Abstract
Velopharyngeal dysfunction (VPD) occurs when the muscular soft palate (velum) and lateral pharyngeal walls are physically unable to separate the oral and nasal cavities during speech production leading to hypernasality and abnormal speech reduction. Because VPD is often associated with overt or submucous cleft palate, it could be present as a subclinical phenotype in families with a history of orofacial clefting. A key assumption to this model is that the overt and subclinical manifestations of the orofacial cleft phenotype exist on a continuum and therefore share common etiological factors. We performed a genome-wide association study in 976 unaffected relatives of isolated CP probands, 54 of whom had VPD. Five loci were significantly (p < 5 × 10−8) associated with VPD: 3q29, 9p21.1, 12q21.31, 16p12.3 and 16p13.3. An additional 15 loci showing suggestive evidence of association with VPD were observed. Several genes known to be involved in orofacial clefting and craniofacial development are located in these regions, such as TFRC, PCYT1A, BNC2 and FREM1. Although further research is necessary, this could be an indication for a potential shared genetic architecture between VPD and cleft palate, and supporting the hypothesis that VPD is a subclinical phenotype of orofacial clefting.
Introduction
Velopharyngeal dysfunction (VPD) refers to an inability to close the opening between the nasal cavity and the oral cavity during speech. This occurs because the muscular soft palate (velum) and lateral pharyngeal walls are physically unable to make a sufficient seal of the oral cavity from the nasal cavity during speech production. As a consequence, air tends to escape into the nasal cavity during speech, resulting in hypernasality and excess air emissions. The causes of VPD are heterogeneous and are the basis of three subtypes of VPD: velopharyngeal incompetency (caused by a lack of neuromotor competency), velopharyngeal mislearning (caused by maladaptive articulatory habits) and velopharyngeal insufficiency (VPI, caused by insufficient tissue or mechanical restriction)1,2. For example, a congenitally short palate and/or deep nasopharynx can alter the geometry of the velopharyngeal apparatus, such that the soft palate is no longer able to effectively create a seal against the posterior wall of the nasopharynx2,3. Although VPD can occur as the result of surgical procedures, such as adenoidectomies, the most common congenital cause of VPD is cleft palate (CP) or submucous cleft palate (smCP), which can occur as isolated malformations or as part of a syndrome4. After primary palatal repair surgeries, approximately 30% of CP patients require additional surgery for VPD1.
VPD can also occur in the absence of an overt orofacial cleft5, and some cases have been reported with autosomal-dominant inheritance6,7. Detailed phenotyping in these “isolated” VPD cases shows that these result from structural deficiencies in the anatomical components that comprise the velopharyngeal mechanism. The genetic basis of isolated VPD is poorly understood and the autosomal-dominant families have yet to be genetically mapped. However, given the association with CP and the structural deficiencies of the palate, genes and pathways implicated in the pathogenesis of secondary palate clefting may provide some clues8,9.
The purpose of the current study is to examine the influence of common genetic variants on VPD. To accomplish this, we performed a genome-wide association study (GWAS) on a sample of unaffected relatives from families with a history of CP or smCP, who had been assessed for VPD.
Materials and Methods
Participants
Our study sample consisted of 976 relatives within three degrees of relatedness of probands with an isolated CP (437 male, 539 female; mean age = 29.7 ± 16.29) and who were not affected with an overt CP by both self-report and in-person assessment of their cleft status. The participants were recruited as part of the larger Pittsburgh Orofacial Cleft Study10 at different US and international sites: Pittsburgh (n = 281), St. Louis (n = 51), Texas (n = 299), Colorado (n = 39), Hungary (n = 99), Colombia (n = 16), Philippines (n = 171), and Puerto Rico (n = 20).
Speech assessment
Structured and spontaneous speech samples were recorded for all participants using a Canon 7D camera (Canon USA, Melville, NY). The structured speech paragraphs in English, Spanish and Tagalog can be found in the online supplemental material. Medical and surgical history was documented for all subjects, with a particular focus on speech pathology and palatal surgery. Using the Pittsburgh Weighted Speech Scale11, the speech samples were rated for the presence of VPD by an experienced speech and language pathologist (MF). A speech score was given based on the presence of audible nasal emission and nasal turbulence, nasality, phonation and articulation patterns. Subjects with a score higher than three (which is the cut-off for clinical significance) were considered to have VPD. Using this threshold, a total of 54 participants (20 males, 34 females) were diagnosed with VPD.
Genotyping, quality control, population structure and imputation
DNA was extracted from saliva or blood, and genotyped for 541787 SNPs on an Illumina HumanCore + Exome array plus 15890 SNPs of custom content covering candidate genes for overt clefts. Genetic data cleaning and quality control analyses were performed as described previously12. In brief, samples were interrogated for genetic sex, chromosomal aberrations, relatedness, genotype call rate, and batch effects. SNPs were interrogated for call rate, discordance among 72 duplicate samples, Mendelian errors among HapMap controls (parent-offspring trios), deviations from Hardy-Weinberg equilibrium, and sex differences in allele frequencies and heterozygosity. Filters applied to genotyped SNPs are described in Supplementary Table S1.
Imputation of non-genotyped variants was performed via IMPUTE213, using haplotypes from the 1000 Genomes Project Phase 3 as the reference. We converted imputed probabilities to most-likely genotypes using a genotype probability threshold of 0.9. We then filtered out imputed SNPs with an info score of <0.5. Masked variant analysis, in which genotyped SNPs were imputed in order to assess imputation quality, indicated high accuracy of imputation. Genetic association with VPD was tested for genotyped and imputed SNPs with MAF >5% and which did not show evidence of extreme deviation for the Hardy-Weinberg equilibrium.
Association analysis
Genetic association with VPD was tested for SNPs with MAF >5% using a mixed-models approach as implemented in EMMAX14, which explicitly models the variance due to the kinship (comprising both the family relatedness and population structure) in the sample. 54 unaffected relatives of patients with CP were diagnosed with VPD and compared to 922 unaffected relatives of patients with CP, who are not showing VPD. Sex, age, age2, and site (as a proxy for language) were included as covariates. Principal components of ancestry were not included because variation due to population structure was already explicitly modeled by the kinship matrix. Autosomal SNP genotypes were modeled additively. For SNPs on the X-chromosome, genotypes were coded as 0, 1, and 2 for females, and were coded as 0 or 2 for males in order to maintain the same scale between sexes. The conventional Bonferroni-corrected threshold of 5 × 10−8 was set for genome-wide statistical significance; 5 × 10−6 was the threshold for suggestive hits.
Functional annotation
Potential genes of interest were identified based on physical proximity of ±500 kb from the lead SNP at each genome-wide significant locus. These genes were queried in the following online databases: The Mouse Genome Informatics (MGI) database15, which was used to annotate expression in relevant tissues and phenotypic consequences, the VISTA enhancer database16, which was used to annotate active enhancer elements in relevant tissues, and OMIM and PubMed, which were used to annotate human phenotypic information. The following genes were considered to be of interest during annotation: genes involved in orofacial clefting, in speech pathology caused by structural and central differences, and in craniofacial development.
Data availability
SNPs used in the current study are available in the dbGAP repository (https://www.ncbi.nlm.nih.gov/gap; accession number phs000774.v1.p1).
Compliance with Ethical Standards
Written informed consent was obtained from all individual participants in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards: University of Pittsburgh IRB #PRO09060553 and IRB0405013 (covering both the Pittsburgh and Hungary sites); UT Health Committee for the Protection of Human Subjects #HSC-DB-09-0508 and #HSC-MS-03-090; Colorado Multiple Institutional Review Board #10-0055, Washington University in St. Louis HRPO #03-087, University of the Philippines Manilla, IRB00002908, FWA00018728; University of Puerto Rico Medical Sciences Campus IRB, IRB protocol 0640111 l. The “Fundacion Clinica Noel de Medellin” IRB approved the study protocol for the subjects in Colombia, an IRB approval number is not available for this site.
Results
Genetic association
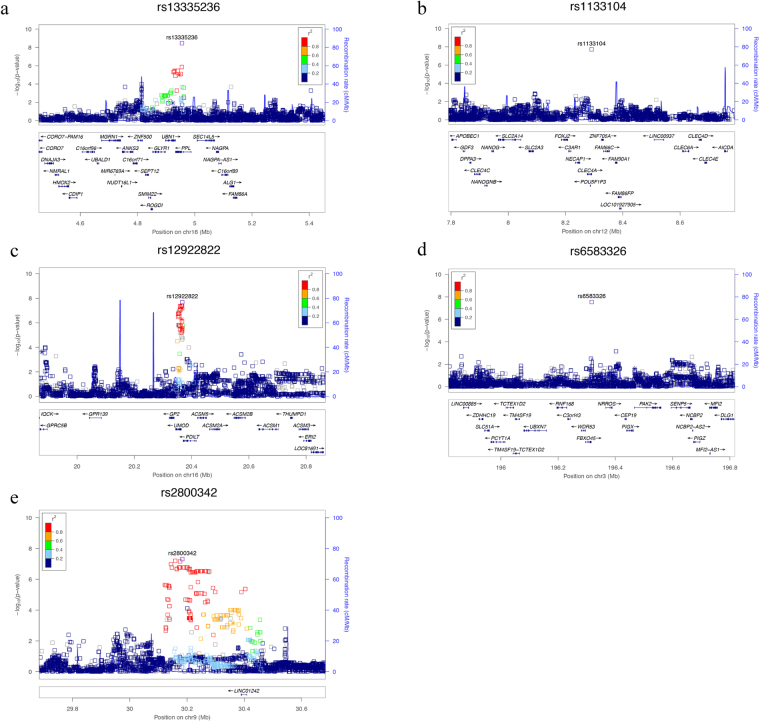
Our GWAS revealed five genome-wide significant associations at loci 3q29 (lead SNP: rs6583326, p = 2.86 × 10−8), 9p21.1 (lead SNP: rs2800342, p = 4.88 × 10−8), 12q21.31 (lead SNP: rs1133104, p = 1.96 × 10−8), 16p12.3 (lead SNP: rs12922822, p = 2.08 × 10−8) and 16p13.3 (lead SNP: rs13335236, p = 3.50 × 10−9) (Table 1). Two of these SNPs were intragenic: SNP rs1133104 (12q21) is located in the last exon of CLEC4A and rs13335236 (16p13) is located intronic in PPL. The LocusZoom plots17 of these results are shown in Fig. 1, and the Manhattan and QQ plots are shown in Supplementary Figure S1. In addition, 15 loci showed a suggestive association (p < 5 × 10−6) with VPD (Table 1). LocusZoom plots of all suggestive associations are displayed in Supplementary Figure S2 and results are described in Supplementary Table S2.
Table 1.
Genome-wide significant (p < 5*10E-08) and suggestive (p < 5*10E-06) associations with VPD.
| Locus | SNP | Chr | BP position | P | A1 | A2 | MAF | Annotated genes |
|---|---|---|---|---|---|---|---|---|
| 3p24.3 | rs13095954 | 3 | 18108126 | 1.05E-07 | G | C | 0.07358 | SATB1 |
| 3q29 | rs6583326 | 3 | 196317308 | 2.86E-08 | T | C | 0.1434 | TFRC, PCYT1A |
| 4q22.3 | rs11727234 | 4 | 96807975 | 6.95E-07 | G | T | 0.05155 | — |
| 5p13.1 | rs56276612 | 5 | 40427385 | 1.19E-07 | T | G | 0.09129 | — |
| 6p12.2 | rs10948810 | 6 | 54176227 | 4.08E-07 | C | T | 0.05169 | — |
| 6q26 | rs3900809 | 6 | 161135202 | 2.94E-07 | G | A | 0.05169 | MAP3K4, PARK2 |
| 8q21.2 | rs34927688 | 8 | 8622794 | 7.59E-07 | G | A | 0.07439 | — |
| 9p21.1 | rs2800342 | 9 | 30183553 | 4.88E-08 | T | A | 0.05983 | — |
| 9p22.2 | rs1538101 | 9 | 16710870 | 3.39E-07 | G | A | 0.05169 | BNC2 |
| 9p22.3 | rs72702916 | 9 | 14484998 | 1.47E-07 | C | G | 0.07164 | FREM1 |
| 11q25 | rs56160206 | 11 | 133921939 | 8.02E-08 | CA | C | 0.1263 | GLB1L2, B3GAT1 |
| 12q21.31 | rs1133104 | 12 | 8291122 | 1.96E-08 | G | T | 0.1712 | — |
| 14q13.1 | rs73257280 | 14 | 32270651 | 6.48E-07 | C | CT | 0.05113 | — |
| 14q23.3 | chr14:66527789 | 14 | 66527789 | 4.48E-07 | T | A | 0.05236 | — |
| 16p12.1 | rs77085399 | 16 | 22002367 | 9.89E-07 | C | T | 0.16 | — |
| 16p12.3 | rs12922822 | 16 | 20367645 | 2.08E-08 | T | C | 0.1498 | — |
| 16p13.3 | rs13335236 | 16 | 4955256 | 3.50E-09 | C | T | 0.05733 | NAGPA, RBFOX1 |
| 18q21.32 | rs11877326 | 18 | 56503556 | 2.64E-07 | A | G | 0.07174 | — |
| 18q22.1 | rs66549549 | 18 | 62628423 | 4.39E-07 | T | G | 0.05215 | — |
| 22q12.1 | rs9613645 | 22 | 28947402 | 7.57E-08 | T | A | 0.08383 | TTC28, KREMEN1 |
Figure 1.
(a–e) LocusZoom plots of genome-wide significant (p < 5*10E-08) associations. LocusZoom plots show the association (left y-axis; log10-transformed p-values). Genotype SNPs are represented by stars, imputed SNPs are represented by squares. Shading of the points represent the linkage disequilibrium (r2, based on the 1000 Genomes Project) between each SNP and the top SNP. The blue overlay shows the recombination rate (right y-axis). Positions of genes are shown below the plot.
Several possible biologically relevant candidate genes (e.g., PCYT1A, FREM1) were located at the five genome-wide significant loci. To ensure a more comprehensive evaluation, genes within 500 kb of each lead SNP were queried for possible roles in orofacial clefting, speech development, and/or development of the nasopharyngeal region. Corroborating evidence, such as expression in relevant tissues or putative roles in relevant human syndromes, was found for eight of the 20 loci as discussed below.
Discussion
This GWAS of VPD identified five genome-wide significant associations and 15 suggestive associations. We were not able to replicate these results, because, to our best knowledge, no suitable replication cohort is available. Although the genetic basis of VPD is largely unknown, several of these loci were located near potentially relevant candidate genes, including some previously implicated in orofacial clefting. PCYT1A, located 350 kb upstream of the lead SNP at the 3q29 locus, has been shown to be associated with increased risk for NSCL/P, through an epistatic interaction with BHMT18. Furthermore, a microdeletion in this locus is furthermore associated with a delayed development, especially in speech19. The association at this locus, however, is based on a single imputed SNP. We also observed borderline associations with several variants in TTC28 (locus 22q12.1). Conte and colleagues found an association between copy number variants in TTC28 and orofacial development20. A microdeletion in the same genetic region was found in a child with Pierre-Robin Sequence (including cleft palate) and Neurofibromatosis type 221. Moreover, the ttc28 mouse mutant shows cranial abnormalities with abnormal maxillary morphology, further suggesting the potential for this gene to impact palatogenesis.
Several other associated loci contained candidate genes known to be involved more generally with craniofacial development. For instance, FREM1 (near locus 9p22.3) has been shown to play a role in the fusion of the nasal processes during gestation22, and was implicated in human upper lip morphology in a recent GWAS of facial shape23. In humans, mutations in this gene result in several Mendelian conditions affecting the midline facial structures, such as BNAR syndrome (OMIM #608980) and trigonocephaly (OMIM #190440)22,24. Trigonocephaly is in 34% of the cases associated with speech and/or language delay25. KREMEN1 (near locus 22q12.2) is a modulator of WNT signaling, crucial for neural tube closure26. TFRC, located 500 kb from the lead SNP of locus 3q29, is involved in craniofacial morphogenesis by regulating TGFß and BMP signaling activation27.
Interestingly, one of the identified loci contained genes involved in the neural control of speech production. NAGPA at locus 16p13.3 is involved in stuttering28 and focal epilepsy with speech disorders29, respectively. Neurophysiological dysfunction is a known cause of VPD4, so it is possible that variants in these genes might influence the movement of the muscles comprising the soft palate.
We have hypothesized that isolated VPD may represent a subclinical phenotype in families with a history of orofacial clefting10. In the context of orofacial clefting, subclinical phenotypes can be conceptualized as incomplete (or intermediate) expressions of the risk factors for the overt defect or as pleiotropic expressions in related tissues/structures. Such subclinical phenotypes have now been extensively documented in the clinically unaffected relatives of affected individuals within families affected with orofacial clefts10,30. A key assumption is that the overt and subclinical manifestations of the orofacial cleft phenotype share common etiological factors. If this model is correct, then at least some of the genes that underlie orofacial cleft susceptibility, particularly those involved in clefts of the secondary palate, may also underlie VPD.
If the hypothesis is correct that CP and VPD (partially) share their genetic etiology, it may be valuable to investigate the effect of genes known to be involved in the etiology of CP, in subjects with VPD. However, our understanding of the genetic basis of isolated CP is largely incomplete. Associations between CP and SNPs in FAF131, FOXE132, and GRHL39,33 have been described. No variants in or near these genes were associated with VPD in our study cohort. This does not preclude the possibility that additional (yet to be identified) variants involved in CP will contribute to VPD and vice versa. Unlike overt CP, smCP is usually not immediately diagnosed at birth. A recent candidate gene study of six SNPs in loci strongly associated with orofacial clefting, did not show any association between these SNPs and smCP34, leaving the genetic basis of smCP unknown. Since there is a high prevalence of VPD in patients with smCP, the genetic loci identified in this study are also good candidate genes for both overt CP and smCP.
This study represents the first attempt to identify genetic variants associated with VPD. Although we observed several promising associations, we are not currently able to independently replicate any of these signals due to a lack of additional datasets. Our study was also limited by a lack of objective assessments of VPD, such as acoustic nasalence data during speech assessed by nasometry, or visualization of the velopharyngeal mechanism during speech assessed by video nasopharyngeal endoscopy. Including these types of assessments may yield additional insights into the genetic basis of VPD. Furthermore, the presence of smCP could not be determined in this dataset. Thus, it is possible that the presence of VPD is actually due to undiagnosed smCP. Although we hypothesize that VPD may be a subclinical phenotype of CP, we did not find strong evidence of association between the signals identified in this study and CP.
Electronic supplementary material
Acknowledgements
The authors want to thank the dedicated staff, collaborators, and participants for their contribution to the study, with a special remembrance of Dr. Andrew Czeizel’s contributions. This work was supported by grants from the National Institutes of Health (X01-HG00784 [MLM, EF], R01-DE016148 [MLM, SMW], R21-DE016930 [MLM], R00-DE025060 [EJL], K99-DE024571 [CJB], S21-MD001830 [CJB], U54-MD007587 [CJB]. Genotyping and data cleaning was provided via a contract to the Center for Inherited Disease Research: HHSN268201200008I. Additional support provided by: an intramural grant from the Research Institute of the Children’s Hospital of Colorado [FWD]; operating cost support in the Philippines was provided by the Institute of Human Genetics, National Institutes of Health, University of the Philippines, Manila [CP].
Author Contributions
J.C., J.R.: analysis, results interpretation, manuscript writing. M.L.K., E.F., J.R.S.: statistical guidance. M.F., J.T.H., C.P., F.W.D., C.J.B.: data collection. M.F.: speech sampling. B.E., J.A.: data management. E.F., E.J.L., J.R.S., S.M.W., M.L.M.: conceptual ideas, results interpretation, manuscript revision.
Competing Interests
The authors declare no competing interests.
Footnotes
Jonathan Chernus and Jasmien Roosenboom contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26880-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Seth M. Weinberg, Email: smwst46@pitt.edu
Mary L. Marazita, Email: marazita@pitt.edu
References
- 1.Naran S, Ford M, Losee JE. What’s New in Cleft Palate and Velopharyngeal Dysfunction Management? Plast. Reconstr. Surg. 2017;139:1343e–1355e. doi: 10.1097/PRS.0000000000003335. [DOI] [PubMed] [Google Scholar]
- 2.Kummer A. Types and Causes of Velopharyngeal Dysfunction. Semin. Speech Lang. 2011;32:150–158. doi: 10.1055/s-0031-1277717. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro RS. Velopharyngeal insufficiency starting at puberty without adenoidectomy. Int. J. Pediatr. Otorhinolaryngol. 1980;2:255–60. doi: 10.1016/0165-5876(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 4.Kummer AW, Marshall JL, Wilson MM. Non-cleft causes of velopharyngeal dysfunction: Implications for treatment. Int. J. Pediatr. Otorhinolaryngol. 2015;79:286–295. doi: 10.1016/j.ijporl.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Peterson-Falzone SJ. Velopharyngeal inadequacy in the absence of overt cleft palate. J. Craniofac. Genet. Dev. Biol. Suppl. 1985;1:97–124. [PubMed] [Google Scholar]
- 6.Kannu P, Aftimos S, Winship I. Autosomal dominant velopharyngeal insufficiency: father-to-son transmission confirmed. Clin. Genet. 2003;64:522–3. doi: 10.1046/j.1399-0004.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 7.Vantrappen G, et al. Autosomal dominant isolated velopharyngeal insufficiency. Clin. Genet. 2002;61:74–6. doi: 10.1034/j.1399-0004.2002.610115.x. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney W, Lanier S, Purnell C, Gosain A. Genetics of Cleft Palate and Velopharyngeal Insufficiency. J. Pediatr. Genet. 2015;4:009–016. doi: 10.1055/s-0035-1554978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leslie EJ, et al. A multi-ethnic genome-wide association study identifies novel loci for non-syndromic cleft lip with or without cleft palate on 2p24.2, 17q23 and 19q13. Hum. Mol. Genet. 2016;25:ddw104. doi: 10.1093/hmg/ddw104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg SM, et al. The Pittsburgh Oral-Facial Cleft Study: Expanding the Cleft Phenotype. Background and Justification. Cleft Palate-Craniofacial J. 2006;43:7–20. doi: 10.1597/04-122R1.1. [DOI] [PubMed] [Google Scholar]
- 11.McWilliams BJ, et al. A comparative study of four methods of evaluating velopharyngeal adequacy. Plast. Reconstr. Surg. 1981;68:1–10. doi: 10.1097/00006534-198107000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Leslie EJ, et al. Identification of Functional Variants for Cleft Lip with or without Cleft Palate in or near PAX7, FGFR2, and NOG by Targeted Sequencing of GWAS Loci. Am. J. Hum. Genet. 2015;96:397–411. doi: 10.1016/j.ajhg.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang HM, et al. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 2010;42:348–54. doi: 10.1038/ng.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eppig JT. Mouse Genome Informatics (MGI) Resource: Genetic, Genomic, and Biological Knowledgebase for the Laboratory Mouse. ILAR J. 2017;58:17–41. doi: 10.1093/ilar/ilx013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visel A, Minovitsky S, Dubchak I, Pennacchio LA. VISTA Enhancer Browser–a database of tissue-specific human enhancers. Nucleic Acids Res. 2007;35:D88–D92. doi: 10.1093/nar/gkl822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pruim RJ, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–7. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mostowska A, Hozyasz KK, Biedziak B, Misiak J, Jagodzinski PP. Polymorphisms located in the region containing BHMT and BHMT2 genes as maternal protective factors for orofacial clefts. Eur. J. Oral Sci. 2010;118:325–332. doi: 10.1111/j.1600-0722.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- 19.Ballif BC, et al. Expanding the clinical phenotype of the 3q29 microdeletion syndrome and characterization of the reciprocal microduplication. Mol. Cytogenet. 2008;1:8. doi: 10.1186/1755-8166-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conte F, et al. Systematic analysis of copy number variants of a large cohort of orofacial cleft patients identifies candidate genes for orofacial clefts. Hum. Genet. 2016;135:41–59. doi: 10.1007/s00439-015-1606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson TB, et al. Microdeletion del(22)(q12.2) encompassing the facial development-associated gene, MN1 (meningioma 1) in a child with Pierre-Robin sequence (including cleft palate) and neurofibromatosis 2 (NF2): a case report and review of the literature. BMC Med. Genet. 2012;13:19. doi: 10.1186/1471-2350-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alazami AM, et al. FREM1 mutations cause bifid nose, renal agenesis, and anorectal malformations syndrome. Am. J. Hum. Genet. 2009;85:414–8. doi: 10.1016/j.ajhg.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MK, et al. Genome-wide association study of facial morphology reveals novel associations with FREM1 and PARK2. PLoS One. 2017;12:e0176566. doi: 10.1371/journal.pone.0176566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vissers LELM, et al. Heterozygous Mutations of FREM1 Are Associated with an Increased Risk of Isolated Metopic Craniosynostosis in Humans and Mice. PLoS Genet. 2011;7:e1002278. doi: 10.1371/journal.pgen.1002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelleher MO, et al. Behavioral, developmental, and educational problems in children with nonsyndromic trigonocephaly. J. Neurosurg. Pediatr. 2006;105:382–384. doi: 10.3171/ped.2006.105.5.382. [DOI] [PubMed] [Google Scholar]
- 26.Kimura-Yoshida C, Mochida K, Ellwanger K, Niehrs C, Matsuo I. Fate Specification of Neural Plate Border by Canonical Wnt Signaling and Grhl3 is Crucial for Neural Tube Closure. EBioMedicine. 2015;2:513–27. doi: 10.1016/j.ebiom.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei R, et al. Transferrin receptor facilitates TGF-β and BMP signaling activation to control craniofacial morphogenesis. Cell Death Dis. 2016;7:e2282. doi: 10.1038/cddis.2016.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raza MH, et al. Mucolipidosis types II and III and non-syndromic stuttering are associated with different variants in the same genes. Eur. J. Hum. Genet. 2016;24:529–34. doi: 10.1038/ejhg.2015.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner SJ, Morgan AT, Perez ER, Scheffer IE. New Genes for Focal Epilepsies with Speech and Language Disorders. Curr. Neurol. Neurosci. Rep. 2015;15:35. doi: 10.1007/s11910-015-0554-0. [DOI] [PubMed] [Google Scholar]
- 30.Roosenboom J, et al. Review: Facial endophenotypes in non-syndromic orofacial clefting. B-ENT. 2015;11:173–182. [PubMed] [Google Scholar]
- 31.Ghassibe-Sabbagh M, et al. FAF1, a gene that is disrupted in cleft palate and has conserved function in zebrafish. Am. J. Hum. Genet. 2011;88:150–61. doi: 10.1016/j.ajhg.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludwig KU, et al. Strong Association of Variants around FOXE1 and Orofacial Clefting. J. Dent. Res. 2014;93:376–381. doi: 10.1177/0022034514523987. [DOI] [PubMed] [Google Scholar]
- 33.Mangold E, et al. Sequencing the GRHL3 Coding Region Reveals Rare Truncating Mutations and a Common Susceptibility Variant for Nonsyndromic Cleft Palate. Am. J. Hum. Genet. 2016;98:755–762. doi: 10.1016/j.ajhg.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiter R, et al. A post GWAS association study of SNPs associated with cleft lip with or without cleft palate in submucous cleft palate. Am. J. Med. Genet. Part A. 2015;167:670–673. doi: 10.1002/ajmg.a.36891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
SNPs used in the current study are available in the dbGAP repository (https://www.ncbi.nlm.nih.gov/gap; accession number phs000774.v1.p1).