Abstract
Backgrounds/Aims
This study attempted to identify risk factors for development of post-hepatectomy hepatic failure (PHF) and its effect on long-term survival of patients with liver metastases from colorectal cancer.
Methods
We carried out a retrospective study of 143 patients who had been diagnosed with liver metastases from colorectal cancer and who had undergone hepatectomy between 2003 and 2010. We allocated these patients to PHF and non-PHF groups, using the definition of the International Study Group of Liver Surgery, and compared the clinical factors of the two groups, using Cox regression and Kaplan-Meier analysis to evaluate the differences in overall survival (OS) and recurrence-free survival (RFS) between these groups.
Results
The PHF group comprised 19 patients (13.3%); all had Grade A PHF. Independent risk factors for development of PHF were metachronous liver metastases and major hepatectomy. The differences between the PHF and non-PHF groups in OS or RFS were not statistically significant; however, the PHF group tended to have a worse prognosis. Multivariate analysis revealed significant associations between OS and the factors of poor differentiation of the primary colorectal cancer, major hepatectomy, and positive resection margin.
Conclusions
Major hepatectomy is an important risk factor for PHF in patients with liver metastases from colorectal cancer. The pathological characteristics of the primary tumor are more important as predictors than is Grade A PHF.
Keywords: Liver metastasis from colorectal cancer, Hepatectomy, International Study Group of Liver Surgery, Hepatic failure
INTRODUCTION
Liver metastases are present in 14 to 25% of patients with colorectal cancer at the time of diagnosis and develop in about 60% of such patients during their lifetime.1 Although most patients are not candidates for surgery, the optimal treatment for liver metastases is reportedly complete surgical resection, which achieves 5-year overall survival rates of 50%.2,3
Advances in preoperative chemotherapy and surgical techniques, such as portal vein ligation or embolization, have recently made more active surgical interventions possible for lesions that were previously considered unresectable.4,5 Post-hepatectomy hepatic failure (PHF), one of most feared and serious complications, is frequent, even though the prospects for survival and length of survival after liver resection have been improved.6 Nevertheless, the clinical significance of PHF in patients with liver metastases from colorectal cancer has not been well examined.
In contrast, PHF in patients with hepatocellular carcinoma (HCC) has been well studied. Several studies reported that underlying liver disease, major hepatectomy, blood loss, and transfusion were risk factors for developing PHF.7,8 In addition, PHF in patients with HCC was significantly associated with postoperative complications and long-term survival. The mechanism by which PHF affects tumor progression or recurrence is unclear. One explanation is that PHF forces active regeneration of remnant liver, which could trigger growth of microscopic HCC and eventually cause recurrence of tumors after hepatectomy.9
Thus, we assumed that PHF would increase tumor recurrence rates and reduce the survival time of patients with liver metastases from colorectal cancer. We therefore assessed the clinical significance of PHF in these patients by examining risk factors for development of PHF and its correlation with long-term survival.
MATERIALS AND METHODS
Patients
Consecutive patients undergoing partial hepatectomy for liver metastases from colorectal cancer between March 2003 and December 2010 were considered for this study. Patients were included regardless of whether they had synchronous or metachronous liver metastases and simultaneous or staged surgery. The following exclusion criteria were applied: history of previous hepatectomy; surgery not intended to cure; and presence of extrahepatic metastases. The resulting study cohort comprised 143 patients, 19 (13.2%) of whom were classified as the PHF group and 124 patients (86.8%) as the non-PHF group. Data were collected prospectively on medical records and reviewed retrospectively.
Definition of post-hepatectomy hepatic failure
The definition of the International Study Group of Liver Surgery (ISGLS) was used.10 The ISGLS defines PHF as a postoperative acquired deterioration in the ability of the liver to maintain its synthetic, excretory, and detoxifying functions that is characterized by an increased international normalized ratio and concomitant hyperbilirubinemia on or after postoperative day 5. This definition applies to patients with normal or abnormal preoperative liver function.
Hepatectomy
Of all 143 patients, 94 (65.7%) had synchronous liver metastases. Among them, 63 patients underwent simultaneous liver resection (67.0%) and 31 patients underwent staged resection (33.0%), which consisted of hepatectomy delayed until after resection of the primary colorectal cancer. For synchronous liver metastases, the decision whether to perform staged or simultaneous resection was made at the surgeon's discretion. Most patients with initially resectable synchronous liver metastases underwent simultaneous resection. However, patients who required extended resection because of the number or size of liver metastases underwent staged resection.
Contrast-enhanced multidetector abdominal CT or liver magnetic resonance imaging (MRI) using hepatocyte-specific contrast was done before hepatectomy to assess underlying liver fibrosis, resectability of tumors, boundary of resection, and vascular anatomy, including its relationship with tumors. Patients were operated on by means of either a right subcostal or midline incision or laparoscopically, according to the type of hepatectomy. Intraoperative sonography was done in all cases to make an adequate resection margin more easily and to detect occult tumors. Pringle maneuver was performed as needed, which consisted of hepatic inflow occlusion for 15 minutes, followed by 5 minutes of reperfusion until the parenchymal transection was complete. Liver transection was performed by the Kelly clamp crush method or by using a Cavitron Ultrasonic Surgical Aspirator. One patient in the PHF group and three patients in the non-PHF group received intraoperative radiofrequency ablation (RFA) combined with hepatectomy. These four patients were considered to undergo R0 resection according to the final description of a serial follow-up CT by radiologists and their clinical course. After surgery, selected patients were managed in the intensive care unit (ICU) and transferred to the general ward when they were clinically stable.
Clinical factors
To identify the risk factors for PHF and their prognostic effects, the background characteristics, primary colorectal cancer, liver metastasis-related factors, and long-term outcomes, were compared between the PHF and non-PHF groups. Primary colorectal cancer characteristics that were evaluated comprised TNM staging according to the Seventh Edition of the American Joint Committee on Cancer (AJCC) and the histological degree of differentiation. Liver metastasis-related factors assessed included metachronous metastasis, pre-hepatectomy chemotherapy, number and sizes of tumors, and resection margin. Pre-hepatectomy chemotherapy was defined as that administered within the two months prior to the hepatectomy, regardless of the type of regimen. Major hepatectomy was defined as the resection of three or more Couinaud segments.11
Postoperative follow-up and long-term outcomes
After discharge from hospital, patients underwent clinical follow-up every 3 to 6 months for the first five years and yearly thereafter. The follow-up included a physical exam, laboratory tests including tumor markers, and a computed tomography scan. Overall survival (OS) was defined as the time from curative hepatectomy to death or last follow-up. Recurrence-free survival (RFS) was defined as the time from curative hepatectomy to the first locoregional or systemic recurrence, no matter whether there were synchronous or metachronous liver metastases.
Statistical analyses
Continuous data are expressed as the median and range. Clinical characteristics were compared using independent samples t-tests and χ2 tests. Logistic regression analysis was performed to identify risk factors and effects of PHF. Cox regression and Kaplan-Meier analysis were used to compare OS and RFS between groups. Each independent factor was examined by univariate and multivariate analyses. A p value <0.05 was considered to dente statistically significant. SPSS Statistics ver. 20.0 was used for all statistical analyses (SPSS, Chicago, IL, USA).
RESULTS
Patient characteristics
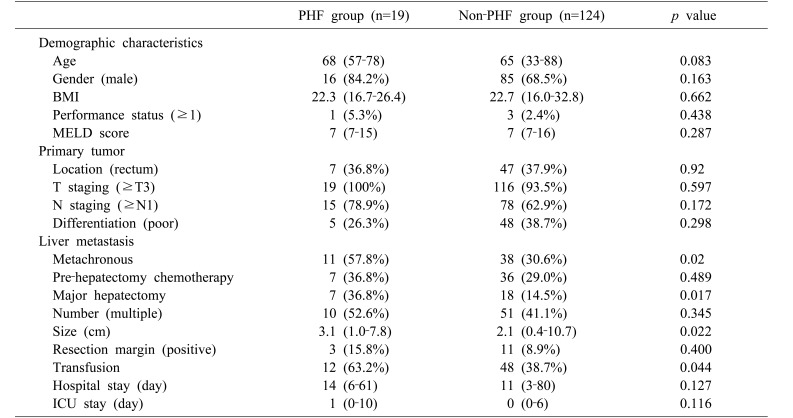
Nineteen of the enrolled patients (13.2%) had grade A PHF, which, according to the ISGLS definition, does not affect the clinical course. No patients were diagnosed with grade B or C PHF. Background characteristics are summarized in Table 1. No significant differences were found in clinical and primary tumor characteristics between the PHF and non-PHF groups. For liver metastasis-associated factors, metachronous metastases were more frequent in the PHF (57.8%) than in the non-PHF group (30.6%, p=0.02). Pre-hepatectomy chemotherapy (36.8% vs. 29.0%, p=0.489) and positive resection margin (15.8% vs. 8.9%, p=0.4) did not differ significantly between the PHF and non-PHF groups. Major hepatectomy was performed significantly more often in the PHF (36.8%) than in the non-PHF group (14.5%, p=0.017). Multiplicity of tumors did not differ significantly between the PHF (52.6%) and non-PHF groups (41.1%, p=0.345). Median tumor diameter was significantly larger in the PHF (median 3.1 cm, range 1.0–7.8 cm) than in the non-PHF group (median 2.1 cm, range 0.4–10.7 cm; p=0.022). Also, 12 patients (63.2%) in the PHF group and 48 (38.7%) in the non-PHF group needed transfusions (p=0.044).
Table 1. Baseline patient and tumor characteristics.
Values were presented as median (range) for continuous data; n (%) for categorical data. Performance status was according to the grade of the Eastern Cooperative Oncology Group (ECOG)
PHF, post-hepatectomy hepatic failure; BMI, body mass index; MELD, modified end-stage liver disease; ICU, intensive care unit
Risk factors for post-hepatectomy hepatic failure
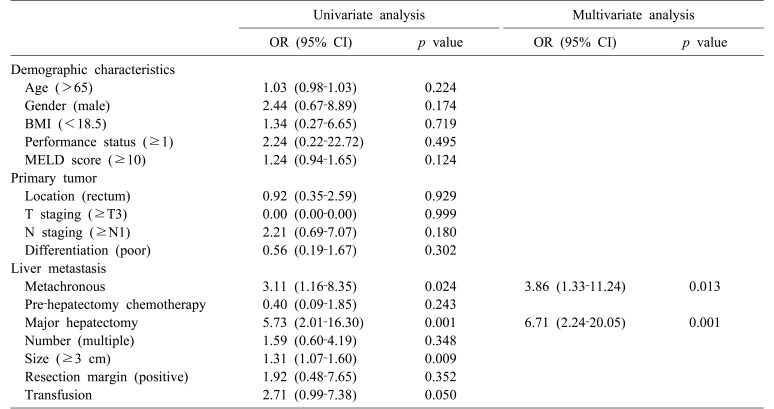
Metachronous metastases (OR, 3.11; 95% CI, 1.16–8.35; p=0024), major hepatectomy (OR, 5.73; 95% CI, 2.01–16.30; p=0.001), and tumor size (OR, 1.31; 95% CI, 1.07–1.60; p=0.009) were identified as predictors of PHF by univariate analysis (Table 2). Multivariate analysis was performed on factors with p≤0.2 by univariate analysis; these included sex, Model for End-stage Liver Disease (MELD) score (≥10), N staging (≥N1), metachronous metastases, major hepatectomy, tumor size (≥3 cm), and transfusion. Metachronous liver metastases (OR, 3.86; 95% CI, 1.33–11.24; p=0.013) and major hepatectomy (OR, 6.71; 95% CI, 2.24–20.05; p=0.001) were found by multivariate analysis to be significantly associated (Table 2).
Table 2. Univariate and multivariate analyses of risk factors for post-hepatectomy hepatic failure.
BMI, body mass index; MELD score, modified end-stage liver disease score; OR, odds ratio; CI, confidence interval
Long-term outcome
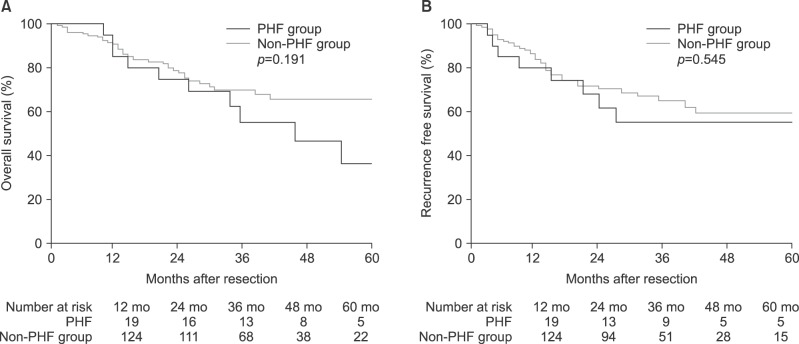
The median duration of follow-up was 28 months (range 3–95). The 1-, 3-, and 5-year OS rates for all patients were 91.6%, 67.0%, and 56.7%, respectively, and 1-, 3-, and 5-year RFS rates 82.5%, 61.3%, and 56.8%, respectively. Long-term survival by study group is shown in Fig. 1. The 1-, 3-, and 5-year OS rates were 84.2%, 60.1%, and 32.9%, respectively, for the PHF group; and 90.3%, 68.2%, and 63.8%, respectively, for the non-PHF group (p=0.191). Additionally, the 1-, 3-, and 5-year RFS rates were 78.9%, 53.0%, and 53.0%, respectively, for the PHF group; and 83.0%, 63.0%, and 57.3%, respectively, for the non-PHF group (p=0.545). No significant differences in OS or RFS were found between the PHF and non-PHF groups.
Fig. 1. (A) Kaplan-Meier curves showing risk of overall survival by PHF and non-PHF groups. (B) Recurrence-free survival by PHF and non-PHF groups. PHF, post-hepatectomy hepatic failure.
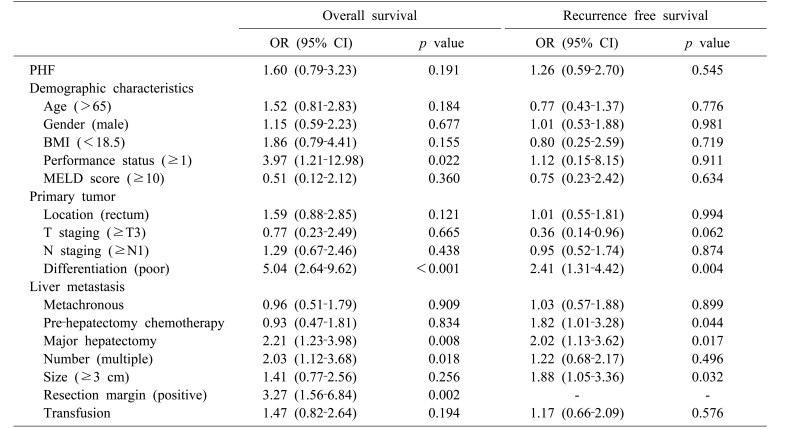
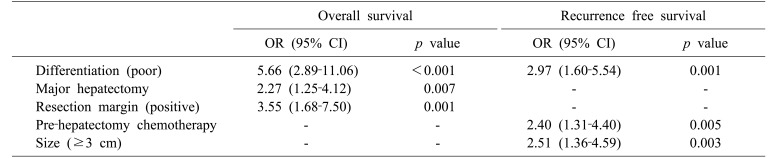
Cox regression analysis was performed to identify risk factors for long-term survival and to investigate the hypothesis that PHF affects long-term survival. Results of univariate analysis for OS and RFS are shown in Table 3: PHF did not affect long-term survival. Multivariate analysis included factors with p≤0.2 by univariate analysis. Poor differentiation of primary colorectal cancer was found to be an independent risk factor for both OS and RFS (OR, 5.66; 95% CI, 2.89–11.06; p<0.001 and OR, 2.97; 95% CI, 1.60–5.54; p=0.001, respectively). Major hepatectomy (OR, 2.27; 95% CI, 1.25–4.12; p=0.007) and positive resection margin (OR, 3.55; 95% CI, 1.68–7.50; p=0.001) were significantly associated with OS, whereas prehepatectomy chemotherapy (OR, 2.40; 95% CI, 1.31–4.40; p=0.005) and tumor size (OR, 25.1; 95% CI, 1.36–4.59; p=0.003) were independent risk factors for RFS (Table 4).
Table 3. Univariate analysis of risk factors for overall survival and recurrence-free survival.
PHF, post-hepatectomy hepatic failure; BMI, body mass index; MELD, modified end-stage liver disease; OR, odds ratio; CI, confidence interval
Table 4. Multivariate analysis of risk factors for overall survival and recurrence-free survival.
OR, odds ratio; CI, confidence interval
DISCUSSION
Regardless of the reason for liver resection, PHF is a predominant cause of postoperative morbidity and mortality after elective hepatic resection.6,12 Although survival rates after liver resection have improved during the past 10 years as a result of more accurate preoperative evaluation of hepatic functional reserve and of improvements in surgical techniques and perioperative management,8,13 PHF can still result from the underlying liver disease, functional hepatic reserve, or remnant liver volume.14 Furthermore, PHF is clinically important because it decreases long-term survival and may prevent use of some treatment options for intrahepatic recurrence.15
There have been many attempts to define PHF. First, Beaujon Hospital's team reported that a combination of prothrombin time of less than 50% and total bilirubin concentration of more than 50 µmol/L (50–50 criteria) on postoperative day 5 predict mortality rate of more than 50% after liver resection.16 Second, Mullen et al.17 reported that peak serum bilirubin >7 mg/dl is the most powerful predictor of 90-day mortality after major hepatectomy. Finally, the ISGLS definition is characterized by an increased international normalized ratio and concomitant hyperbilirubinemia on or after postoperative day 5.10 Skrzypczyk et al.18 found that the ISGLS definition is less accurate than the other two criteria in identifying patients at risk of major complications or death after hepatectomy. However, despite its lower predictive value for early postoperative mortality, we chose to use the ISGLS definition in this study because it includes more patients whose PHF is less severe. We considered that such patients also need active regeneration of remnant liver and that their statistics likely influence the overall rated for recurrence and survival, even though their liver damage is transient and reversible.
Meanwhile, several studies on patients with HCC have identified that PHF is associated with postoperative complications and long-term outcomes.7,8 In HCC, underlying liver diseases, such as fibrosis and cirrhosis, provide an ongoing field of cancerization that can cause new metachronous HCC after resection.19 Furthermore, in patients with PHF after resection of HCC, the remnant liver must undergo vigorous regeneration beyond its normal metabolic demand to overcome the effects of surgical resection.20 This process facilitates the growth and malignant transformation of microscopic HCC and thus affects the long-term prognosis.9,21 In contrast, as indicated by the MELD scores in this study, patients with colorectal cancer generally have well-preserved liver function without fibrosis or cirrhosis. We therefore wondered how the process of recovery from PHF would affect these patients; published studies on this topic are lacking. Thus, we here aimed to clarify risk factors for developing PHF and correlate these factors with OS and RFS in patients with liver metastases from colorectal cancer.
In this study, PHF occurred in 13.2% of patients, a finding that fits well with previous reports of rates of 3–30%.6,7,22 Interestingly, no patients were diagnosed with grade B or C PHF. We speculate that preservation of maximal liver remnants and optimal postoperative care with active ICU treatment in selected patients are the keys to preventing worse grades of PHF. Furthermore, since grade A PHF could not be the direct cause of death, this study, in which all patients in PHF group were grade A, would better analyze the effect of PHF on oncologic outcome. According to multivariate analysis, independent risk factors for PHF were metachronous liver metastases and major hepatectomy. To identify how they affected PHF, additional analysis according to the synchronicity of liver metastases was performed. No clinical variables, including tumor characteristics, major hepatectomy, and prehepatectomy chemotherapy, reached statistical significance between the groups. However, if prehepatectomy chemotherapy is redefined as that administered regardless of the timing, patients with metachronous metastases had received it more often than had those with synchronous metastases (65.3% vs. 35.1%, p=0.017). Thus, it appears that the cumulative effects of chemotherapy prior to hepatectomy might affect the incidence of PHF. Furthermore, several studies have reported major hepatectomy as a risk factor for developing PHF.7,23 Major hepatectomy often leads to PHF because of the small remnant liver volume and the long time required for the liver to recover to its preoperative functional status.
We next investigated whether PHF affects OS and RFS. As shown in Fig. 1, although the PHF group appeared to have lower OS and RFS, these differences were not statistically significant. Given that this was a retrospective study that may have had a selection bias, interpretation of the results requires particular care. Because major hepatectomy is a risk factor for development of both PHF and OS, enrollment of a larger number of patients could identify PHF as a significant risk factor for long-term outcomes. A study of 193 patients with liver metastases from colorectal cancer found that PHF did not affect the 3-month survival rate, but was associated with a worse 2-year survival.22 The short follow-up period was also a limitation of our study.
We performed multivariate and Cox regression analysis to identify the risk factors for long-term survival and found that differentiation of the primary colorectal cancer is a significant risk factor for both OS and RFS. Previous studies have reported that poor differentiation of the primary tumor is an important factor affecting the survival of patients with liver metastases from colorectal cancer.24 In our study, risk factors for OS also included major hepatectomy and positive resection margin, confirming that positive resection margin is an important prognostic factor for survival, as shown by others.25
One limitation of this study concerns pre-hepatectomy chemotherapy. Previous studies have reported that preoperative chemotherapy that caused steatohepatitis or sinusoidal obstruction is associated with an increased incidence of post-hepatectomy complications.26,27 In our study, only 43 patients (30.0%) received pre-hepatectomy chemotherapy, which comprised oxaliplatin-based chemotherapy in 39 patients (90.6%) and irinotecan-based chemotherapy in two patients (4.6%). The lack of normal distribution of the data made it unsuitable for statistical analysis. Prehepatectomy chemotherapy did not seem to affect PHF, possibly because we could not perform subgroup analysis by type of chemotherapy. Furthermore, patients who had received pre-hepatectomy chemotherapy had worse RFS than those who did not. There are two possible ways of interpreting this result. First, our data was collected over a long period, during which the indications for chemotherapy changed. Second, a selection bias characterized by patients who received prehepatectomy chemotherapy and who tended to have more aggressive tumors may have influenced our findings.
In conclusion, we identified major hepatectomy as an independent risk factor for both developments of PHF and poor OS in patients with liver metastases from colorectal cancer. Tumor-related factors, such as differentiation of the primary tumor and size of metastasis, are more important factors for predicting length of survival than is occurrence of grade A PHF.
References
- 1.Spolverato G, Ejaz A, Azad N, Pawlik TM. Surgery for colorectal liver metastases: the evolution of determining prognosis. World J Gastrointest Oncol. 2013;5:207–221. doi: 10.4251/wjgo.v5.i12.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones RP, Jackson R, Dunne DF, Malik HZ, Fenwick SW, Poston GJ, et al. Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br J Surg. 2012;99:477–486. doi: 10.1002/bjs.8667. [DOI] [PubMed] [Google Scholar]
- 3.Akgül Ö, Çetinkaya E, Ersöz Ş, Tez M. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol. 2014;20:6113–6122. doi: 10.3748/wjg.v20.i20.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatri VP, Chee KG, Petrelli NJ. Modern multimodality approach to hepatic colorectal metastases: solutions and controversies. Surg Oncol. 2007;16:71–83. doi: 10.1016/j.suronc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Chen JX, Ran HQ, Sun CQ. Associating microwave ablation and portal vein ligation for staged hepatectomy for the treatment of huge hepatocellular carcinoma with cirrhosis. Ann Surg Treat Res. 2016;90:287–291. doi: 10.4174/astr.2016.90.5.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narita M, Oussoultzoglou E, Bachellier P, Jaeck D, Uemoto S. Post-hepatectomy liver failure in patients with colorectal liver metastases. Surg Today. 2015;45:1218–1226. doi: 10.1007/s00595-015-1113-7. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima K, Fukumoto T, Kuramitsu K, Kido M, Takebe A, Tanaka M, et al. Assessment of ISGLS definition of posthepatectomy liver failure and its effect on outcome in patients with hepatocellular carcinoma. J Gastrointest Surg. 2014;18:729–736. doi: 10.1007/s11605-013-2423-y. [DOI] [PubMed] [Google Scholar]
- 8.Iguchi K, Hatano E, Yamanaka K, Tanaka S, Taura K, Uemoto S. The impact of posthepatectomy liver failure on the recurrence of hepatocellular carcinoma. World J Surg. 2014;38:150–158. doi: 10.1007/s00268-013-2247-7. [DOI] [PubMed] [Google Scholar]
- 9.Shi JH, Huitfeldt HS, Suo ZH, Line PD. Growth of hepatocellular carcinoma in the regenerating liver. Liver Transpl. 2011;17:866–874. doi: 10.1002/lt.22325. [DOI] [PubMed] [Google Scholar]
- 10.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Couinaud C. Liver lobes and segments: notes on the anatomical architecture and surgery of the liver. Presse Med. 1954;62:709–712. [PubMed] [Google Scholar]
- 12.Kim SH, Kang DR, Lee JG, Kim DY, Ahn SH, Han KH, et al. Early predictor of mortality due to irreversible posthepatectomy liver failure in patients with hepatocellular carcinoma. World J Surg. 2013;37:1028–1033. doi: 10.1007/s00268-013-1959-z. [DOI] [PubMed] [Google Scholar]
- 13.Cescon M, Vetrone G, Grazi GL, Ramacciato G, Ercolani G, Ravaioli M, et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249:995–1002. doi: 10.1097/SLA.0b013e3181a63c74. [DOI] [PubMed] [Google Scholar]
- 14.Schreckenbach T, Liese J, Bechstein WO, Moench C. Posthepatectomy liver failure. Dig Surg. 2012;29:79–85. doi: 10.1159/000335741. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura N, Hatano E, Iguchi K, Seo S, Taura K, Uemoto S. Posthepatectomy liver failure affects long-term function after resection for hepatocellular carcinoma. World J Surg. 2016;40:929–936. doi: 10.1007/s00268-015-3345-5. [DOI] [PubMed] [Google Scholar]
- 16.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. discussion 828-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 862-864. [DOI] [PubMed] [Google Scholar]
- 18.Skrzypczyk C, Truant S, Duhamel A, Langlois C, Boleslawski E, Koriche D, et al. Relevance of the ISGLS definition of posthepatectomy liver failure in early prediction of poor outcome after liver resection: study on 680 hepatectomies. Ann Surg. 2014;260:865–870. doi: 10.1097/SLA.0000000000000944. discussion 870. [DOI] [PubMed] [Google Scholar]
- 19.Bilimoria MM, Lauwers GY, Doherty DA, Nagorney DM, Belghiti J, Do KA, et al. Underlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma. Arch Surg. 2001;136:528–535. doi: 10.1001/archsurg.136.5.528. [DOI] [PubMed] [Google Scholar]
- 20.van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malagó M, Jalan R, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767–780. doi: 10.1111/j.1478-3231.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 21.Harun N, Nikfarjam M, Muralidharan V, Christophi C. Liver regeneration stimulates tumor metastases. J Surg Res. 2007;138:284–290. doi: 10.1016/j.jss.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Vibert E, Pittau G, Gelli M, Cunha AS, Jamot L, Faivre J, et al. Actual incidence and long-term consequences of posthepatectomy liver failure after hepatectomy for colorectal liver metastases. Surgery. 2014;155:94–105. doi: 10.1016/j.surg.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro HS, Costa WL, Jr, Diniz AL, Godoy AL, Herman P, Coudry RA, et al. Extended preoperative chemotherapy, extent of liver resection and blood transfusion are predictive factors of liver failure following resection of colorectal liver metastasis. Eur J Surg Oncol. 2013;39:380–385. doi: 10.1016/j.ejso.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Arru M, Aldrighetti L, Castoldi R, Di Palo S, Orsenigo E, Stella M, et al. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93–103. doi: 10.1007/s00268-007-9285-y. [DOI] [PubMed] [Google Scholar]
- 25.Mbah NA, Scoggins C, McMasters K, Martin R. Impact of hepatectomy margin on survival following resection of colorectal metastasis: the role of adjuvant therapy and its effects. Eur J Surg Oncol. 2013;39:1394–1399. doi: 10.1016/j.ejso.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]