Abstract
Objective
The choice between primary debulking surgery (PDS) and neoadjuvant chemotherapy (NAC) in advanced ovarian cancer remains controversial. We evaluated NAC use in our center before and after results from a randomized trial were published, with the aim to determine the impact of changes in the neoadjuvant strategy on survival in advanced-stage ovarian cancer.
Methods
We retrospectively investigated the clinical course of 435 patients with ovarian, tubal, or peritoneal carcinoma (International Federation of Gynecology and Obstetrics [FIGO] stage III or IV). According to the period of treatment, we stratified patients into a control group (n=216; diagnosed between 2006 and 2010; 83.8% underwent PDS) and a study group (n=219; diagnosed between 2011 and 2014; 48.9% received NAC followed by interval debulking surgery [IDS]).
Results
There were no between-group differences in age, body mass index, histology findings, or tumor grade. Compared to patients in the control group, those in the study group were more likely to receive NAC followed by IDS as first-line treatment (48.9% vs. 16.2%; p<0.001), cytoreductive surgery to no-residual disease (21.5% vs. 10.2%; p<0.001), or radical surgery (57.5% vs. 35.6%; p<0.001). However, there was no between-group difference in postoperative morbidity. Kaplan-Meier analysis showed no between-group differences in progression-free or overall survival (p=0.449 and 0.952, respectively).
Conclusion
NAC incorporation resulted in increased optimal cytoreduction rates although no significant differences in survival outcomes were noted. NAC is advantageous for patients with high perioperative morbidity or unresectable disease.
Keywords: Ovarian Neoplasms; Neoadjuvant Therapy; Debulking Surgical Procedure; Chemotherapy, Adjuvant
INTRODUCTION
Ovarian cancer is the leading cause of death from gynecologic malignancy [1]. In Korea, ovarian cancer incidence and mortality rates have recently increased [2,3]. Primary debulking surgery (PDS) followed by platinum-based chemotherapy is currently the standard treatment for advanced-stage ovarian cancer [4]. Recently, several randomized clinical trials have shown that neoadjuvant chemotherapy (NAC) followed by interval debulking surgery (IDS) is not inferior to PDS with respect to survival, postoperative morbidity, and mortality in women with stage III or IV ovarian cancer [5,6,7,8]. Therefore, NAC followed by IDS is considered a feasible alternative approach in advanced-stage ovarian cancer [9].
To date, few studies have examined the impact of the increased adoption of NAC as a primary treatment for advanced-stage ovarian cancer in clinical practice, where the choice between PDS and NAC remains controversial. Two meta-analyses investigated the outcomes of NAC but reported contradictory findings [10,11]. In our institution, PDS has been the standard treatment strategy for patients with advanced-stage ovarian cancer. In addition, NAC has been used for patients who were considered unsuitable for optimal PDS or who had significant medical comorbidities.
The European Organization for Research and Treatment of Cancer (EORTC) trial [6] demonstrated that survival outcomes after NAC were comparable to those of PDS. The median progression-free survival (PFS) was 12 months for both the PDS and NAC groups, whereas median overall survival (OS) was 29 and 30 months for the PDS and NAC groups, respectively. Importantly, the NAC group had higher rates of complete gross resection and lower rates of major postoperative complications. Since the publication of the EORTC 55971 trial outcomes, our institution increased the utilization of NAC in patients with advanced-stage ovarian cancer, fallopian tube cancers, or primary peritoneal carcinomas.
The purpose of the present study was to compare the survival outcomes before and after the increased adoption of NAC in our center's practice of treating advanced-stage ovarian cancer. We hypothesized that this paradigm shift would lead to an increased rate of no gross residual disease without increasing the rates of major perioperative complications.
MATERIALS AND METHODS
1. Study population
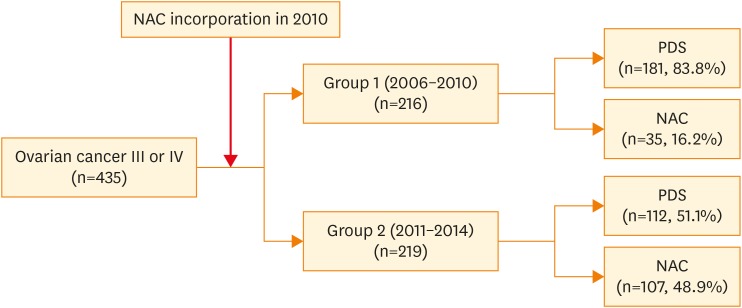
We performed a retrospective review of the medical records of patients with pathology-confirmed International Federation of Gynecology and Obstetrics (FIGO) stage III or IV ovarian cancer treated at Yonsei Cancer Hospital, who received PDS or NAC between 2006 and 2014. The following patients were excluded: patients who did not undergo PDS or IDS after NAC (n=9); patients lost to follow-up (n=21); patients whose medical records were not available (n=12). The final study population comprised 435 patients.
NAC has been used in our institution since late 2010. Therefore, we stratified the patients into 2 groups according to the period in which they were treated. Group 1 (control group) consisted of 216 patients who had been diagnosed between January 1, 2006 and December 31, 2010; most patients in this group underwent PDS. Group 2 (study group) consisted of 219 patients who were diagnosed between January 1, 2011 and December 31, 2014, during which time our center had adopted increased utilization of NAC for advanced-stage ovarian cancer, fallopian tube cancer, and primary peritoneal carcinomas.
The diagnosis was established based on open surgery, laparoscopic, or imaging-biopsy samples or based on fine-needle aspiration samples obtained from the tumor site or ascites/effusion. All surgical procedures were performed by one of the 5 gynecologic oncology surgeons at our institute. The histological diagnoses were based on World Health Organization criteria, and all microscope slides were reviewed by 2 experienced gynecologic pathologists. NAC was performed if at least one of the following 3 criteria was met: 1) presence of pulmonary and/or hepatic parenchymal metastases on imaging studies before surgery; 2) medically inoperable cancer; and/or 3) optimal cytoreduction infeasible because of high tumor burden (Fagotti score ≥8) as observed on diagnostic laparoscopy [12,13]. Standard surgical procedures included the following: sampling of free fluid or peritoneal washings for cytology; thorough inspection of the abdomen and pelvis, including the upper abdominal viscera, diaphragm, and retroperitoneal spaces; and hysterectomy, bilateral oophorectomy and omentectomy, pelvic/para-aortic lymph node dissection, and appendectomy. Radical surgery included bowel resection, diaphragm or other peritoneal surface stripping, splenectomy, partial hepatectomy, partial gastrectomy, or partial cystectomy with or without ureteroneocystostomy, cholecystectomy, and/or distal pancreatectomy [14,15,16]. Surgical complexity was classified, per previously published protocols, as low, intermediate, or high [17]. A different score was assigned to each surgical procedure ranging from 1 to 3 according to the complexity of the procedure. The surgical procedure assigned to 1 are hysterectomy, bilateral salpingo-oophorectomy, omentectomy, pelvic lymphadenectomy, para-aortic lymphadenectomy, abdominal peritoneum stripping, small bowel resection, small bowel resection, 2 are large bowel resection, diaphragm stripping/resection, splenectomy, liver resection, and 3 is recto-sigmoidectomy with anastomosis. Perioperative complications were graded using the Memorial Sloan-Kettering Cancer Center's (MSKCC) surgical secondary events grading system [18]. Major complications were defined as events with MSKCC grade ≥3. Perioperative morbidity was defined as any adverse events (AEs) related to treatment occurring within 30 days of surgery. Operative mortality was defined as death occurring within 30 days after surgery (MSKCC grade 5). The present study was reviewed and approved by the Institutional Review Board of Yonsei Cancer Hospital (registration number: 4-2017-0998).
2. Statistical analysis
SPSS statistical software (version 21.0; IBM Corp., Armonk, NY, USA) was used for the statistical analyses. Descriptive statistics were used for demographic data and are summarized as the median (range) or frequency (percentage). Differences in the patient characteristics associated with the 2-time intervals were assessed using the χ2 or Mann-Whitney U test. The study endpoints included PFS and OS. PFS was defined as the interval between the date of diagnosis and the date of the first recurrence. OS was defined as the interval between the date of diagnosis and the date of death. The date of recurrence was defined as the date of disease detection via radiology during follow-up. An increase in cancer antigen 125 (CA-125) levels without clinical signs of relapse was not regarded as progression but commonly necessitated radiological examinations. PFS and OS were analyzed using the Kaplan-Meier method and log-rank test. Factors identified as significant in the univariate analyses were entered into multivariate analysis. Cox regression analysis was used to evaluate the effects of the prognostic factors, expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). For all analyses, p<0.05 was considered to indicate statistical significance.
RESULTS
A total of 435 consecutive patients undergoing PDS or NAC at our institution were evaluated over the study period. A comparison of patient and clinical characteristics between group 1 and group 2 is shown in Table 1. There were no significant between-group differences in patient characteristics such as age, body mass index, tumor grade, or histologic type. The distribution of FIGO stages was significantly different between the 2 groups: group 1, 64 (29.6%) stage IV patients; group 2, 126 (57.5%) stage IV patients (p<0.001). Performance status was also different between the 2 groups, with 2.3% and 19.7% of patients having American Society of Anesthesiologists (ASA) score ≥3 in groups 1 and 2, respectively (p<0.001). The median CA-125 level was significantly different between the 2 groups: group 1, 897.1 U/mL (range, 8.7–30,008.8); group 2, 1,474.1 U/mL (range, 12.9–30,000.0; p=0.003).
Table 1. Patient and clinical characteristics.
| Characteristics | Group 1 (n=216) | Group 2 (n=219) | p | |
|---|---|---|---|---|
| Age (yr) | 56 (22–83) | 55 (26–79) | 0.779 | |
| BMI (kg/m2) | 22.9 (16.0–40.3) | 22.5 (16.4–34.4) | 0.530 | |
| FIGO stage | <0.001 | |||
| III | 152 (70.4) | 93 (42.5) | ||
| IV | 64 (29.6) | 126 (57.5) | ||
| Tumor grade | 0.214 | |||
| 1 | 16 (7.4) | 11 (5.0) | ||
| 2 | 72 (33.4) | 62 (28.3) | ||
| 3 | 107 (49.5) | 113 (51.6) | ||
| Not available | 21 (9.7) | 33 (15.1) | ||
| Histologic type | 0.696 | |||
| Serous | 174 (80.6) | 179 (82.1) | ||
| Endometrioid | 10 (4.6) | 5 (2.3) | ||
| Mucinous | 14 (6.5) | 14 (6.4) | ||
| Clear cell | 9 (4.2) | 12 (5.5) | ||
| Other | 9 (4.2) | 9 (3.7) | ||
| ASA score | <0.001 | |||
| 1 | 128 (59.3) | 60 (27.4) | ||
| 2 | 77 (35.6) | 110 (50.2) | ||
| 3 | 5 (2.3) | 42 (19.2) | ||
| 4 | 0 (0) | 1 (0.5) | ||
| Not available | 6 (2.8) | 6 (2.7) | ||
| Median CA-125 level (U/mL) | 897.1 (8.7–30,008.8) | 1,474.1 (12.9–30,000.0) | 0.003 | |
| NAC | <0.001 | |||
| Yes | 35 (16.2) | 107 (48.9) | ||
| No | 181 (83.8) | 112 (51.1) | ||
| Chemotherapy regimen | 0.006 | |||
| Paclitaxel+carboplatin | 156 (72.2) | 171 (78.1) | ||
| Docetaxel+carboplatin | 25 (11.6) | 36 (16.4) | ||
| Paclitaxel+carboplatin+bevacizumab | 1 (0.5) | 3 (1.4) | ||
| Paclitaxel+cisplatin | 2 (0.9) | 3 (1.4) | ||
| IP chemotherapy | 28 (13.0) | 0 (0) | ||
| Others | 2 (0.9) | 2 (0.9) | ||
| Not available | 2 (0.9) | 4 (1.8) | ||
| Cycles of total chemotherapy | 0.005 | |||
| ≤6 | 158 (73.1) | 132 (60.3) | ||
| >6 | 58 (26.9) | 87 (39.7) | ||
Values are presented as median (range) or number (%).
ASA, American Society of Anesthesiologists; BMI, body mass index; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics; IP, intraperitoneal; NAC, neoadjuvant chemotherapy.
Among the entire cohort, 142 of 435 patients (32.6%) received NAC and 293 of 435 patients (67.4%) underwent PDS. In groups 1 and 2, 16.2% and 48.9% of patients, respectively, underwent NAC (p<0.001). The change in NAC use between 2006 and 2014 is shown in Fig. 1. Regarding the chemotherapy regimen, the use of intraperitoneal (IP) chemotherapy was different between the 2 groups (13.0% vs. 0%, p=0.006). The median number of chemotherapy cycles was 6 (range, 1–12 cycles) in both groups. In groups 1 and 2, 58 (26.9%) and 87 (39.7%) patients, respectively, received more than 6 cycles of chemotherapy, with a significant difference between the 2 groups (p=0.005). Across the study period, the rate of no-gross residual disease increased from 10.2% to 21.5% (p<0.001). This was followed by an increase in the rate of radical surgery from 35.6% in group 1 to 57.5% in group 2 (p<0.001). Surgical complexity was also higher in group 2 (high-surgical complexity in 3.7% and 12.8% of patients from groups 1 and 2, respectively; p=0.002). There were 2 (3.7%) and 1 (0.5%) 30-day postoperative deaths (grade 5 events) in groups 1 and 2, respectively. There were 10 (4.7%) and 13 (6.0%) grade 3 or 4 AEs in group 1 and 2, respectively. Major postoperative morbidity (restricted to grade 3–5) was not different between the 2 groups (5.6% vs. 6.4%, p=0.465) despite the increase in surgical complexity (Table 2).
Fig. 1.
Change in NAC use between 2006 and 2014.
NAC, neoadjuvant chemotherapy; PDS, primary debulking surgery.
Table 2. Classification of postoperative outcomes according to the Memorial Sloan-Kettering Cancer Center's surgical secondary events grading system.
| Variables | Group 1 (n=216) | Group 2 (n=219) | p | |
|---|---|---|---|---|
| Complication grade* | 0.068 | |||
| 0 | 102 (47.2) | 130 (59.4) | ||
| 1 | 15 (6.9) | 9 (4.1) | ||
| 2 | 79 (36.6) | 62 (28.4) | ||
| 3 | 10 (4.7) | 10 (4.6) | ||
| 4 | 0 (0) | 3 (1.4) | ||
| 5 | 2 (3.7) | 1 (0.5) | ||
| Not available | 8 (3.7) | 4 (1.8) | ||
| Major complications | 0.465 | |||
| 0–2 | 196 (90.7) | 201 (91.8) | ||
| 3–5 | 12 (5.6) | 14 (6.4) | ||
| Not available | 8 (3.7) | 4 (1.8) | ||
| Residual disease | <0.001 | |||
| No gross | 22 (10.2) | 47 (21.5) | ||
| ≤1.0 cm | 94 (43.5) | 111 (50.7) | ||
| >1.0 cm | 52 (24.1) | 13 (5.9) | ||
| Not available | 48 (22.2) | 48 (21.9) | ||
| PDS | <0.001 | |||
| No gross | 16 (8.8) | 17 (15.2) | ||
| ≤1.0 cm | 77 (42.5) | 60 (53.6) | ||
| >1.0 cm | 49 (27.1) | 8 (7.1) | ||
| Not available | 39 (21.5) | 27 (24.1) | ||
| NAC | 0.466 | |||
| No gross | 6 (17.1) | 30 (28.0) | ||
| ≤1.0 cm | 17 (48.6) | 51 (47.7) | ||
| >1.0 cm | 3 (8.6) | 5 (4.7) | ||
| Not available | 9 (25.7) | 21 (19.6) | ||
| Radical surgery† | <0.001 | |||
| None | 139 (64.4) | 93 (42.5) | ||
| Any radical surgery | 77 (35.6) | 126 (57.5) | ||
| Surgical complexity score groups‡ | 0.002 | |||
| 1 | 1 (0.5) | 0 (0) | ||
| 2 | 207 (95.8) | 191 (87.2) | ||
| 3 | 8 (3.7) | 28 (12.8) | ||
Values are presented as number (%).
NAC, neoadjuvant chemotherapy; PDS, primary debulking surgery.
*According to the Memorial Sloan-Kettering Cancer Center's surgical secondary events grading system [16]; †Radical surgery included any of following: bowel surgery, cholecystectomy, diaphragm peritonectomy/resection, distal pancreatectomy video-assisted thoracoscopic surgery, splenectomy, liver resection, supraclavicular fossa resection, ureter resection, and others; ‡According to Aletti et al [17].
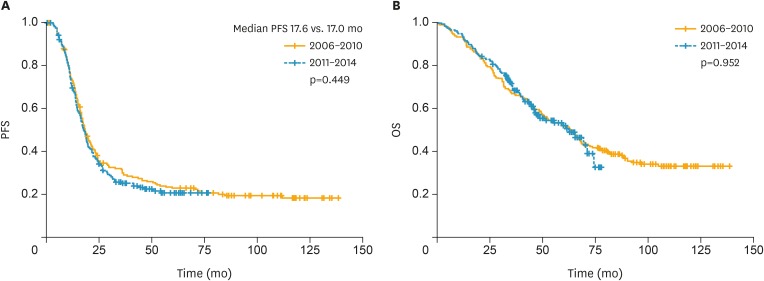
The Kaplan-Meier curves for OS and PFS are shown in Fig. 2. The median follow-up period was 44.7 months in all patients. The median PFS was 17.6 and 17.0 months for groups 1 and 2, respectively. The median OS for group 1 was 63.3 months, while the median OS in group 2 was not reached. There were no differences in PFS or OS between the groups (p=0.449 and 0.952, respectively). We performed a subgroup analysis of patients who underwent PDS and NAC according to time period (Supplementary Figs. 1 and 2). There were no differences in PFS or OS between the 2 subgroups of patients who underwent PDS (p=0.873 and 0.624, respectively). Similarly, there were no differences in PFS or OS between the 2 subgroups of patients who underwent NAC during the study period (p=0.206 and 0.122, respectively). In addition, we performed subgroup analysis of residual disease (R0, R≤1.0 cm, R>1.0 cm). Median PFS and OS stratified by residual disease according to year are summarized in Supplementary Table 1 and Kaplan-Meier curves in Supplementary Figs. 3 and 4.
Fig. 2.
Kaplan-Meier curves of PFS (A) and OS (B) according to time period (2006–2010 vs. 2011–2014).
OS, overall survival; PFS, progression-free survival.
The results of the multivariate Cox regression analyses of PFS and OS in all patients are shown in Supplementary Table 2. Residual disease (HR=1.59; 95% CI=1.11–2.27) and high ASA score (HR=1.75; 95% CI=1.12–2.74) were independent prognostic factors associated with a higher risk of progression. In terms of survival, residual disease (HR=2.08; 95% CI=1.24–3.49) and high ASA score (HR=2.02; 95% CI=1.17–3.49) were independent prognostic factors in all patients.
DISCUSSION
In this study, we investigated the impact of the adoption of NAC in our institution's clinical practice on survival outcomes of advanced-stage ovarian cancer over the period 9 years. Our analysis demonstrates that the overall NAC use increased from 16.2% to 48.9% between 2006 and 2014 and that the incorporation of NAC increased the rate of complete gross resection in IDS without significantly increasing perioperative morbidity and mortality. There was no improvement in the survival rates during this 9-year period, and the results of multivariate analysis support our findings.
The EORTC 55971 and Chemotherapy OR Upfront Surgery (CHORUS) randomized controlled trials each showed similar survival rates between PDS and NAC groups but with improved optimal resection rates and less perioperative complications in the NAC groups, leading the authors to conclude that NAC is a reasonable treatment option for advanced-stage ovarian cancer [6,7]. However, these trials have been criticized because a high number of patients who received PDS had >1 cm of residual disease after surgery.
At our institution, the incorporation of NAC resulted in increased optimal cytoreduction rates and a similar rate of AEs despite more radical surgery. There was no significant difference in survival outcomes between the 2 groups, although the rate of radical surgery was typically higher in our institution. Various factors potentially associated with increased extent of debulking surgery should be considered, including NAC and extended surgery for advanced-stage ovarian cancer. Primarily, the use of NAC has improved the cytoreduction rates achievable with IDS by reducing the disease burden [6,7]. Another important factor was the improvement of surgical skills in our institution during the study period. Specifically, we incorporated radical surgeries in an attempt to decrease residual disease at the time of PDS or IDS. Previous studies reported that radical surgery resulted in higher rates of complete gross resection and optimal resection (residual disease ≤1 cm) in advanced ovarian cancer [15,19,20]. At our institution, the rate of radical surgery increased from 35.6% in group 1 patients to 57.5% in group 2 patients. MSKCC reported a rate of 38% for extensive upper abdominal procedures in PDS, which is not superior to the rate of radical surgery at our institution. It is noteworthy that the complete resection rates also increased from 10.2% in group 1 to 21.5% in group 2. However, the perioperative complication rate did not differ between the 2 groups even after the incorporation of NAC. These findings are in contrast to the results of previous studies of NAC [6,7,8]. Fagotti et al. [8] showed that perioperative morbidity rates were more favorable for NAC than for PDS in advanced-stage ovarian cancer patients. Vergote et al. [6] and Kehoe et al. [7] demonstrated less treatment-related perioperative morbidity and mortality in NAC-treated patients than in those who received PDS. However, these studies did not report improvement in surgical methods during the study period. At our institution, the rate of radical surgery increased during the study period, which may have affected the rate and nature of perioperative complications. Radical debulking procedures required for optimal cytoreduction are often associated with significant morbidity and mortality [15,19].
The survival outcomes in our study were similar to those reported from the EORTC and CHORUS trials. The median PFS and OS for patients who underwent PDS were 17.7 and 50.5 months, respectively, in our study, compared with 12 and 29 months, respectively, in the EORTC trial and 10.7 and 22.6 months, respectively, in the CHORUS trial. In the subgroup analysis, we found no difference in survival outcomes between groups 1 and 2 for the patients who underwent PDS and NAC following IDS. These findings suggest that the improvement in surgical methods did affect survival outcome in this study, which is in agreement with the findings of the EORTC and CHORUS trials. The 2 groups in the present study received almost the same chemotherapy regimens. Basically, primary combination taxane and platinum-based treatment was administered in both groups and may have contributed to the similar survival outcomes.
One limitation of the present study is the retrospective nature of data collection. Moreover, we included patients who received IP chemotherapy. This is important when considering why survival outcomes did not appear substantially different across the time periods. Several studies showed that IP chemotherapy improved survival outcomes [21,22,23]. Suidan et al. [24] compared patients receiving primary intravenous (IV)/IP chemotherapy after PDS with those receiving consolidation IV/IP chemotherapy after PDS. Their results revealed improved OS in the primary IV/IP chemotherapy group (78.8 vs. 57.5 months for consolidation chemotherapy). Considering these data, it is possible that IP chemotherapy could have impacted survival outcomes in 13% of patients in group 1.
Our analysis demonstrates that the incorporation of NAC did not affect survival (PFS or OS) or perioperative morbidity and mortality after PDS, but was associated with increased rates of no-gross residual disease. The similar rates of perioperative morbidity and mortality despite more radical surgery and the higher rate of complete resection to no-residual disease suggest that NAC followed by IDS may be important for patients with advanced ovarian cancer. Further studies should evaluate the outcomes of patients treated with NAC in high-volume centers with experience in PDS. Comparative effectiveness research is required to clarify the effects of a paradigm shift toward widespread use of NAC.
Footnotes
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2016R1D1A1B03931916). The study was also supported by a new Faculty Research Seed Grant from Yonsei University College of Medicine for 2017 (2017-32-0033).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: L.Y.J., L.J.Y., K.S.
- Data curation: L.Y.J., C.Y.S., L.J.Y., K.S.
- Formal analysis: L.Y.J., L.J.Y., K.S.
- Funding acquisition: K.S.
- Investigation: L.Y.J., K.S.W., K.S.
- Methodology: L.Y.J., C.Y.S., K.S.
- Project administration: L.Y.J., K.S.
- Resources: L.Y.J., C.Y.S., L.J.Y., K.S.
- Software: L.Y.J., C.Y.S.
- Supervision: L.Y.J., L.J.Y., N.E.J., K.S., K.Y.T.
- Validation: L.Y.J.
- Visualization: L.Y.J., L.J.Y., N.E.J., K.S.W., K.S., K.Y.T.
- Writing - original draft: L.Y.J., K.S.
- Writing - review & editing: L.Y.J., K.S.W., K.S.
SUPPLEMENTARY MATERIALS
Survival outcomes in patients according to year (2006–2010 vs. 2011–2014) stratified by residual disease
Multivariate Cox regression analysis of PFS and OS
Kaplan-Meier curves of PFS (A) and OS (B) according to time period (2006–2010 vs. 2011–2014) in patients who underwent PDS.
Kaplan-Meier curves of PFS (A) and OS (B) according to time period (2006–2010 vs. 2011–2014) in patients who received NAC.
Kaplan-Meier curves of PFS (A) and OS (B) stratified by residual disease in 2006–2010.
Kaplan-Meier curves of PFS (A) and OS (B) stratified by residual disease in 2011–2014.
References
- 1.Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J Reprod Med. 2005;50:426–438. [PubMed] [Google Scholar]
- 2.Lee JY, Kim EY, Jung KW, Shin A, Chan KK, Aoki D, et al. Trends in gynecologic cancer mortality in East Asian regions. J Gynecol Oncol. 2014;25:174–182. doi: 10.3802/jgo.2014.25.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999–2010. J Gynecol Oncol. 2013;24:298–302. doi: 10.3802/jgo.2013.24.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RJ, Jr, Alvarez RD, Armstrong DK, Burger RA, Castells M, Chen LM, et al. Ovarian cancer, version 3.2012. J Natl Compr Canc Netw. 2012;10:1339–1349. doi: 10.6004/jnccn.2012.0140. [DOI] [PubMed] [Google Scholar]
- 5.Onda T, Matsumoto K, Shibata T, Sato A, Fukuda H, Konishi I, et al. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0602. Jpn J Clin Oncol. 2008;38:74–77. doi: 10.1093/jjco/hym145. [DOI] [PubMed] [Google Scholar]
- 6.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 7.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 8.Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome. Eur J Cancer. 2016;59:22–33. doi: 10.1016/j.ejca.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Schott AF, Hayes DF. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:1747–1749. doi: 10.1200/JCO.2011.41.3161. [DOI] [PubMed] [Google Scholar]
- 10.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103:1070–1076. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol. 2009;16:2315–2320. doi: 10.1245/s10434-009-0558-6. [DOI] [PubMed] [Google Scholar]
- 12.Fagotti A, Vizzielli G, Fanfani F, Costantini B, Ferrandina G, Gallotta V, et al. Introduction of staging laparoscopy in the management of advanced epithelial ovarian, tubal and peritoneal cancer: impact on prognosis in a single institution experience. Gynecol Oncol. 2013;131:341–346. doi: 10.1016/j.ygyno.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Fagotti A, Ferrandina G, Fanfani F, Garganese G, Vizzielli G, Carone V, et al. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am J Obstet Gynecol. 2008;199:642.e1–642.e6. doi: 10.1016/j.ajog.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Levine DA, Poynor EA, Aghajanian C, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol. 2006;103:1083–1090. doi: 10.1016/j.ygyno.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR) Gynecol Oncol. 2007;106:69–74. doi: 10.1016/j.ygyno.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol. 2011;120:23–28. doi: 10.1016/j.ygyno.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004;94:650–654. doi: 10.1016/j.ygyno.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107:77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 20.Ren Y, Jiang R, Yin S, You C, Liu D, Cheng X, et al. Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: an observational study. BMC Cancer. 2015;15:583. doi: 10.1186/s12885-015-1525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 23.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 24.Suidan RS, St Clair CM, Lee SJ, Barlin JN, Long Roche KC, Tanner EJ, et al. A comparison of primary intraperitoneal chemotherapy to consolidation intraperitoneal chemotherapy in optimally resected advanced ovarian cancer. Gynecol Oncol. 2014;134:468–472. doi: 10.1016/j.ygyno.2014.07.090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival outcomes in patients according to year (2006–2010 vs. 2011–2014) stratified by residual disease
Multivariate Cox regression analysis of PFS and OS
Kaplan-Meier curves of PFS (A) and OS (B) according to time period (2006–2010 vs. 2011–2014) in patients who underwent PDS.
Kaplan-Meier curves of PFS (A) and OS (B) according to time period (2006–2010 vs. 2011–2014) in patients who received NAC.
Kaplan-Meier curves of PFS (A) and OS (B) stratified by residual disease in 2006–2010.
Kaplan-Meier curves of PFS (A) and OS (B) stratified by residual disease in 2011–2014.