Abstract
The inability to elicit strong and durable cellular responses is a major obstacle in the development of successful vaccines, in particular those against malaria. In this regard, the generation of novel adjuvants that will potently boost cell-mediated immunity induced by candidate vaccines is helpful. We and others have found a glycolipid, called α-galactosylceramide (α-GalCer), which could be presented on CD1d expressed by antigen-presenting cells (APCs) and stimulate natural killer T (NKT) cells. This triggers the activation/maturation of APCs, particularly dendritic cells (DCs). By activating NKT cells and subsequently DCs, α-GalCer has been shown to enhance adaptive immune responses, particularly of CD8 + T cells, induced by the vaccines. More recently, we identified an analogue of α-GalCer, which can display a potent adjuvant activity in conjunction with malaria vaccines in mice and non-human primates. It is anticipated that CD1d-binding, NKT cell-stimulating glycolipids will be tested as adjuvants in humans in the near future.
Keywords: glycolipid, adjuvant, natural killer T cell, CD1d, malaria vaccine, dendritic cell
Introduction
The development of effective vaccines remains the key to eradicating many prevalent global pathogens, such as malaria. In order to develop successful vaccines against these diseases, one has to elicit powerful and long-lasting protective immunity consisting of humoral and cellular responses, as both are shown to be essential for effectively eliminating pathogens. The inability to elicit potent, durable, and protective T-cell responses, particularly CD8 + T-cell responses, has been a major obstacle in developing successful vaccines.
Many efforts to identify a new adjuvant have been undertaken in order to overcome the limitations of current vaccines 1– 5. An adjuvant is a compound that helps enhance immune responses elicited by vaccines. For example, a weak immunogen requires an adjuvant for the enhancement of its immunogenicity. In some cases of viral vectored vaccines, the pre-existing immunity to the vector itself has been shown to reduce their immunogenicity 6, 7, and hence the use of adjuvants that circumvent the pre-existing immunity might be important to augment the efficacy of the vaccines.
Antigen-presenting cells (APCs) express a non-polymorphic major histocompatibility complex (MHC) class I–like molecule, called CD1d, which presents lipids to unconventional T cells, called invariant natural killer T ( iNKT) cells, and stimulate them through their invariant α/β T-cell receptor (TCR) 8– 10. Upon stimulation, the iNKT cells rapidly secrete cytokines, such as interferon-gamma (IFN-γ), and, together with CD40-CD40L interactions, iNKT cells induce maturation and activation of APCs 9.
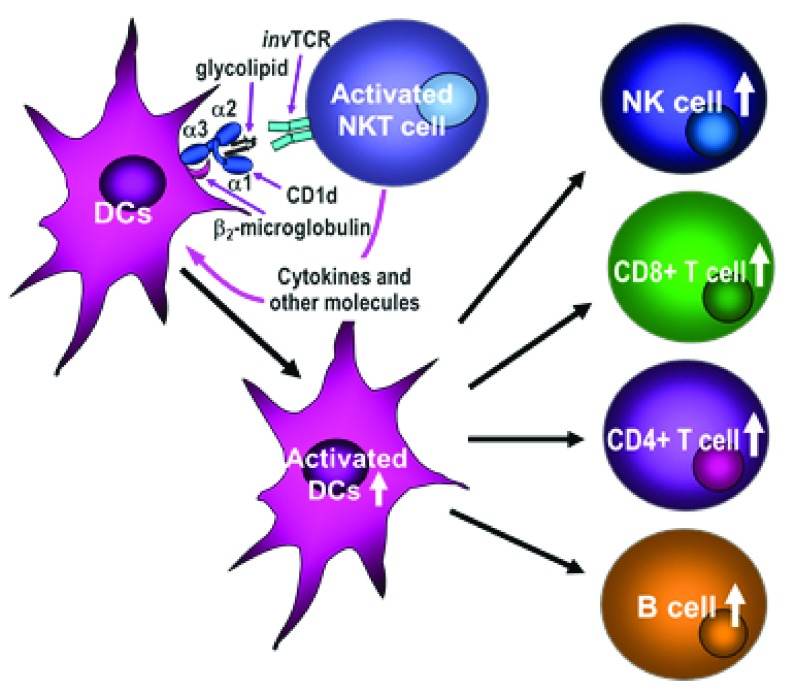
Alpha-galactosylceramide (α-GalCer), which is a well-known glycolipid that binds CD1d 11– 14, activates iNKT cells to rapidly produce large quantities of Th1 and Th2 cytokines and subsequently induces the activation of a cascade of various immuno-competent cells, including dendritic cells (DCs), NK cells, B cells, and CD4 + and CD8 + T cells ( Figure 1). α-GalCer has been used not only as a potential direct therapy for cancer and autoimmune and infectious diseases 15– 24 but also as an adjuvant to enhance the efficacy of various existing or new vaccines that include live vector vaccines 25– 30.
Figure 1. Mode of invariant natural killer T (NKT) cell activation by glycolipids and subsequent activation of various immune competent cells.
Glycolipids presented by CD1d molecules activate invariant NKT cells, which in turn induce activation/maturation of dendritic cells (DCs) and subsequent activation of CD8 + T cells, and also natural killer (NK), B, and CD4 + T cells. TCR, T-cell receptor.
It is noteworthy that we have more recently identified a lead clinical candidate synthetic analog of α-GalCer, named 7DW8-5, which elicits the highest level of iNKT-cell response among 100 α-GalCer analogs tested 31. When we co-administered 7DW8-5 or α-GalCer intra-muscularly with a sub-optimal dose of a recombinant adenovirus expressing a major malaria antigen, the circumsporozoite protein of Plasmodium yoelii (PyCSP), we found that 7DW8-5 has a 100-fold higher dose-sparing effect than α-GalCer in displaying the adjuvant effect on the level of PyCSP-specific CD8 + T-cell response induced in mice upon its single immunizing dose 31. In this article, we will review the adjuvant effects of glycolipids, which bind CD1d and stimulate iNKT cells, in the context of certain vaccines.
Identification of a potent CD1d-binding NKT-cell ligand
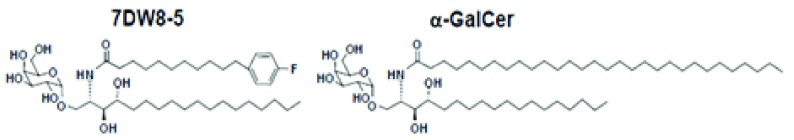
From a focused glycolipid library consisting of about 100 analogs of α-GalCer, we have identified a lead candidate glycolipid, 7DW8-5, which exhibits a more potent biological activity than its parental compound, α-GalCer 31. The formal chemical name of 7DW8-5 is [(2S, 3S, 4R)-1-O-(α-D-galactopyranosyl)-N-(11-(4-fluorophenyl) undecanoyl)-2-amino-1,3,4-octadecanetriol)]. This analog differs from α-GalCer in that it possesses a fluorinated benzene ring at the end of a C8 length fatty acyl chain ( Figure 2). 7DW8-5 was shown to have a much higher binding affinity than α-GalCer for murine and human CD1d molecules and consequently display a stronger stimulatory activity toward iNKT cells and CD1d-expressing DCs. We also determined various functional properties of the two glycolipids. Table 1 contains a summary of published results comparing the properties of two glycolipids: 7DW8-5 and α-GalCer 31– 37.
A potent CD1d-binding NKT-cell ligand as an adjuvant for malaria vaccines
As for its safety, when 7DW8-5 was administered together with a human malaria vaccine to non-human primates, 7DW8-5 not only exhibited a significantly potent adjuvant effect to enhance the malaria vaccine-induced T-cell responses but also displayed no systemic reactogenicity 32. It is noteworthy that 7DW8-5 combined with a TLR4 agonist, monophosphoryl lipid A (MPLA), displays a potent adjuvant effect and enhances the levels of antigen-specific CD8 + T-cell responses with effector memory function and protective immunity to malaria and cancer 35.
Most recently, we have found that after co-administration of the glycolipid with a malaria vaccine based on radiation-attenuated sporozoites (RASs) of a rodent malaria, in contrast to α-GalCer, 7DW8-5 co-localizes with murine RASs in the draining lymph nodes and induces activation/maturation of DCs that present malaria antigens. This cascade of events resulted in enhancing the levels of malaria-specific CD8 + T-cell response and ultimately the level of protective anti-malaria immunity induced by the RAS vaccine ( Table 1) 34. In terms of translational value, it is exciting to report that when 7DW8-5 and an adenovirus-based human malaria vaccine were co-administered to our cutting-edge humanized mice that possess functional human CD8 + T cells and iNKT cells 37, 7DW8-5 was shown to enhance not only the malaria antigen-specific human CD8 + T-cell response but also the efficacy of the vaccine 37. As expected, the adjuvant effect of 7DW8-5 was more potent than that of α-GalCer in humanized mice 37.
Figure 2. Structure of 7DW8-5 and α-galactosylceramide (α-GalCer).
Table 1. Summary comparing the properties between 7DW8-5 and α-galactosylceramide (α-GalCer) 31– 37.
| 1. 7DW8-5 and α-GalCer possess very similar chemical structures; the only difference is the fatty acyl chain, in which a terminal
fluorinated benzene ring is attached for 7DW8-5 ( Figure 2). 2. 7DW8-5 binds mouse and human CD1d molecules with a 30- to 80-fold higher affinity than α-GalCer and stimulates invariant natural killer T ( iNKT) cells in vitro with a 100-fold higher dose-sparing effect than α-GalCer. 3. 7DW8-5 induces the upregulation of major histocompatibility complex (MHC) II and CD86 expression by dendritic cells (DCs) almost two-fold higher than that induced by α-GalCer. 4. 7DW8-5, but not α-GalCer, can co-localize in the draining lymph nodes (dLNs) with mouse malaria vaccine upon co- administration by the intra-muscular route and induce activation/maturation of lymph node–resident DCs. 5. The co-localization of 7DW8-5 with a malaria vaccine in dLNs results in enhancing malaria-specific CD8 + T-cell responses and ultimately protective anti-malaria immunity induced by the vaccine. 6. 7DW8-5 can enhance malaria-specific CD8 + T-cell responses induced by a human malaria vaccine in non-human primates while displaying no systemic reactogenicity. 7. 7DW8-5 displays an adjuvant effect by enhancing protective CD8 + T-cell responses against WT1 tumor in vivo. 8. 7DW8-5 displays an adjuvant effect in human immune system (HIS) mice by enhancing malaria-specific human CD8 + T-cell response and protective efficacy against hybrid rodent malaria parasites that express human malaria antigen. |
Conclusions
Many candidate vaccines evaluated to date fail to achieve protection against certain human pathogens, such as malaria, and this is primarily due to their poor cellular immunogenicity. In this regard, glycolipids, which bind CD1d molecules and stimulate iNKT cells, have been shown to exert an adjuvant effect on liver vector-based vaccines and also to increase the level of CD8 + T-cell responses induced by the vaccines. Our proof-of-concept data on 7DW8-5 in vitro in parallel with normal mice, in a humanized mouse model, and in non-human primates clearly depict this potent glycolipid as a safe and effective immune-boosting adjuvant for a malaria vaccine, which could support future studies of 7DW8-5 as an adjuvant for other vaccines against HIV, cancer, and autoimmune diseases. Furthermore, it is plausible that this glycolipid adjuvant may add value when it is used in combination with existing adjuvants. Therefore, we hope to facilitate the clinical development of 7DW8-5 as a potent adjuvant to aid in the development of successful vaccines, such as a malaria vaccine, in humans in the near future.
Abbreviations
α-GalCer, α-galactosylceramide; APC, antigen-presenting cell; DC, dendritic cell; iNKT, invariant natural killer T; PyCSP, circumsporozoite protein of Plasmodium yoelii; RAS, radiation-attenuated sporozoite
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Tonya Webb, Department of Microbiology and Immunology, University of Maryland School of Medicine, Baltimore, MD, USA
Arnaud Didierlaurent, GSK Vaccines, Rixensart, Belgium
Randy Brutkiewicz, Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN, USA
Ed Lavelle, Adjuvant Research Group, School of Biochemistry and Immunology, Trinity Biomedical Sciences Institute, Trinity College Dublin, Dublin, Ireland
Funding Statement
This work was supported by a grant from the National Institutes of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 4 approved]
References
- 1. O'Hagan DT, Fox CB: New generation adjuvants--from empiricism to rational design. Vaccine. 2015;33 Suppl 2:B14–20. 10.1016/j.vaccine.2015.01.088 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Di Pasquale A, Preiss S, Tavares Da Silva F, et al. : Vaccine Adjuvants: from 1920 to 2015 and Beyond. Vaccines (Basel). 2015;3(2):320–43. 10.3390/vaccines3020320 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. O'Hagan DT, Friedland LR, Hanon E, et al. : Towards an evidence based approach for the development of adjuvanted vaccines. Curr Opin Immunol. 2017;47:93–102. 10.1016/j.coi.2017.07.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. McKee AS, Marrack P: Old and new adjuvants. Curr Opin Immunol. 2017;47:44–51. 10.1016/j.coi.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Shah RR, Hassett KJ, Brito LA: Overview of Vaccine Adjuvants: Introduction, History, and Current Status. Methods Mol Biol. 2017;1494:1–13. 10.1007/978-1-4939-6445-1_1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Lauterbach H, Ried C, Epstein AL, et al. : Reduced immune responses after vaccination with a recombinant herpes simplex virus type 1 vector in the presence of antiviral immunity. J Gen Virol. 2005;86(Pt 9):2401–10. 10.1099/vir.0.81104-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Pine SO, Kublin JG, Hammer SM, et al. : Pre-existing adenovirus immunity modifies a complex mixed Th1 and Th2 cytokine response to an Ad5/HIV-1 vaccine candidate in humans. PLoS One. 2011;6(4):e18526. 10.1371/journal.pone.0018526 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Bendelac A, Rivera MN, Park SH, et al. : Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–62. 10.1146/annurev.immunol.15.1.535 [DOI] [PubMed] [Google Scholar]
- 9. Brossay L, Chioda M, Burdin N, et al. : CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188(8):1521–8. 10.1084/jem.188.8.1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kronenberg M: Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. 10.1146/annurev.immunol.23.021704.115742 [DOI] [PubMed] [Google Scholar]
- 11. Zajonc DM, Cantu C, 3rd, Mattner J, et al. : Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6(8):810–8. 10.1038/ni1224 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Koch M, Stronge VS, Shepherd D, et al. : The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol. 2005;6(8):819–26. 10.1038/ni1225 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Tsuji M: Glycolipids and phospholipids as natural CD1d-binding NKT cell ligands. Cell Mol Life Sci. 2006;63(16):1889–98. 10.1007/s00018-006-6073-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borg NA, Wun KS, Kjer-Nielsen L, et al. : CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448(7149):44–9. 10.1038/nature05907 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Kawano T, Cui J, Koezuka Y, et al. : Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci U S A. 1998;95(10):5690–3. 10.1073/pnas.95.10.5690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kakimi K, Guidotti LG, Koezuka Y, et al. : Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192(7):921–30. 10.1084/jem.192.7.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, et al. : alpha -galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci U S A. 2000;97(15):8461–6. 10.1073/pnas.97.15.8461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hong S, Wilson MT, Serizawa I, et al. : The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7(9):1052–6. 10.1038/nm0901-1052 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Sharif S, Arreaza GA, Zucker P, et al. : Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7(9):1057–62. 10.1038/nm0901-1057 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Jahng AW, Maricic I, Pedersen B, et al. : Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1789–99. 10.1084/jem.194.12.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh AK, Wilson MT, Hong S, et al. : Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1801–11. 10.1084/jem.194.12.1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawakami K, Kinjo Y, Yara S, et al. : Activation of Valpha14 + natural killer T cells by alpha-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect Immun. 2001;69(1):213–20. 10.1128/IAI.69.1.213-220.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chackerian A, Alt J, Perera V, et al. : Activation of NKT cells protects mice from tuberculosis. Infect Immun. 2002;70(11):6302–9. 10.1128/IAI.70.11.6302-6309.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crowe NY, Smyth MJ, Godfrey DI: A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196(1):119–27. 10.1084/jem.20020092 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, et al. : Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195(5):617–24. 10.1084/jem.20011889 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Hermans IF, Silk JD, Gileadi U, et al. : NKT cells enhance CD4 + and CD8 + T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171(10):5140–7. 10.4049/jimmunol.171.10.5140 [DOI] [PubMed] [Google Scholar]
- 27. Fujii S, Shimizu K, Smith C, et al. : Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198(2):267–79. 10.1084/jem.20030324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silk JD, Hermans IF, Gileadi U, et al. : Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114(12):1800–11. 10.1172/JCI22046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Venkataswamy MM, Baena A, Goldberg MF, et al. : Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 2009;183(3):1644–56. 10.4049/jimmunol.0900858 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Kopecky-Bromberg SA, Fraser KA, Pica N, et al. : Alpha-C-galactosylceramide as an adjuvant for a live attenuated influenza virus vaccine. Vaccine. 2009;27(28):3766–74. 10.1016/j.vaccine.2009.03.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li X, Fujio M, Imamura M, et al. : Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci U S A. 2010;107(29):13010–5. 10.1073/pnas.1006662107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Padte NN, Boente-Carrera M, Andrews CD, et al. : A glycolipid adjuvant, 7DW8-5, enhances CD8+ T cell responses induced by an adenovirus-vectored malaria vaccine in non-human primates. PLoS One. 2013;8(10):e78407. 10.1371/journal.pone.0078407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Venkataswamy MM, Ng TW, Kharkwal SS, et al. : Improving Mycobacterium bovis bacillus Calmette-Guèrin as a vaccine delivery vector for viral antigens by incorporation of glycolipid activators of NKT cells. PLoS One. 2014;9(9):e108383. 10.1371/journal.pone.0108383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li X, Kawamura A, Andrews CD, et al. : Colocalization of a CD1d-Binding Glycolipid with a Radiation-Attenuated Sporozoite Vaccine in Lymph Node-Resident Dendritic Cells for a Robust Adjuvant Effect. J Immunol. 2015;195(6):2710–21. 10.4049/jimmunol.1403017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coelho-Dos-Reis JG, Huang J, Tsao T, et al. : Co-administration of α-GalCer analog and TLR4 agonist induces robust CD8 + T-cell responses to PyCS protein and WT-1 antigen and activates memory-like effector NKT cells. Clin Immunol. 2016;168:6–15. 10.1016/j.clim.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li X, Huang J, Kawamura A, et al. : Co-localization of a CD1d-binding glycolipid with an adenovirus-based malaria vaccine for a potent adjuvant effect. Vaccine. 2017;35(24):3171–7. 10.1016/j.vaccine.2017.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li X, Huang J, Kaneko I, et al. : A potent adjuvant effect of a CD1d-binding NKT cell ligand in human immune system mice. Expert Rev Vaccines. 2017;16(1):73–80. 10.1080/14760584.2017.1256208 [DOI] [PMC free article] [PubMed] [Google Scholar]