Abstract
Backgrounds/Aims
Ranson's score (RS) and Glasgow score (GS) have been utilized to stratify the severity of acute pancreatitis (AP). The aim of this study was to validate RS and GS for stratifying the severity of acute pancreatitis and audit our experience of managing AP.
Methods
We conducted a retrospective review of patients treated for AP from July 2009 to September 2016. Final severity was determined using the revised Atlanta classification. Mortality and complications were analyzed.
Results
From July 2009 to September 2016, a total of 675 patients with a diagnosis of AP were admitted at the hospital. Of them, 669 patients who had sufficient data were analyzed. Their average age±SD was 58.7±17.4 years (range, 21–98 years). There was a male preponderance (n=393, 53.8%). A total of 82 (12.3%) patients had eventual severe pancreatitis. RS demonstrated a sensitivity of 92.7% and a specificity of 52.8% with a positive predictive value (PPV) of 21.5% and a negative predictive value (NPV) of 98.1%. GS demonstrated a sensitivity of 76.8% and a specificity of 69.2% with a PPV of 25.8% and a NPV of 95.5%. For severity prediction, areas under the curve (AUCs) for RS and GS were 0.848 (95% CI: 0.819–0.875) and 0.784 (95% CI: 0.750–0.814), respectively (p=0.003). Twelve (1.6%) patients died in the hospital.
Conclusions
RS has higher sensitivity, NPV and AUC for predicting severity of AP than GS.
Keywords: Glasgow score, Ranson score, Scoring, Severe acute pancreatitis
INTRODUCTION
Acute pancreatitis (AP) with an incidence of 50–80 per 100,000 population is a common cause of acute abdominal pain.1 Its management is influenced by etiology, severity, and local resources.2,3 SAP based the revised Atlanta classification4 occurs in 20% of AP cases. It has higher mortality rates than AP.5,6 To optimize outcomes, patients with predicted SAP should be identified early to allow prompt resuscitation, resource allocation, close monitoring, and timely interventions. For these patients, optimal prognostication allows clinicians to inform patients and caregivers on heightened possibility of critical care requirement, increased length of stay with interventions, higher hospital costs, and mortality. Hence, predictive scoring systems are essential to AP management. Many such systems exist. Ranson's score (RS) and Glasgow-Imrie (Glasgow) score (GS) have been traditionally used for severity stratification of AP.7,8 They have been widely validated for their prognostic utility.9,10,11,12,13
Despite their widespread use, both scores have been criticized for their limitations. A meta-analysis including 110 studies has reported that the predictive power of RS in clinical setting is insufficient for prognosticating new admissions.14 Another prospective study has evaluated 137 consecutive patients on admission and at 48 hours using RS, GS, and Acute Physiology and Chronic Health Evaluation (APACHE)-II score and found that none of them could achieve sufficient predictability when used alone.15
Both RS and GS scores have some limitations in clinical setting.16 Interpretation of blood glucose levels in diabetic patients, urea levels in patients with renal dysfunction, and redundancy of performing arterial blood gas analysis in a healthy patient are some of their limitations. It is not uncommon to find patients with predicted SAP clinically behaving as ‘mild’ AP. Furthermore, for patients clinically manifesting SAP, it is essential to have prompt treatment rather than waiting for 48 hours before full stratification. Both RS and GS have been criticized for their requirement of 48 hours for prediction. Many authors have reported prediction models that can provide risk stratification upon admission.17,18 Lastly, multivariable scoring is cumbersome to perform in routine clinical practice. To overcome these limitations, recent guidelines advocate the implementation of organ failure (OF)-based scoring systems.4,5 While it is ideal to predict severity accurately upon admission, this deviates from clinical truth. Hence, experts now advocate 48 hours for OF-based scoring. Although OF-based scoring is being increasingly adopted worldwide, many units continue to use traditional scoring either exclusively or along with OF-based scores.
The search for an ideal scoring system continues. It remains our local practice to continue using traditional scoring systems along with OF-based systems. Conflicting evidence regarding the accuracy and predictive value of RS and GS brings to question their relevance to modern pancreatology. In addition, although presentation, etiology, and complications AP in a multi-ethnic population are more complex than those in other reported populations,19 few such studies exist. Therefore, the primary aim of this study was to validate RS and GS for stratifying the severity of acute pancreatitis in a local clinical setting and audit our experience of managing AP in a multi- ethnic population.
MATERIALS AND METHODS
Methodology
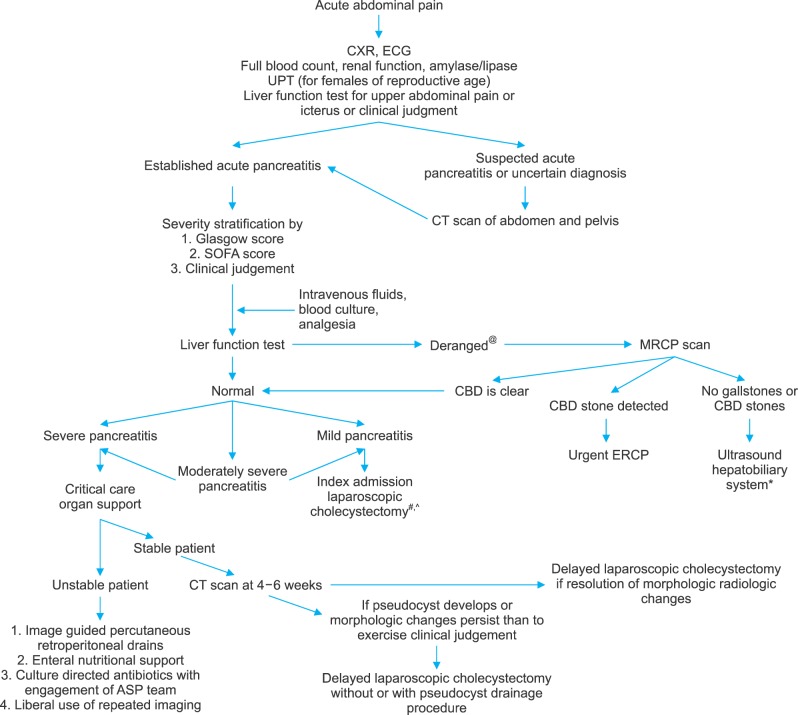
A retrospective cohort study of all patients admitted for AP in the Department of General Surgery between July 2009 and September 2016 was conducted. All patients were prognosticated with RS and GS at 48 hours. Demographic and clinical data were correlated with final severity outcome upon discharge. All patient data were reviewed retrospectively to assign severity stratification according to the revised Atlanta classification.4 Patients with insufficient data to complete any of the three scores were considered for imputation or exclusion. Imputation of variables with up to 20% missing data was done by mean substitution of missing data. Variables with >20% missing data were excluded from analysis. Electronic medical records were reviewed for demographic information, laboratory investigations, imaging, intervention history, morbidity, and mortality. Data on mortality and local complications were collected and correlated with risk scores. Tan Tock Seng Hospital's algorithm of management of AP is shown in Fig. 1. This study was approved by our Institutional Review Board.
Fig. 1. Tan tock seng hospital algorithm for management of acute pancreatitis. CXR, chest X-ray; ECG, electrocardiogram; FBC, full blood count; RP, renal panel; UPT, urine pregnancy test; LFT, liver function test; IVF, intravenous fluids; CT, Computed Tomography; CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography; MRCP, magnetic retrograde cholangiopancreatography; SOFA, sequential organ failure assessment; ASP, antibiotic stewardship programme. @Any form of elevation in bilirubin, alkaline phosphatase, or gamma glutamyl transferase is considered derangement. #Liberal practice of MRCP scan in preference to intraoperative cholangiography. *At least two imaging modalities are done prior to concluding non-biliary aetiology. All patients were offered endoscopic ultrasonography before diagnosis of idiopathic pancreatitis. ^Laparoscopic cholecystectomy is discussed with and offered to all patients with idiopathic pancreatitis.
Definitions
Diagnosis and severity stratification of AP were made in accordance with the revised Atlanta classification.4 The local laboratory did not measure amylase value in excess of 2000 units or lipase value in excess of 400 units. Hence, for these two variables, maximum value was fixed as predetermined. We have adopted Atlanta classification in clinical practice since 2013. Atlanta classification suggests the use of modified Marshall score which defines organ failure as a score of ≥2 for one of the following three systems: respiratory, cardiovascular, and renal. In our department, patients treated for AP are routinely prognosticated via Sequential Organ Failure Assessment (SOFA) score. The SOFA score is a mortality prediction tool based on six organ systems. It is widely used as mortality stratification tool in intensive care units (ICU).20 While SOFA score is not widely adopted for severity stratification or prognostication of SAP, there is evidence of its utility within ICU.21 Analysis of severity stratification using SOFA was included for comparison along with RS and GS.
Etiology of acute pancreatitis
The following aetiologies were documented: gallstones, alcohol, hypertriglyceridemia, hypercalcemia, post endoscopic retrograde cholangiopancreatography (ERCP), autoimmune, drug-induced, and idiopathic (Table 1). Gallstone-associated AP was based on identification of gallstones on any form of imaging. Alcohol-associated AP was based on the presence of a recent alcoholic binge or regular high intake. Hypertriglyceridemia-associated AP was based on the presence of hypertriglyceridemia (TG, defined as serum TG >10 mmol/L) at the time of presentation of AP. Autoimmune AP was diagnosed in accordance with the Mayo Clinic HISORt (Histology, Imaging, Serology, Other organ involvement, Response to steroid therapy, diagnostic criteria for autoimmune pancreatitis) criteria.22 Hypercalcemia was designated as the etiology if serum ionized calcium was >1.3 mmol/L. Drug-induced AP was diagnosed in the absence of the above common etiologies and in the presence of recent intake of hydrochlorothiazide, statins, non-steroidal anti-inflammatory drugs (NSAIDs), and sulfa-based medication. Idiopathic AP was diagnosed if no clear etiology was found. All patients should have at least two normal abdominal ultrasound scans or one ultrasound scan and one magnetic resonance cholangiopancreatography (MRCP) scan prior to excluding gallstone etiology. All patients were offered endoscopic ultrasound (EUS) prior to concluding idiopathic pancreatitis. All patients with idiopathic pancreatitis were offered laparoscopic cholecystectomy.
Table 1. Demographic and clinical profile of patients with acute pancreatitis.
Rightmost column displays results of Chi-square test on demographic and comorbid factors. T2DM, type 2 diabetes mellitus; COPD, chronic obstructive pulmonary disease. 41 (6%) patients, all of whom belonging to the mild or moderately-severe AP group had no recorded data on etiology. Eetiology, “others” includes AP attributed to choledochal cysts, microlithiasis, post-ERCP (endoscopic retrograde cholangiopancreaticography), common bile duct strictures and pancreatic divisum
Ranson's score (RS)
First published in 1974,7 RS was based on 11 parameters. It requires 48 hours to complete scoring. SAP is considered when patients have a cumulative score of ≥3.
Glasgow score (GS)
GS was initially described by Blamey et al.8 in 1984 for severity prognostication of AP. It is scored at 48 hours post-admission. SAP is considered when patients have a score of ≥3.
Comorbidities
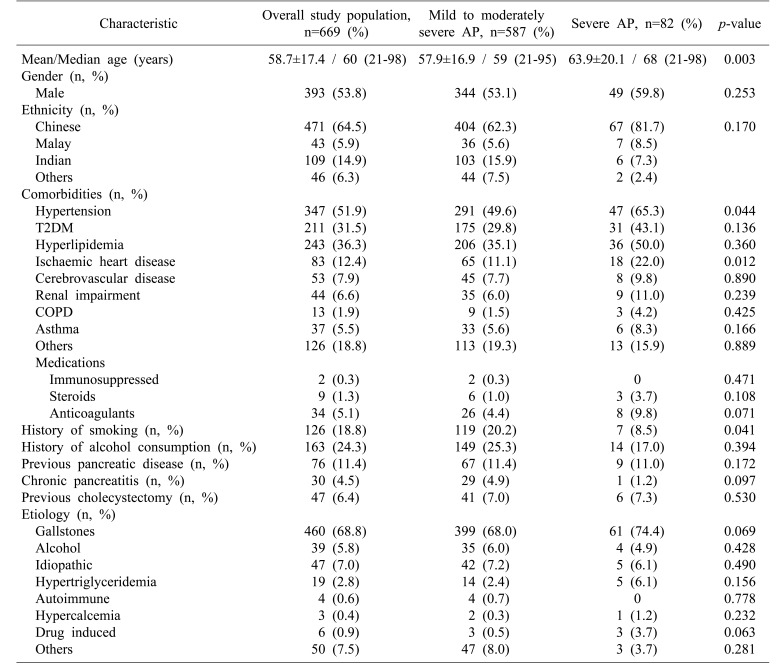
In this study comorbidities included hypertension, type 2 diabetes mellitus (T2DM), hyperlipidemia, ischemic heart disease (IHD), cerebrovascular disease, renal impairment, chronic obstructive pulmonary disease (COPD), and asthma (Table 1). Hypertension was diagnosed based on the eighth Joint National Committee (JNC8) guidelines. T2DM was diagnosed based on measured random serum glucose of ≥11.1 mmol/L or a fasting glucose level of ≥6.0 mmol/L with symptoms of diabetes mellitus and its complications. Hyperlipidemia was diagnosed based on National Cholesterol Education Program (NCEP) guidelines. History of smoking was defined as any personal history of tobacco use or dependence. History of alcohol consumption was regarded as any history of regular alcohol consumption.
Length of stay and mortality
Length of stay (LOS) was defined as the duration in days between the time of admission and the time of discharge. Mortality was defined as death occurring within the same hospital admission.
Statistical analysis
All statistical analyses were conducted via SPSS version 23 (Armonk NY: IBM Corp). Categorical variables are expressed as absolute numbers and proportions. Variance within categorical variables was analysed using chi-square test while continuous variables were analysed using Student's t-test. Multivariate analysis was conducted using logistic regression analysis. Statistical significance was considered at p<0.05. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), positive and negative likelihood ratios (LR+ and LR−, respectively), diagnostic odds ratio, and overall accuracy were calculated for both RS and GS (with ≥3 for either score as the cutoff for SAP). Receiver-operating characteristic (ROC) curves for the prediction of SAP were calculated for both scoring systems. The predictive accuracy of RS or GS was measured by the area under the receiver-operative curve (AUC). Pairwise AUC comparisons were performed between ROC curves for RS and GS via the nonparametric approach developed by DeLong et al.23 AUC ranged from 0.5 to 1.0. For prediction models, AUC values of <0.6, 0.6 to 0.8, 0.8 to 0.9, and >0.9 indicated poor, moderate, good, and excellent discriminatory ability, respectively.24
RESULTS
Demographic and clinical profile
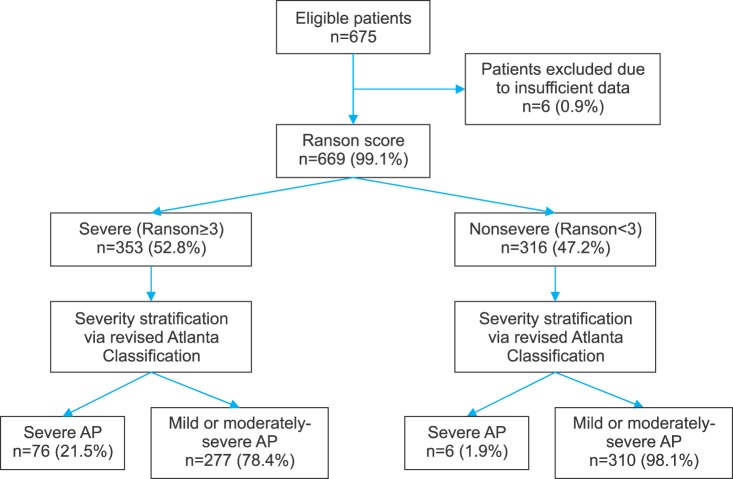
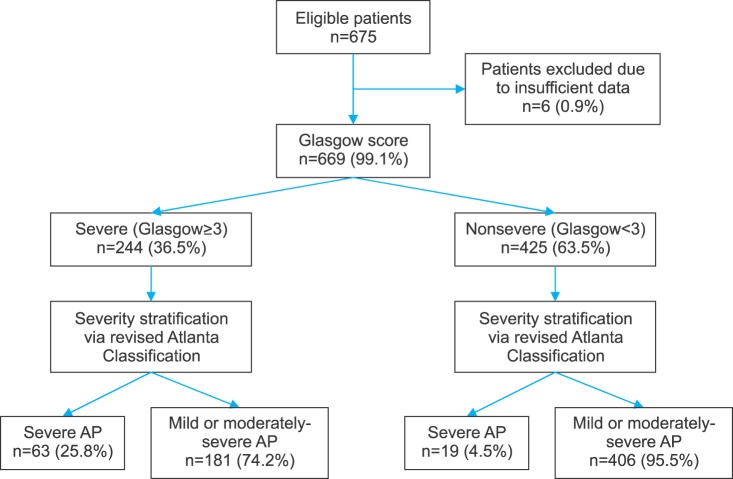
A total of 675 patients with a diagnosis of AP were admitted to Department of General Surgery. Six patients with insufficient data for either scoring system (RS or GS) were excluded. Figs. 2 and 3 reflect patient distribution according to RS and GS, respectively. Of variables studied, C-reactive protein (CRP), prothrombin time (PT), partial thromboplastin time (aPTT), and arterial partial pressure of O2 (PaO2) had over 20% data missing. Thus, they were excluded from analysis. For 12 variables (alanine transaminase [AST], lactate dehydrogenase [LDH], serum amylase, hemoglobin, total white (blood cell) count [TWC], platelet count, albumin, serum total bilirubin, urea, creatinine, alanine transaminase [ALT], and corrected serum calcium), missing data occurred for less than 20% of the sample. Thus, missing values were substituted with mean values. Average age of the 669 patients was 58.7±17.4 years (range, 21–98 years). There was a male preponderance (n=393, 53.8%). Their demographic characteristics are summarized in Table 1.
Fig. 2. Distribution of patients by Ranson score.
Fig. 3. Distribution of patients by Glasgow score.
The three most common comorbidities were hypertension (n=347, 51.9%), hyperlipidemia (n=243, 36.3%), and T2DM (n=211, 31.5%). Seventy-six (11.4%) patients had previous history of pancreatic disease, including 30 (4.5%) with history of chronic pancreatitis. A total of 126 (18.8%) patients had smoking history while 163 (24.3%) patients consumed alcohol. The three most common etiologies of AP were gallstone disease (n=460, 68.8%), alcohol consumption (n=39, 5.8%), and hypertriglyceridemia (n=19, 2.8%). Hypertension (49.6% vs. 65.3%, p=0.044) and IHD (11.1% vs. 22.0%, p=0.012) were found to be significantly more prevalent in patients with SAP. History of smoking was more common in patients with mild-to-moderate AP (20.2% vs. 8.5%, p=0.041) (Table 1). On multivariate analysis, only IHD had significant association with severity (p=0.014).
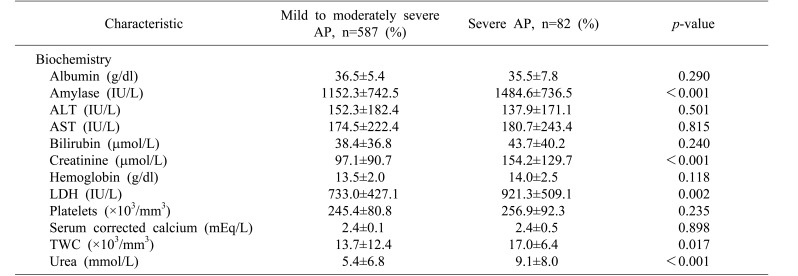
Results of comparison for baseline biochemistry investigations done at admission are shown in Table 2. Of parameters assessed at initial presentation, univariate analysis using Student's t-test identified five variables with significant differences between the non-severe and severe AP groups: serum amylase (mean±SD: 1151.1±753.5 vs. 1484.6±736.5, p<0.001), TWC (13.7±12.5 vs. 17.0±6.4, p=0.017), urea (5.4±6.9 vs. 9.1±8.0, p<0.001), creatinine (97.0±91.5 vs. 154.2±129.7, p<0.001), and LDH (729.8±455.9 vs. 936.7±530.0, p=0.002).
Table 2. Biochemical characteristics of patients with acute pancreatitis.
ALT, alanine transaminase; AST, aspartate transaminase; LDH, lactate dehydrogenase; TWC, total white count
Morbidity and outcomes
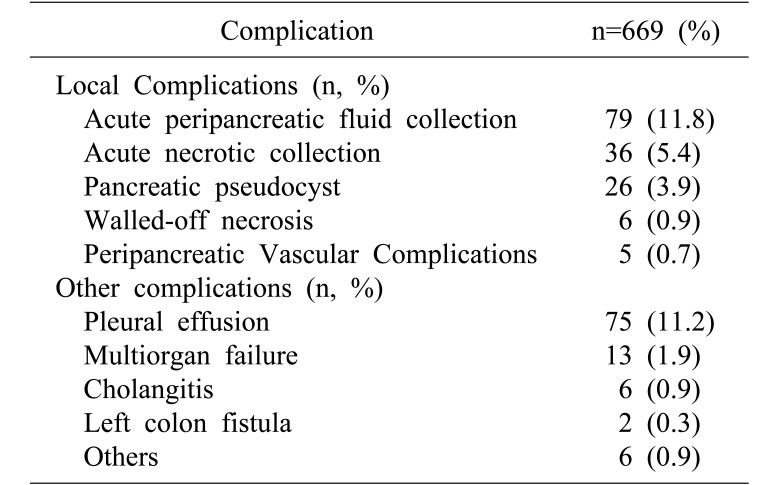
Of patients studied, 160 (23.9%) had imaging findings consistent with local complications, most commonly acute peripancreatic fluid collection (n=79, 11.8%) and acute necrotic collection (n=36, 5.4%). Eighty-seven (13.0%) patients developed other complications, most commonly pleural effusion (n=75, 11.2%) (Table 3).
Table 3. Local and systemic complications in acute pancreatitis.
Included within the category “Others” are: Atrial fibrillation (n=3), new-onset diabetes mellitus (n=1), gram negative rod bacteremia (n=2), ischemic bowel (n=1) and severe type 1 respiratory failure (n=1). Some patients had more than one complication
Mean LOS was 6.8±8.7 days (range, 2–92 days). Ninety-four (14.1%) and 20 (3.0%) patients required admission to high dependency ward (HDU) and ICU, respectively. The most common surgical intervention was cholecystectomy (n=188, 28.1%), followed by percutaneous retroperitoneal drainage (n=10, 1.5%) and necrosectomy (n=7, 1.0%). Ninety-six (14.3%) patients underwent ERCP with stent insertion. Thirteen (1.9%) patients required endoscopic ultrasonography for diagnostic evaluation. The overall incidence of local complications was 23.9% (n=160). Twelve (1.8%) patients died in the hospital.
Patients with severe acute pancreatitis
Eighty-two (12.3%) patients eventually developed SAP. Mean age of patients with SAP was higher than those with mild-moderate AP (63.9±20.1 years vs. 57.9±16.9 years, p=0.003). The most common aetiology for AP was gallstone (n=61, 74.4%). Gender, ethnicity, and the three most common comorbidities were comparable between the two groups (SAP and mild-to-moderate AP). Incidence of previous pancreatic disease (n=9, 11.0%) and chronic pancreatitis (n=1, 1.2%) in overall distribution was similar to each other (p=0.097). SAP group had higher complications (54.9% vs. 19.6%, p<0.001) and mortality (14.6% vs. nil, p<0.001) compared to the non-severe group.
Score evaluation
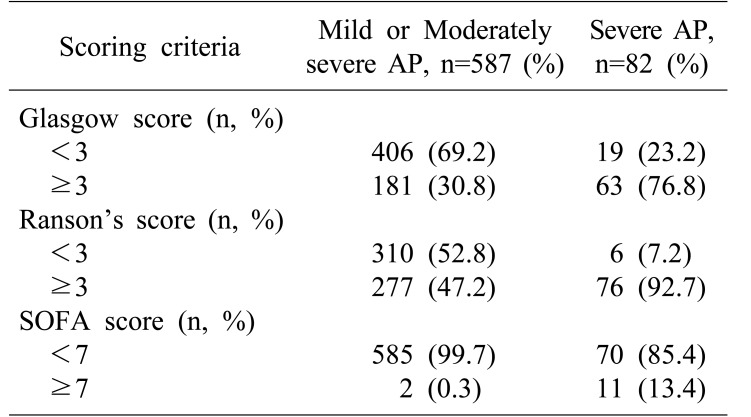
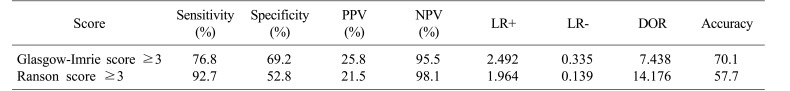
Distribution of RS, GS, and SOFA score is summarized in Table 4. Diagnostic outcomes stratified by initial scoring of both tests are shown in Table 5. RS demonstrated a sensitivity of 92.7%, a specificity of 52.8%, a PPV of 21.5%, and an NPV of 98.1%. Positive and negative likelihood ratios (LR+ and LR−) were 1.964 and 0.139, respectively. Diagnostic odds ratio of RS was 14.18 and its overall accuracy was 57.7%. GS demonstrated a sensitivity of 76.8%, a specificity of 69.2%, a PPV of 25.8%, and an NPV of 95.5%. LR+ and LR− were 2.492 and 0.335, respectively, corresponding to a diagnostic odds ratio of 7.44. The overall accuracy of GS was 70.1%.
Table 4. Distribution of Glasgow, Ranson and SOFA scores in patients with AP.
Results of severity prognostication using Glasgow score, Ranson's score. Results for SOFA score were included for comparison. AP, acute pancreatitis; SOFA, sequential organ failure assessment. Data on SOFA score for one patient was not available
Table 5. Evaluation of Glasgow and Ranson's scores.
PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio; DOR, diagnostic odds ratio. DOR is an indicator of test performance which is independent of prevalence (unlike accuracy) and ranges from zero to infinity. Higher DOR is indicative of better test performance
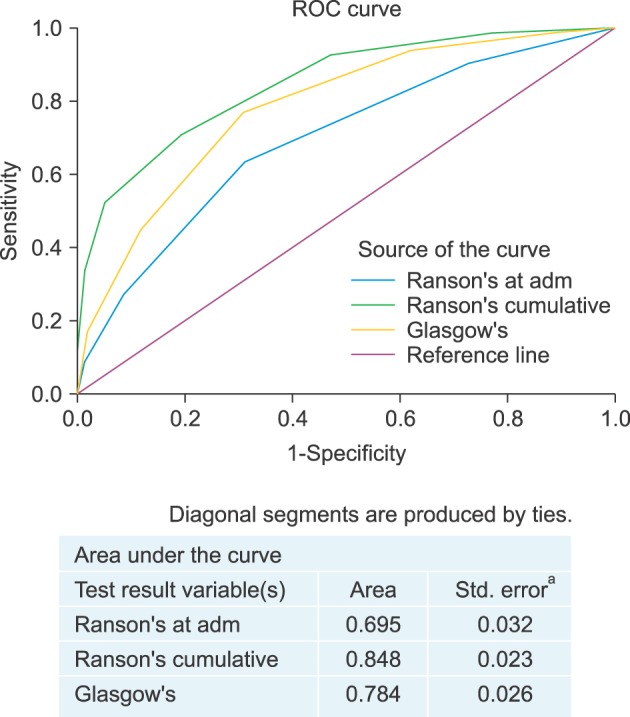
ROC curves for RS and GS are shown in Fig. 4. AUCs for RS and GS were 0.848 (95% CI: 0.819–0.875) and 0.784 (95% CI: 0.750–0.814), respectively, for SAP prediction (p<0.001). Pairwise comparison of ROC curves of GS and RS revealed that AUC of RS was significantly greater than that of GS (p=0.003).
Fig. 4. Area under receiver-operator curves of Ranson's score and Glasgow score.
DISCUSSION
Our study demonstrates that both RS and GS are effective in severity stratification at 48 hours. However, RS has superior diagnostic odds ratio and higher area under the curve for predicting severity of AP than GS in multiethnic Asian population with predominant gallstone aetiology.
Age was found to be significantly higher in the SAP group than that in the mild-to-moderate AP group in our study. The old age of our patients reflects local demography of 30% local population being ≥55 years.25 Ethnicity and distribution of comorbidities within the study population were congruent with those of the local population.26 Gallstone was a predominant aetiology, similar to findings of other studies.8,9,27 However, Westerners have higher incidence of alcoholic pancreatitis.10,15 This is likely due to lower alcohol consumption rate in our patients compared to Westerners. Incidence of SAP (12.3%) in this study was lower than 20–25% estimated by Beger and Rau,5 but comparable to results of more recent studies.12,27 This might be due to changing definitions of SAP with the revised Atlanta classification.4 On univariate analysis, biochemical profiles of SAP patients revealed significant differences in serum amylase, TWC, urea, creatinine, and LDH compared to those of mild-to-moderate AP patients. These biochemical associations have been well-documented in previous studies, showing that increased TWC,3 serum urea,28,29 creatinine,30,31,32 and LDH33,34 are associated with AP severity. Studies have also documented low serum albumin levels in patients with SAP.8,35 However, this was not evident in our study.
One key finding of this study was the high NPV of both scores (i.e., patients who were predicted not to have SAP truly did not have SAP). While the ideal NPV is 100%, these values remain consistent with other studies, highlighting the scores' utility in ruling out SAP.10,27,36 Results from our study are consistent with other reports showing that RS has a higher sensitivity but a lower specificity than GS for SAP prediction.10,14,15 This highlights the primacy of the treating physician's judgment and wisdom in deciding disposition and initial management regardless of scoring.
RS and GS have other inherent limitations. Both scores' low PPV raises a high alert level in many patients who eventually have mild AP, imposing an unnecessary resource burden and anxiety to patients. Chauhan and Forsmark16 have commented that using the revised Atlanta classification reduces the broad spectrum of AP severity to a binary result for the purpose of analysis. However, it fails to realistically capture the full spectrum of the condition. Nonetheless, our study demonstrates high AUC of both scores, emphasizing their suitability as a basic risk-stratification tool.
RS and GS have been compared to other prognostic indices in several studies. Comparative risk prediction tools include the APACHE-II score, Bedside Index of Severe Acute Pancreatitis (BISAP) score, and Harmless Acute Pancreatitis score (HAPS). Robert et al.15 have compared RS and GS scores to APACHE II and CRP and found that APACHE II is superior to RS on admission (p=0.049). This persisted throughout the subsequent five days,15 highlighting the limited prognostic value of RS before 48 hours. In a study on 185 patients with mean age of 57.1 years with 51% male population, Papachristou et al.9 have shown that RS has the highest AUC (94%) for predicting SAP compared to APACHE II score, BISAP score, and Computerised Tomography Severity Index (CTSI). In their study, the incidence of SAP (22% vs 12.3%) and mortality in SAP (17.6% vs 14.5%) were higher compared to our results. A study involving 161 patients with mean age of 62.3 years including 63% males by Cho et al.27 has reported that APACHE-II has higher AUC than RS, BISAP, CRP at 24 hours and CTSI. However, there was no significant difference. In addition, NPV of RS (95.3%) was on par with APACHE-II (95.8%). Their study involved 22% alcoholic and 21% idiopathic pancreatitis aetiologies. This is different from our experience. However, their incidence of SAP (13%) is comparable to ours. Both Papachristou et al.9 and Cho et al.27 have concluded that novel and unique models are needed to further improve the prognostic accuracy in SAP. Recently, many authors have evaluated the role of indices based on acute phase proteins and simple laboratory indices such as neutrophil to lymphocyte ratio (NLR) as predictive tools in SAP. A study including 328 patients with AP of mean age 47.5 years by Han et al.37 has reported that a combination of NLR and fluid sequestration has predictive abilities similar to RS. However, in that study, RS had higher AUC (82.4%) compared to NLR and fluid sequestration measured on days 0, 1, and 2. A study including 169 patients with mean age 54.3 years and 58% males by Yue et al.38 has reported that prealbumin/fibrinogen ratio score has higher AUC than RS (92.3% vs. 86%).38 These results need to be validated prospectively and in other units. A Spanish study by Valverde-López et al.39 including 269 patients with mean age 64.6 years and 50% males has reported that BISAP outperforms RS. However, AUCs for RS in predicting severity (85%) and mortality (94%) were high.39 Their study emphasized the role of BUN at 48 hours and serum lactate as simple biomarkers for severity prediction.
These findings prompt several questions. Firstly, given all scores possess a margin of error, should they be considered relevant given that ultimately clinical judgment triumphs all? Secondly, given traditional scoring systems have sufficient prognostic accuracy, is there a need to develop novel and unique indices? Thirdly, is there a need to continue investing efforts to develop an ‘upon-admission scoring system’ for severity or is the 48-hour time-frame essential for accurate severity prediction?
Regarding the first question, while these criteria cannot replace clinical judgment, they have value in providing objective stratification in doubtful cases, providing means for standardized reporting and auditing, and providing a platform for patient communication and future research.
For the second question, while novel indices such as BISAP, HAPS, and APACHE-II allow prognostication at admission, we believe scores at admission contradict the underlying pathophysiology of AP as a disease in evolution that requires daily review for severity reassessment. Severity is thus a ‘continuous phenomenon’ rather than a ‘time-point’. Many studies have suggested that the 48-hour time frame is essential to accurately predict severity.1,3,4,5,37,38,39 Further, the quality of clinical care and treatment response contribute to clinical outcomes. One may thus consider the 48-hour requirement to complete RS as an inherent strength, which we have failed to acknowledge since the original report published by Ranson J H et al. in 1974. Recommendations by the recent Atlanta symposium similarly endorse the 48-hour timeframe for severity stratification, highlighting the importance of prognosticating AP accurately rather than prematurely.
For the third question, one must differentiate whether the system is designed for binary prediction (i.e., mild versus severe) or multiple stages of disease severity. For the latter, the 48-hour window seems essential. For the former, it is possible that composite scoring systems which incorporate ‘scoring on admission’ with ‘physician-determined severity’ should enable accurate and reliable severity prediction. Without the subjective component of ‘physician determined severity’, an adequately powered study to develop an objective severity stratification score is not feasible as the overarching aim in care of patients with SAP is to improve outcomes (i.e., reduce mortality). Merely predicting severity is only half of the process. Assuming a 15% rate of SAP, mortality of SAP of 15% and a dropout rate of 10%, approximately 5,000 patients with AP needs to be enrolled in a prospective trial to demonstrate mortality reduction to 10% with 80% power and two-tailed alpha of 5%.
Our study has several limitations. First, patients treated for AP under non-surgical departments were excluded. This might explain the paucity of post-ERCP AP patients. Second, this study did not account for certain laboratory limitations. In particular, for serum amylase, our laboratory cannot quantify beyond 2000 IU/L. Despite this, serum amylase was still significantly greater in the SAP group compared to that in the mild-moderate severe group, rendering this limitation negligible. Further, serum amylase is elevated in a myriad of other pathologies and its magnitude of rise is neither associated with severity nor predictive of mortality.40 Third, gallstone-associated AP appeared to be underreported here, with local incidence falling short of the reported 80% prevalence in other studies.19,41 This might be due to inconsistencies in radiologic reporting and approximately 6% (n=41) patients in our study had no established aetiology. However, the overall distribution of gallstone etiology in our study remained congruent with that reported in other studies. Lastly, this was a single center retrospective study with missing data requiring imputation and exclusion (non-reporting) of certain important variables (e.g., C-reactive protein). We believe these limitations are in part compensated by large sample and computerized data recording system.
In conclusion, in the prediction of SAP, RS and GS have high NPV. RS has a higher AUC than GS. The 48-hour required for risk stratification by traditional scoring systems is their inherent strength rather than weakness. It is very plausible that scientific endeavors to investigate novel and unique scoring systems will have limited ability to improve outcomes of SAP with clinical impact. We foresee that the use of traditional scoring systems is a reasonable alternative to generic OF-based scoring systems. Further studies comparing the utility of both scores with organ failure-based systems are needed before traditional scoring systems are considered outdated and obsolete. Future effort needs to be invested in collaborative multicenter prospective studies aiming to identify simple bed side and/or biochemical variables routinely done as part of clinical care.
References
- 1.Clancy TE, Benoit EP, Ashley SW. Current management of acute pancreatitis. J Gastrointest Surg. 2005;9:440–452. doi: 10.1016/j.gassur.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Tenner S, Baillie J, DeWitt J, Vege SS American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415. 1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 3.Banks PA, Freeman ML Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 4.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 5.Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol. 2007;13:5043–5051. doi: 10.3748/wjg.v13.i38.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelat VG, Diddapur RK. Minimally invasive retroperitoneal pancreatic necrosectomy in necrotising pancreatitis. Singapore Med J. 2007;48:e220–e223. [PubMed] [Google Scholar]
- 7.Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69–81. [PubMed] [Google Scholar]
- 8.Blamey SL, Imrie CW, O'Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut. 1984;25:1340–1346. doi: 10.1136/gut.25.12.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papachristou GI, Muddana V, Yadav D, O'Connell M, Sanders MK, Slivka A, et al. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435–441. doi: 10.1038/ajg.2009.622. quiz 442. [DOI] [PubMed] [Google Scholar]
- 10.Simoes M, Alves P, Esperto H, Canha C, Meira E, Ferreira E, et al. Predicting acute pancreatitis severity: comparison of prognostic scores. Gastroenterology Res. 2011;4:216–222. doi: 10.4021/gr364w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana V, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476–1482. doi: 10.1053/j.gastro.2012.03.005. quiz e15-e16. [DOI] [PubMed] [Google Scholar]
- 12.Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, et al. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612–619. doi: 10.1038/ajg.2011.438. [DOI] [PubMed] [Google Scholar]
- 13.Williams M, Simms HH. Prognostic usefulness of scoring systems in critically ill patients with severe acute pancreatitis. Crit Care Med. 1999;27:901–907. doi: 10.1097/00003246-199905000-00023. [DOI] [PubMed] [Google Scholar]
- 14.De Bernardinis M, Violi V, Roncoroni L, Boselli AS, Giunta A, Peracchia A. Discriminant power and information content of Ranson's prognostic signs in acute pancreatitis: a meta-analytic study. Crit Care Med. 1999;27:2272–2283. doi: 10.1097/00003246-199910000-00035. [DOI] [PubMed] [Google Scholar]
- 15.Robert JH, Frossard JL, Mermillod B, Soravia C, Mensi N, Roth M, et al. Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers. World J Surg. 2002;26:612–619. doi: 10.1007/s00268-001-0278-y. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan S, Forsmark CE. The difficulty in predicting outcome in acute pancreatitis. Am J Gastroenterol. 2010;105:443–445. doi: 10.1038/ajg.2009.623. [DOI] [PubMed] [Google Scholar]
- 17.Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201–205. doi: 10.1016/s0140-6736(89)90381-4. [DOI] [PubMed] [Google Scholar]
- 18.Leese T, Shaw D, Holliday M. Prognostic markers in acute pancreatitis: can pancreatic necrosis be predicted. Ann R Coll Surg Engl. 1988;70:227–232. [PMC free article] [PubMed] [Google Scholar]
- 19.Kandasami P, Harunarashid H, Kaur H. Acute pancreatitis in a multi-ethnic population. Singapore Med J. 2002;43:284–288. [PubMed] [Google Scholar]
- 20.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 21.Adam F, Bor C, Uyar M, Demırağ K, Çankayalı İ. Severe acute pancreatitis admitted to intensive care unit: SOFA is superior to Ranson's criteria and APACHE II in determining prognosis. Turk J Gastroenterol. 2013;24:430–435. doi: 10.4318/tjg.2013.0761. [DOI] [PubMed] [Google Scholar]
- 22.Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–1016. doi: 10.1016/j.cgh.2006.05.017. quiz 934. [DOI] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 24.Anbalakan K, Chua D, Pandya GJ, Shelat VG. Five year experience in management of perforated peptic ulcer and validation of common mortality risk prediction models - are existing models sufficient? A retrospective cohort study. Int J Surg. 2015;14:38–44. doi: 10.1016/j.ijsu.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Department of Statistics, Ministry of Trade and Industry, Republic of Singapore; Department of Statistics, Ministry of Trade and Industry, Republic of Singapore, editors. Population trends, 2016. 12th ed. Singapore: Department of Statistics, Singapore; 2016. Population; pp. 3–17. [Google Scholar]
- 26.Health Information Division, Ministry of Health, Singapore; Ministry of Health, Singapore, editors. Primary care survey 2010. Singapore: Ministry of Health, Singapore; 2010. Morbidity and biographic profile of patients; pp. 6–14. [Google Scholar]
- 27.Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol. 2015;21:2387–2394. doi: 10.3748/wjg.v21.i8.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu BU, Bakker OJ, Papachristou GI, Besselink MG, Repas K, van Santvoort HC, et al. Blood urea nitrogen in the early assessment of acute pancreatitis: an international validation study. Arch Intern Med. 2011;171:669–676. doi: 10.1001/archinternmed.2011.126. [DOI] [PubMed] [Google Scholar]
- 29.Wu BU, Johannes RS, Sun X, Conwell DL, Banks PA. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology. 2009;137:129–135. doi: 10.1053/j.gastro.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 30.Talamini G, Uomo G, Pezzilli R, Rabitti PG, Billi P, Bassi C, et al. Serum creatinine and chest radiographs in the early assessment of acute pancreatitis. Am J Surg. 1999;177:7–14. doi: 10.1016/s0002-9610(98)00296-7. [DOI] [PubMed] [Google Scholar]
- 31.Lankisch PG, Weber-Dany B, Maisonneuve P, Lowenfels AB. High serum creatinine in acute pancreatitis: a marker for pancreatic necrosis? Am J Gastroenterol. 2010;105:1196–1200. doi: 10.1038/ajg.2009.688. [DOI] [PubMed] [Google Scholar]
- 32.Muddana V, Whitcomb DC, Khalid A, Slivka A, Papachristou GI. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. 2009;104:164–170. doi: 10.1038/ajg.2008.66. [DOI] [PubMed] [Google Scholar]
- 33.Hirota M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, et al. JPN guidelines for the management of acute pancreatitis: severity assessment of acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:33–41. doi: 10.1007/s00534-005-1049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hritz I, Hegyi P. Early Achievable Severity (EASY) index for simple and accurate expedite risk stratification in acute pancreatitis. J Gastrointestin Liver Dis. 2015;24:177–182. doi: 10.15403/jgld.2014.1121.242.easy. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Cui Z, Li H, Saleen AF, Zhang D, Miao B, et al. Nosocomial mortality and early prediction of patients with severe acute pancreatitis. J Gastroenterol Hepatol. 2010;25:1386–1393. doi: 10.1111/j.1440-1746.2010.06376.x. [DOI] [PubMed] [Google Scholar]
- 36.Ueda T, Takeyama Y, Yasuda T, Matsumura N, Sawa H, Nakajima T, et al. Simple scoring system for the prediction of the prognosis of severe acute pancreatitis. Surgery. 2007;141:51–58. doi: 10.1016/j.surg.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Han C, Zeng J, Lin R, Liu J, Qian W, Ding Z, et al. The utility of neutrophil to lymphocyte ratio and fluid sequestration as an early predictor of severe acute pancreatitis. Sci Rep. 2017;7:10704. doi: 10.1038/s41598-017-10516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yue W, Liu Y, Ding W, Jiang W, Huang J, Zhang J, et al. The predictive value of the prealbumin-to-fibrinogen ratio in patients with acute pancreatitis. Int J Clin Pract. 2015;69:1121–1128. doi: 10.1111/ijcp.12682. [DOI] [PubMed] [Google Scholar]
- 39.Valverde-López F, Matas-Cobos AM, Alegría-Motte C, Jiménez-Rosales R, Úbeda-Muñoz M, Redondo-Cerezo E. BISAP, RANSON, lactate and others biomarkers in prediction of severe acute pancreatitis in a European cohort. J Gastroenterol Hepatol. 2017;32:1649–1656. doi: 10.1111/jgh.13763. [DOI] [PubMed] [Google Scholar]
- 40.Shah KS, Shelat VG, Jogai S, Trompetas V. Primary gallbladder lymphoma presenting with perforated cholecystitis and hyperamylasaemia. Ann R Coll Surg Engl. 2016;98:e13–e15. doi: 10.1308/rcsann.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan YHA, Rafi S, Tyebally Fang M, Hwang S, Lim EW, Ngu J, et al. Validation of the modified Ranson versus Glasgow score for pancreatitis in a Singaporean population. ANZ J Surg. 2015;87:700–703. doi: 10.1111/ans.13139. [DOI] [PubMed] [Google Scholar]