum, serum-free samples were significantly affected by time. Furthermore, a qRT-PCR analysis showed a similar trend regarding serum in samples of infectious FCV treated with steam vapor, i.e., lower reductions among serum-free samples.
TABLE 3.
Virucidal efficacy of steam vapor with TANCS against FCV on glass measured by plaque assay and qRT-PCR
| Contact time (s) | Reductiona |
|||
|---|---|---|---|---|
| Plaque assay (log10 PFU) |
qRT-PCR (log10 copies) |
|||
| With 5% serumb | Control without 5% serumc | With 5% serumb | Control without 5% serumc | |
| 10 | >4.93 A | >4.11 A | 2.31 ± 0.25 AA | 0.70 ± 0.32 AB |
| 30 | >4.93 A | >4.11 A | 1.92 ± 0.21 AA | 0.20 ± 0.10 AB |
| 60 | >4.93 A | >4.11 A | 1.94 ± 0.07 AA | 0.30 ± 0.38 ABB |
| 90 | >4.93 A | >4.11 A | 1.93 ± 0.32 AA | 1.03 ± 0.36 BB |
The data are expressed as means ± SDs from 9 replicates in 3 independent experiments. Values with different nonsuperscript letters in the same column are significantly different (P < 0.05), whereas values with different superscript letters in the same row for each detection method are significantly different.
FCV stocks concentrated via ultrafiltration were diluted in complete Dulbecco's modified Eagle's medium with 5% low-endotoxin (<10 EU/ml) fetal bovine serum. Recovered control was 5.99 log10 ± 0.20 log10 PFU.
FCV stocks concentrated via ultrafiltration were diluted in 1× phosphate-buffered saline. Recovered control was 5.17 log10 ± 0.16 log10 PFU.
Efficacy of SDC and steam vapor on carpets.
Table 4 shows the efficacy of SDC and steam vapor with TANCS against FCV on wool and nylon carpet carriers treated for 60 min and 90 s, respectively. The initial levels of FCV recovered from wool and nylon carpet during SDC testing were 5.11 ± 0.06 log10 and 5.20 ± 0.22 log10 PFU, respectively. SDC reduced FCV by 1.82 log10 and 3.62 log10 PFU on wool and nylon carpet carriers, respectively, within 60 min. On the other hand, the initial levels of FCV recovered from wool and nylon carpet during steam vapor testing were 5.38 ± 0.19 log10 and 5.26 ± 0.07 log10 PFU, respectively. Steam vapor reduced FCV by 3.80 log10 and 3.68 log10 PFU on wool and nylon carpet carriers, respectively. The efficacy of SDC was affected significantly by the carpet type, whereas no significant surface effect was observed across steam vapor treatments. An analysis by qRT-PCR demonstrated little reduction among SDC and steam vapor treatments. However, there was an effect of surface type among both treatments. Specifically, significantly greater log10 copy reductions were observed on nylon carpet carriers than on wool carpet carriers.
TABLE 4.
Virucidal efficacy of silver dihydrogen citrate and steam vapor with TANCS against FCV on wool and nylon carpet measured by plaque assay and qRT-PCR
| Treatmenta | Contact time | Surface | Reductionb |
|
|---|---|---|---|---|
| Plaque assay (log10 PFU) | qRT-PCR (log10 copies) | |||
| SDC | 60 min | Wool | 1.82 ± 0.19 A | −0.06 ± 0.26 A |
| Nylon | 3.62 ± 0.32 B | 0.49 ± 0.27 B | ||
| Steam | 90 s | Wool | 3.80 ± 0.16 B | 0.03 ± 0.17 A |
| Nylon | 3.68 ± 0.09 B | 0.39 ± 0.23 B | ||
FCV stocks concentrated via ultrafiltration were diluted in complete Dulbecco's modified Eagle's medium with 5% low-endotoxin (<10 EU/ml) fetal bovine serum.
The data are expressed as means ± SDs from 15 replicates in 3 independent experiments. Values with different letters in the same column are significantly different (P < 0.05).
SDC and steam vapor effects on carpet appearance.
Figure 1 illustrates the effects of both SDC and steam vapor immediately after application and after 60 min and 24 h of drying. After the application of SDC and scrubbing, suds and a white film appeared over the wool and nylon carriers but dissipated within 60 min. After 24 h, no visual effects were observed, although the carriers had a sticky residue. On the other hand, after 90 s of steam treatment, the carriers appeared wet, with minor abrasion to the carriers. After 60 min, wool and nylon carriers appeared dry but the surface fibers still appeared to have minor abrasion.
FIG 1.
Effect of silver dihydrogen citrate and steam vapor on the appearance of wool (E to H and M to P) and nylon (A to D and I to L) carpet carriers between 0 and 24 h.
DISCUSSION
An effective environmental hygiene program is important for the prevention and control of HuNoV outbreaks (4). The efficacies of a variety of technologies and chemistries have been tested against HuNoV and their surrogates (8). However, a limited number of these interventions have been evaluated for their efficacy on carpets. In this study, we adapted a standardized method for testing the efficacy of disinfection technologies against viruses on carpet. Our results demonstrate the efficacy of a liquid disinfectant, SDC in suspension, on glass and carpets, as well as the efficacy of steam vapor on glass and carpets, against the EPA-approved HuNoV surrogate FCV.
Cytotoxicity and neutralization tests are critical steps for the successful evaluation of chemistries intended for virucidal efficacy testing. A previous study investigating SDC against HuNoV used Dey-Engley neutralizing broth to quench silver ions and increase the solution pH (12). However, Dey-Engley broth is considered a universal neutralizing broth because it contains a variety of chemicals, such as sodium thiosulfate, sodium bisulfite, and sodium thioglycolate. Because of its complexity, this broth was not filterable via ultrafiltration, a tool essential for the detection of viruses on carpet with our method, and necessitated the development of a targeted filterable neutralizer. We successfully developed a filterable sodium thioglycolate-based neutralizer on the basis of work by Liau et al. (17), who demonstrated silver ion's affinity for thiol-containing groups. While the sodium thioglycolate component was necessary for quenching silver ions, sodium bicarbonate and HEPES buffer were added to eliminate cytotoxicity caused by low pH, whereas the nonionic surfactant, Tween 80, was used to assist with the recovery of FCV.
In suspension, SDC could reach the EPA standard for antiviral efficacy against FCV (4-log10 PFU reduction) within 1 min, both with and without 5% serum present. This contrasts with previous results with SDC against HuNoV (12). The results from Manuel et al. (12) suggested that 5 min is needed to reach a 4-log10 reduction of HuNoV RNA under a pristine condition, whereas a 5% soil load only reduced HuNoV RNA copy numbers by ca. 2.5 log10 in 30 min. This discrepancy can be attributed to FCV's known susceptibility to low pH solutions compared to that of HuNoV (18). Additionally, during suspension testing, the efficacy of SDC was significantly lower in treatments with serum than in treatments without serum between 1 and 10 min. The reduced efficacy of liquid chemistries when organic soil is present in a sample has been documented (12, 19). For instance, Manuel et al. (12), while studying SDC's efficacy against HuNoV, found similar results with and without a soil component. Furthermore, the CDC recommends the use of higher concentrations of bleach, i.e., 5,000 ppm, for soiled surfaces compared to 1,000 ppm for a precleaned surface. This is also why the EPA requires one-step cleaning products to incorporate a 5% soil load within a sample matrix during efficacy testing (6). In this study, the lower efficacy under 5% serum conditions is not surprising, as SDC has an affinity for thiol-containing groups that are present in fetal bovine serum (FBS), i.e., the amino acids cysteine and methionine.
The results from our glass carrier test with SDC demonstrated its efficacy against infectious FCV, albeit SDC's efficacy is reduced compared to its efficacy in suspension. Generally, suspension tests overestimate the efficacy of a technology compared to that from hard-surface testing. As previously postulated, this likely is due to the adsorption and aggregation of virions on the surface, which are less accessible than free unbound viruses in suspension, which have more exposure (20, 21). Regardless, hard-surface testing simulates in-use conditions better than in suspension testing. Consistent with suspension tests, FCV was inactivated beyond our limit of detection. However, the inactivation was not as immediate as in suspension testing, because SDC tests took up to 30 min to achieve a >4-log10 reduction in the presence of serum. Nevertheless, serum-free samples treated with SDC met our limit of detection within 10 min due to a lower recovery after desiccation.
In contrast to SDC, steam vapor demonstrated rapid inactivation of infectious FCV by achieving a >4.11-log10 PFU reduction in 10 s on glass carriers. The inactivation time is a critical factor, because typical contact times for liquid disinfectants are between 1 and 10 min and surfaces must be thoroughly wet. Steam vapor with TANCS technology appears to provide synergism between heat and the municipal tap water containing natural impurities, such as calcium carbonate, that can be crystalized with heat (22). In addition to the steam vapor, these crystals are thought to provide an additional hurdle for microorganisms by interacting with the virus surface, although this has yet to be confirmed. Regardless, our results support a previous finding that steam vapor can reduce viruses beyond the EPA standard in short contact times, i.e., <10 s (14).
Currently, there are no standardized methods for the evaluation of disinfectants intended for carpets contaminated with viruses. The EPA requires soft-surface disinfectants to meet a minimum of a 3-log10 reduction (23). SDC could meet this requirement in 60 min on nylon but not wool carpet carriers, with a significantly higher reduction found on nylon carpets. To our knowledge, only one other study has evaluated liquid chemistries against viruses inoculated on carpet. Malik et al. (9) indicated that of the disinfectants tested, only 2.6% activated glutaraldehyde was effective on synthetic carpets, i.e., olefin, polyester, nylon, and blended carpets. Although this may be true, activated glutaraldehyde, at that level, may not be safe for application on soft surfaces, because glutaraldehyde is listed as category I and III for primary eye irritation and acute dermal exposure, respectively, according to the EPA's toxicity rating, whereas SDC falls within category IV, the lowest EPA toxicity rating (24).
It is especially important to consider the toxicity of the chemicals being applied to soft surfaces. Previous work on soft surfaces with chemicals, such as quaternary ammonium compounds (QUAT) and chlorine, has demonstrated that some soft surfaces can adsorb and sequester active ingredients (24). In addition to toxic residues, their absorption ability may reduce the efficacy of some disinfectants. For instance, McNeil et al. (25) found that some soft surfaces, e.g., gauze and yarn, could adsorb up to >40% of a QUAT, while a similar study using chlorine determined that cotton could adsorb up to 98% of an 800-ppm chlorine solution. Altogether, these results may explain the difference in SDC's efficacies against FCV on wool and nylon carpets, as wool has been demonstrated to absorb ca. 2 times more liquid than nylon (26). Because of this, it is likely more silver ions were sequestered by wool fibers than by nylon fibers, which decreased the amount of free silver ions available to interact with FCV.
Heat treatments are effective at inactivating viruses on both hard and soft surfaces and have no toxicity or residues. This may be why steam treatments have been recommended by multiple governmental agencies. Be that as it may, their efficacy has never been demonstrated on carpets. In this study, steam vapor with TANCS met the EPA's 3-log10 reduction standard for soft surfaces in 90 s (>3.68 log10 PFU), although FCV was not inactivated below the limit of detection as it was when dried on a glass surface. Depending upon the environment, FCV has been shown to survive significantly better on both wool and nylon carpet fibers than on glass (26). It is possible these fibers do not transfer heat as efficiently as glass, but more importantly, FCV is adsorbed and trapped within the substrata of these multilayered fibers, which occludes the capsid and may prevent inactivation.
Under all conditions within this study, SDC and steam vapor were not efficacious against FCV's RNA. While not our study's aim, this discrepancy may provide evidence of SDC and steam vapor's mode of action against FCV. Unlike some HuNoV studies, we chose to not treat samples with RNase (12). This method aids in the removal of exogenous nucleic acids that may inflate the copy number. By not applying RNase and measuring the infectivity of surrogates, we can gain a better understanding of the disinfectant technology's mode of action under certain conditions. Taken alone, the observed difference in log10 reductions between infectious and molecular data for both technologies could suggest viral aggregation, the preservation of the target amplicon after lysis, or that these technologies target the capsid. Viral aggregation would inflate the copy numbers, but in return, would indicate both technologies' limited efficacies. Similarly, amplicon preservation after lysis would suggest a limitation of our qRT-PCR detection method. However, our data juxtaposed with those of other literature strongly suggest that both technologies work primarily against the viral capsid. The heat provided by the steam vapor presumably denatures the capsid and eventually begins to degrade FCV RNA. For example, infectious murine norovirus was found to be sensitive to high temperatures, i.e., 24 to 85°C, but its RNA was significantly reduced only after applying an RNase treatment (27). Different from steam vapor, the citrate in SDC may change the particle's morphology while the silver ions attack cysteine residues important for capsid stabilization and formation (11, 12). A previous study using a similar disinfecting technology found that citrate altered a HuNoV virus-like particle's morphology and its ability to bind to human blood group antigens (11). But a follow-up study only found a 25% reduction in capsid protein and suggested that the silver ion either potentiates or synergistically impacts the efficacy of citrate (12).
Counter to the trend observed in infectious data, lower FCV RNA log10 reductions were reported in serum-free samples than in samples with serum, regardless of the treatment or test type. The serum-free samples were diluted in inert phosphate-buffered saline (PBS), whereas serum-treated samples contain FBS and a variety of complex molecules and compounds that may have reduced the amplification of or degraded exogenous genomic RNA, but this has not been confirmed.
Appearance is also a critical factor to consider when developing disinfectants intended for soft surfaces. Although some may be efficacious, many chemistries can be damaging to soft surfaces. Our qualitative data showed little change in the appearance of the wool and nylon carpets, regardless of treatment type. This suggests, if efficacious, these technologies can be applied with limited damage to wool and nylon surfaces.
A limitation of our study is the recovery of FCV from control glass carriers. Regardless of disinfection technology, lower FCV titers were recovered from serum-free samples (ca. 1 log10). Carriers for both treatments were dried, recovered, and disinfected under the same conditions. But, it is commonly known that organic soils can provide protection to enteric viruses. It is likely the reduced organic load and 30% relative humidity (RH) contributed to higher inactivation during desiccation than in controls that contained 5% serum. Although serum-free samples treated with both SDC and steam vapor were inactivated below our limit of detection, the difference between recovered controls represents a limitation to our study, as we cannot statistically compare serum and serum-free treatments after FCV concentrations have fallen below the limit of detection.
Conclusion.
In summary, ASTM E2966-14 can be adapted for testing the efficacy of disinfectants against viruses on contaminated carpets with few modifications. SDC was found to be efficacious against infectious FCV in suspension, on glass, and on nylon carpet. However, SDC is less efficacious against infectious FCV in the presence of serum and on wool carpet. On the other hand, steam vapor with TANCS was efficacious on all surfaces tested and exhibited no loss to its efficacy in the presence of serum. Furthermore, treatments with these technologies do not affect the esthetic appearance of the carpets. Altogether, these results suggest that surfaces should be thoroughly precleaned for SDC to become efficacious, while steam vapor with TANCS demonstrated rapid inactivation of FCV and could be an appropriate disinfection technology for virally contaminated natural and synthetic carpets.
MATERIALS AND METHODS
Virus propagation, cell culture, and plaque assay.
A stock of feline calicivirus (FCV) strain F9 was propagated by infecting 90% confluent monolayers of Crandell-Rees feline kidney (CRFK) cells (ATCC CCL-94; American Type Culture Collection, Manassas, VA, USA) at a multiplicity of infection (MOI) of 0.01 in complete Eagle's modified essential medium (Corning, Corning, NY, USA) supplemented with 10% low-endotoxin heat-inactivated FBS (Seradigm, VWR International, Radnor, PA, USA), 100 U/liter penicillin (HyClone, GE, Boston, MA, USA), and 100 μg/liter streptomycin (HyClone). CRFK cells were incubated at 37°C and 5% CO2 (Symphony, VWR International, Radnor, PA, USA) until a complete cytopathic effect was observed (1 to 3 days). FCV was harvested from cell lysates by three cycles of freeze-thawing followed by centrifugation for 10 min at 5,000 × g and 4°C and then extracted with chloroform as previously described (28). FCV (ca. 9 log PFU/ml) stocks were aliquoted and stored at −80°C.
Infectious FCV was quantified by standard plaque assays as previously described with modifications (29). Briefly, CRFK cells were seeded in 6-well dishes at 2.5 × 105 viable cells/well and incubated until they were ca. 90% confluent (2 days). FCV samples were serially diluted in an infection medium (complete Dulbecco's modified Eagle's medium [CDMEM]) containing 5% FBS (CDMEM-5), if needed. During the plaque assay, 0.2 ml of an FCV sample was added to each monolayer in addition to 0.3 ml of CDMEM-5 and immediately rocked twice. After a 1-h absorption phase, 2 ml of 1:1 mixtures of 3% Seaplaque agarose (Lonza, Switzerland) and 2× Temin's modified Eagle medium (MEM) was added to each well and incubated until visible plaque formation (1 to 3 days). The 2× MEM was supplemented with 10% low-endotoxin heat-inactivated FBS, 100 U/liter penicillin, 100 μg/liter streptomycin, 10 mM HEPES (HyClone), and 1 mM nonessential amino acids (NEAA; HyClone). FCV plaques were visualized by staining agarose plugs with a 0.03% neutral red solution (Carolina Biological, Burlington, NC) mixed with 1× PBS and were enumerated on a light box (Futura light box, Logan Electric, Bartlett, IL, USA). FCV plaque assays contained a stock suspension of FCV virus and CDMEM-5 as a positive and negative control, respectively, to test for cell line permissiveness and contamination. CRFK cells were passaged fewer than 25 times.
RNA extraction and qRT-PCR.
Viral extraction was performed as previously described with minor modifications (30). Viral RNA was extracted from 0.15 ml of a sample or virus stock with an ENZA viral RNA kit (Omega Bio-Tek, Norcross, GA, USA) per the manufacturer's instructions. Viral RNA was extracted on the day of recovery experiments and stored at −80°C prior to use. A Kapa SYBR fast universal one-step qRT-PCR kit (Kapa Biosystems, Wilmington, MA, USA) was used to detect FCV on a Realplex2 Mastercycler platform (Eppendorf, Hauppauge, NY, USA). The forward and reverse primer sequences for FCV RT-qPCR analysis were GCCATTCAGCATGTGGTAGTAACC and GCACATCATATGCGGCTCTG, respectively (31). The standard curve for FCV was prepared by performing a 7-step 10-fold dilution of virus stocks. Log reductions (equation below) of virus RNA were calculated as previously described (31) as follows:
where CT,t is the cycle threshold (CT) for the experimental group, CT,c is the cycle threshold for the control recovered at time zero, and K is the slope obtained from plotting the CT values versus the log10 of the RNA copy numbers used for presenting the standard curve (31).
Preparation of surface samples.
Wool-level loop and nylon multilevel loop carpets (SDL Atlas, Rock Hill, SC, USA) were selected from ASTM standard F655-13 (32). The carpet fiber characteristics, e.g., absorption capacity and zeta potential, were described elsewhere (26). The carpets contained no finishes, e.g., antimicrobial or soil retardant, and were cut into 5-cm-by-5-cm carriers with a mechanical cutting die (model 1500; Freeman Schwabe, Batavia, OH) (courtesy of Daniel Price, Interface Inc., Atlanta, GA, USA). After cutting, the carpet carriers were dusted by hand to remove loose fibers, wrapped in aluminum foil, and autoclaved on a 30-min dry cycle.
Disinfection technologies.
Two disinfection technologies, i.e., SDC and steam vapor (2300SB with TANCS; Advanced Vapor Technologies, Seattle, WA, USA), were tested. SDC contained 0.003% silver ion stabilized in 4.846% citric acid (pH 2). The steam vapor device with a TANCS processer was filled with tap water and connected to a hose with a ca. 2.5-cm-diameter cleaning head. A standard cotton terry cloth (41 cm by 48 cm) was autoclaved, folded to yield 4 layers, wrapped around the cleaning head, held with a rubber band, and changed between samples. Before each use, the system was preheated by saturating the hose line and cleaning head with steam (ca. 20 s). During application, the cleaning head was vertically guided across a surface while providing a temperature between 99.17°C ± 0.04°C and a pressure of 83 to 138 kPa per the manufacturer's specifications.
Cytotoxicity and neutralization testing.
SDC cytotoxicity and neutralization testing was conducted in three phases and in accordance with methods outlined in ASTM 2197-11 (33). Two SDC neutralizers were tested: neutralizer 1 (2.2 g/liter NaHCO3, 1 g/liter C2H3NaO2S, 0.01 M PBS, and 0.02% Tween 80), and neutralizer 2 (4.4 g/liter NaHCO3, 3 g/liter C2H3NaO2S, 10 mM HEPES, 0.01 M PBS, and 0.02% Tween 80). In phase 1, a cytotoxicity control test was completed. SDC was diluted (1:10 and 1:20) in both neutralizers, applied (500 μl/well) to CRFK monolayers, and incubated for 60 min at 37°C. After the incubation, the monolayers were examined under an inverted microscope (ACCU-SCOPE, Commack, NY, USA) for any apparent cytotoxicity, e.g., cell detachment and rounding. In phase 2, SDC neutralization as used within the testing parameter for virucidal activity was validated. Briefly, SDC was diluted (1:10 and 1:20) in both neutralizers and spiked with 10 to 100 PFU of FCV, and then the infectivity was measured against a control (CDMEM-5 spike with 10 to 100 PFU). In phase 3, both neutralizers were tested to check for the interference of SDC with infectivity. This was completed by conducting a cytotoxicity control test (phase 1) immediately followed by a validation of SDC neutralization as used within the testing parameter (phase 2) with the same CRFK monolayers. Three independent experiments were conducted for each phase, with 3 monolayers per dilution.
Quantitative suspension test.
The efficacy of SDC, with and without 5% FBS, was tested in accordance with ASTM standard E1052-11 with minor modifications (34). Briefly, an FCV stock was diluted in CDMEM-5 or 1× PBS to yield a ca. 7 log PFU/ml concentration. A 100-μl volume of each inoculum was combined with 900 μl of SDC for contact times of 1, 5, 10, and 30 min. The samples were neutralized by mixing 100 μl of the sample with 900 μl of a neutralizer containing 4.4 g/liter NaHCO3 plus 3 g/liter C2H3NaO2S plus 10 mM HEPES plus 0.01 M PBS plus 0.02% Tween 80. The verifications of SDC neutralization and the elimination of cytotoxicity were completed in the same volume format as that described above and in accordance with the recommendations in ASTM 1052-11 (34). Separate aliquots for each sample were prepared for infectivity and qRT-PCR analysis and frozen at −80°C.
Quantitative glass carrier test.
The efficacies of SDC and steam vapor against FCV on a hard surface, with and without 5% FBS, were tested in accordance with ASTM standard E1053-11 with modifications (35). Briefly, glass coverslips (25 mm by 25 mm) in a glass petri dish (Corning, Corning, NY, USA) were inoculated with 25 μl FCV (ca. 7 log PFU/sample) and dried for 1 h in a 30% RH chamber (480 HP; VWR International, Radnor, PA, USA). After drying, virus films were incubated with 200 μl of SDC for 1, 5, 10, and 30 min. At each time point, 1.8 ml of a neutralizing broth, mentioned above, was pipetted onto the coverslip. On the other hand, steam vapor was applied for 10, 30, 60, and 90 s. The samples treated with steam vapor were neutralized by applying 2 ml of a 4°C chilled neutralizer (0.01 M PBS plus 0.02% Tween 80) to the glass surface. The verifications of SDC neutralization and the elimination of cytotoxicity were completed in the same format as that described above in accordance with recommendations in ASTM 1053-11 (34). In both experiments, the samples were recovered as previously described, aliquoted for infectivity and qRT-PCR analyses, and frozen at −80°C (18).
Quantitative carpet carrier test.
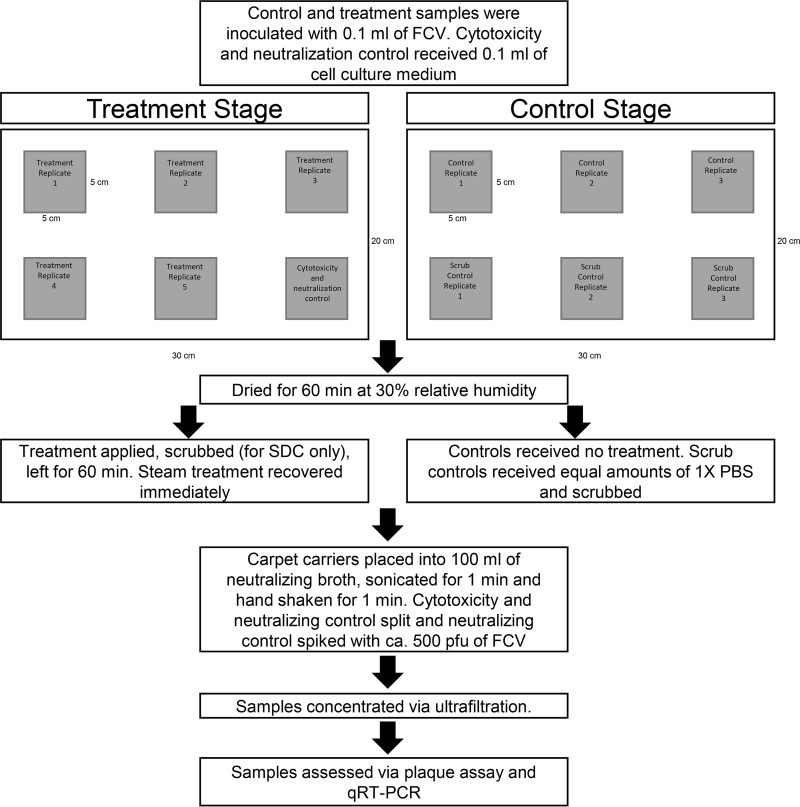
The efficacies of SDC and steam vapor against FCV inoculated onto carpets were tested in accordance with ASTM E2966-14 with modifications (Fig. 2) (16). The inocula were diluted in CDMEM-5 containing ca. 7 log PFU/ml of FCV. Wool and nylon experimental and control carpet carriers (5 cm by 5 cm), each contained in a petri dish, were inoculated with 100 μl of the FCV inoculum, while the cytotoxicity and neutralization controls received 100 μl of CDMEM-5 only. The carpet carriers were then dried for 1 h at 30% RH. For SDC treatments, the experimental carpet carriers were sprayed 5 times (6.85 ± 0.21 ml), scrubbed clockwise and counterclockwise for 30 s each with an SDC-saturated surgical scrub brush (1.23 ± 0.41 ml) (Becton Dickinson, Franklin Lakes, NJ, USA), and left at ambient conditions for 1 h before being neutralized. In contrast, steam vapor was applied to the experimental carpet carriers for a 90-s contact time with a vertical rocking motion before being neutralized. The six-carrier control stage for both technologies consisted of two controls: unscrubbed and scrubbed control populations. The unscrubbed control population received no treatment. For SDC, the scrubbed controls were treated similarly to the experimental carriers but with inert 1× PBS and scrubbed. For the steam vapor control, the steamer head was applied for 90 s with no heat.
FIG 2.
Flow chart for testing the efficacy of disinfectants against FCV on carpets.
To recover FCV, the dried inoculated carpet carriers were aseptically transferred to a 500-ml bottle (Thermo Fisher Scientific, Waltham, MA, USA) containing 100 ± 1 ml of their respective neutralizing broths, sonicated for 1 min at 40 KHz in a sonication bath (FS110; Fisher Scientific International, Pittsburgh, PA, USA), and hand shaken for 1 min. Next, the carpet carriers were aseptically removed from the bottles using sterile tweezers, and the liquid samples were frozen at −80°C. On a separate day, the frozen samples were thawed in a water bath at 37°C (IR35; New Brunswick Scientific, New Brunswick, NJ), transferred to 50-ml conical tubes (VWR International, Radnor, PA), and centrifuged at 4,000 × g for 15 min at 4°C to pellet any debris (Allegra X-30R; Beckman Coulter, Brea, CA, USA). Next, the liquid suspension (ca. 100 ml) was concentrated (1.58 ± 0.46 ml) via ultrafiltration (Amicon Ultra-15 30K; Millipore, Billerica, MA, USA) at 4,000 × g for 15 min at 4°C. Each sample was concentrated over 6 to 7 centrifugation cycles, as the ultrafiltration tube only held 15 ml. After centrifugation, the supernatants, contained in the filtration unit trap, were pooled, vortexed, weighed, aliquoted, and stored at −80°C prior to infectivity and qRT-PCR analyses. Prior to infectivity testing, all samples were diluted 1:4 with CDMEM-5 to eliminate potential cytotoxicity.
Qualitative appearance test.
The wool and nylon carpet carriers were treated with SDC and steam vapor as described above. The carriers were photographed with a camera (AX53; Sony, Minato, Tokyo, Japan) at time zero, 60 min, and 24 h.
Statistical analysis.
All experiments had nine replicates in three independent experiments, except for the carpet experiment, which had 15 replicates in three independent experiments. The log reductions were calculated by log N/N0, where N is the average from the treatment samples and N0 is the average from each technology's scrubbed control population. Statistical analysis was performed using a one-way multiple-comparison t test to test the effect of time and serum. All results are expressed as means ± standard deviations. Statistical significance was defined as a P value of ≤0.05. Statistical analyses were conducted using JMP (JMP Pro 12.2.0, SAS Inc., Cary, NC).
ACKNOWLEDGMENTS
We thank Guohui Huang at Clemson University for his technical assistance with cell culture, and Advanced Vapor Technologies (Seattle, WA) for providing the steam vapor device.
This research was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture (USDA; agreement 2011-68003-30395), and The Procter and Gamble Company.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the USDA or The Procter and Gamble Company.
REFERENCES
- 1.Glass RI, Parashar UD, Estes MK. 2009. Norovirus gastroenteritis. N Engl J Med 361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY. 2016. Global economic burden of norovirus gastroenteritis. PLoS One 11:e0151219. doi: 10.1371/journal.pone.0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosa KM, Cates SC, Hall AJ, Brophy JE, Fraser A. 2014. Knowledge of norovirus prevention and control among infection preventionists. Am J Infect Control 42:676–678. doi: 10.1016/j.ajic.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopman B, Gastañaduy P, Park GW, Hall AJ, Parashar UD, Vinjé J. 2012. Environmental transmission of norovirus gastroenteritis. Curr Opin Virol 2:96–102. doi: 10.1016/j.coviro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Cheesbrough JS, Green J, Gallimore CI, Wright PA, Brown DW. 2000. Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol Infect 125:93–98. doi: 10.1017/S095026889900432X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall AJ, Vinjé J, Lopman B, Park GW, Yen C, Gregoricus N, Parashar UD, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases. 2011. Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep 60:1–18. [PubMed] [Google Scholar]
- 7.Quinn MM, Henneberger PK, Braun B, Delclos GL, Fagan K, Huang V, Knaack JLS, Kusek L, Lee SJ, Le Moual N, Maher KAE, McCrone SH, Mitchell AH, Pechter E, Rosenman K, Sehulster L, Stephens AC, Wilburn S, Zock JP. 2015. Cleaning and disinfecting environmental surfaces in health care: toward an integrated framework for infection and occupational illness prevention. Am J Infect Control 43:424–434. doi: 10.1016/j.ajic.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Yeargin T, Buckley D, Fraser A, Jiang X. 2016. The survival and inactivation of enteric viruses on soft surfaces: a systematic review of the literature. Am J Infect Control 44:1365–1373. doi: 10.1016/j.ajic.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Malik YS, Allwood PB, Hedberg CW, Goyal SM. 2006. Disinfection of fabrics and carpets artificially contaminated with calicivirus: relevance in institutional and healthcare centres. J Hosp Infect 63:205–210. doi: 10.1016/j.jhin.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Occupational Safety and Health Administration. 2006. Best practices for the safe use of glutaraldehyde in health care. OSHA 3258-08N. Occupational Safety and Health Administration, U.S. Department of Labor, Washington, DC. [Google Scholar]
- 11.Koromyslova AD, White PA, Hansman GS. 2015. Treatment of norovirus particles with citrate. Virology 485:199–204. doi: 10.1016/j.virol.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Manuel CS, Moore MD, Jaykus LA. 2017. Efficacy of a disinfectant containing silver dihydrogen citrate against GI.6 and GII.4 human norovirus. J Appl Microbiol 122:78–86. doi: 10.1111/jam.13331. [DOI] [PubMed] [Google Scholar]
- 13.Occupational Safety and Health Administration. 2008. OSHA fact sheet–noroviruses. Occupational Safety and Health Administration, U.S. Department of Labor, Washington, DC. [Google Scholar]
- 14.Tanner BD, Rock R. 2009. Reduction in infection risk through treatment of microbially contaminated surfaces with a novel, portable, saturated steam vapor disinfection system. Am J Infect Control 37:20–27. doi: 10.1016/j.ajic.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg L. 2005. Virucidal effectiveness test–feline calicivirus (surrogate for Norwalk and Norwalk-like viruses) on unglazed, clay tiles. Advanced Vapor Technologies, LLC, Everett, WA. [Google Scholar]
- 16.ASTM International. 2014. Standard test method for quantitative assessment of sanitizing solutions for carpet. ASTM E2966-14. ASTM International, West Conshohocken, PA. [Google Scholar]
- 17.Liau SY, Read DC, Pugh WJ, Furr JR, Russell AD. 1997. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett Appl Microbiol 25:279–283. doi: 10.1046/j.1472-765X.1997.00219.x. [DOI] [PubMed] [Google Scholar]
- 18.Cannon JL, Papafragkou E, Park GW, Osborne J, Jaykus LA, Vinjé J. 2006. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J Food Prot 69:2761–2765. doi: 10.4315/0362-028X-69.11.2761. [DOI] [PubMed] [Google Scholar]
- 19.Chiu S, Skura B, Petric M, McIntyre L, Gamage B, Isaac-Renton J. 2015. Efficacy of common disinfectant/cleaning agents in inactivating murine norovirus and feline calicivirus as surrogate viruses for human norovirus. Am J Infect Control 43:1208–1212. doi: 10.1016/j.ajic.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Gerba CP. 1984. Applied and theoretical aspects of virus adsorption to surfaces. Adv Appl Microbiol 30:133–168. [DOI] [PubMed] [Google Scholar]
- 21.Park GW, Boston DM, Kase JA, Sampson MN, Sobsey MD. 2007. Evaluation of liquid- and fog-based application of Sterilox hypochlorous acid solution for surface inactivation of human norovirus. Appl Environ Microbiol 73:4463–4468. doi: 10.1128/AEM.02839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer W. February 2006. Systems and methods for disinfecting and sterilizing by applying steam vapor containing low zeta potential mineral crystals. U.S. patent 7,547,413.
- 23.U.S. Environmental Protection Agency. 2013. Product performance test guidelines. OSCPP 810.2400. Disinfectants and sanitizers for use on fabrics and textiles-efficacy data recommendations. Office of Chemical Safety and Pollution Prevention, U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- 24.U.S. Environmental Protection Agency. 2007. Reregistration eligibility decision for glutaraldehyde. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- 25.McNeil E, Greenstein M, Stuart LS, Goldsmith MT. 1960. Some problems involved in the use of quaternary ammonium compounds as fabric disinfectants. Appl Microbiol 8:156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckley D, Fraser A, Huang G, Jiang X. 2017. Recovery optimization and survival of human norovirus surrogates, feline calicivirus and murine norovirus on carpet. Appl Environ Microbiol 83:e01336-17. doi: 10.1128/AEM.01336-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo K, Lee JE, Lim MY, Ko G. 2012. Effect of temperature, pH, and NaCl on the inactivation kinetics of murine norovirus. J Food Prot 75:533–540. doi: 10.4315/0362-028X.JFP-11-199. [DOI] [PubMed] [Google Scholar]
- 28.Hwang S, Alhatlani B, Arias A, Caddy SL, Christodoulou C, Cunha JB, Emmott E, Gonzalez-Hernandez MB, Kolawole A, Lu J, Rippinger C, Sorgeloos F, Thorne L, Vashist S, Goodfellow IG, Wobus CE. 2014. Murine norovirus: propagation, quantification, and genetic manipulation. Curr Protoc Microbiol 33:15K.2.1–15K.2.61. doi: 10.1002/9780471729259.mc15k02s33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bidawid S, Malik N, Adegbunrin O, Sattar SA, Farber JM. 2003. A feline kidney cell line-based plaque assay for feline calicivirus, a surrogate for Norwalk virus. J Virol Methods 107:163–167. doi: 10.1016/S0166-0934(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 30.Yeargin T, Fraser A, Guohui H, Jiang X. 2015. Recovery and disinfection of two human norovirus surrogates, feline calicivirus and murine norovirus, from hard nonporous and soft porous surfaces. J Food Prot 78:1842–1850. doi: 10.4315/0362-028X.JFP-14-515. [DOI] [PubMed] [Google Scholar]
- 31.Park GW, Barclay L, Macinga D, Charbonneau D, Pettigrew CA, Vinjé J. 2010. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII.4 norovirus. J Food Prot 73:2232–2238. doi: 10.4315/0362-028X-73.12.2232. [DOI] [PubMed] [Google Scholar]
- 32.ASTM International. 2015. Standard specification for test carpets and pads for vacuum cleaner testing. ASTM International, West Conshohocken, PA. [Google Scholar]
- 33.ASTM International. 2011. Standard quantitative disk carrier test method for determining bactericidal, virucidal, fungicidal, mycobactericidal, and sporicidal activities of chemicals. ASTM E2197. ASTM International, West Conshohocken, PA. [Google Scholar]
- 34.ASTM International. 2011. Standard test method to assess the activity of microbicides against viruses in suspension. ASTM E1052-11. ASTM International, West Conshohocken, PA. [Google Scholar]
- 35.ASTM International. 2011. Standard test method to assess virucidal activity of chemicals intended for disinfection of inanimate, nonporous environmental surfaces. ASTM E1053-11. ASTM International, West Conshohocken, PA. [Google Scholar]