Abstract
Math Anxiety (MA) is characterized by a negative emotional response when facing math-related situations. MA is distinct from general anxiety and can emerge during primary education. Prior studies typically comprise adults and comparisons between high- versus low-MA, where neuroimaging work has focused on differences in network activation between groups when completing numerical tasks. The present study used voxel-based morphometry (VBM) to identify the structural brain correlates of MA in a sample of 79 healthy children aged 7–12 years. Given that MA is thought to develop in later primary education, the study focused on the level of MA, rather than categorically defining its presence. Using a battery of cognitive- and numerical-function tasks, we identified that increased MA was associated with reduced attention, working memory and math achievement. VBM highlighted that increased MA was associated with reduced grey matter in the left anterior intraparietal sulcus. This region was also associated with attention, suggesting that baseline differences in morphology may underpin attentional differences. Future studies should clarify whether poorer attentional capacity due to reduced grey matter density results in the later emergence of MA. Further, our data highlight the role of working memory in propagating reduced math achievement in children with higher MA.
Introduction
Math anxiety (MA) is characterised by negative emotional response such as fear and tension when facing math-related situations, which cannot be reduced to either general anxiety or test anxiety1. It disrupts mathematical performance irrespective of gender2, and can emerge in the primary school years3,4. Depending on the extent of MA, the negative impact of MA could have far-reaching consequences beyond academic achievements5. Given that a significant variation in the level of MA is contributed by genetic factors6, understanding the neurocognitive basis of individual differences in MA may shed light on its causal pathway.
To date, there are a limited number of neuroimaging studies, which are mainly based on adults and comparisons of neural response between groups of high and low levels of MA. In adults, high- compared with low-level MA has been shown to be associated with increased activity in bilateral posterior insula, brain areas linked with threat and pain processing, when anticipating mathematical tasks7. High- compared to low-level MA has also been associated with stronger deactivation within the default mode network during tasks that require additional inhibitory functions, possibly reflecting depletion of working memory resources4. Further, increased activity in frontoparietal regions in high-level MA adults when anticipating mathematical tasks has been shown to correspond with reduced performance deficits, suggesting the role of cognitive control in MA8. In children9, high-level MA has been associated with hyperactivity in the right amygdala when solving mathematical problems, and increased connectivity between this and the ventromedial prefrontal cortex, areas implicated in the processing and regulation of negative emotions. Compared with low-level MA, high-level MA has been linked with decreased activity in brain areas involved in working memory and attention, including the dorsolateral prefrontal cortex, and reduced activity in posterior parietal areas, critical to numerical processing. Furthermore, brain stimulation over the dorsolateral prefrontal cortex has shown the ability to improve arithmetic performance and reduced cortisol level in those with high-level MA10. To our knowledge, there are no prior published studies assessing the link between brain structure and MA in children or adults.
A recent review11 highlighted that there are specific aspects of numerical and executive function that might explain varying degrees of MA: lower working memory capacity, reduced attentional control, lower inhibitory control and a deficit in low-level numerical representations. Furthermore, increasing levels of MA may negatively affect mathematical achievement, via disruption of core executive functions and/or deficit in low-level numerical representations. Using data collected from a larger study, we sought to test the strength of association made by those predictions in that review, to better understand the neural and cognitive factors that are associated with the degree of anxiety towards mathematics. We aimed to identify the structural brain correlates of the level of MA in a typical school population, and to determine how brain structure mediates the relationship between those cognitive functions that are most strongly predictive of the level of MA. Much of the prior literature has consisted of between-group comparisons and, whilst these have provided important insights on the plausible neural mechanisms of MA, the neural and cognitive architecture that contributes to individual differences in MA, and its association with mathematical achievement, remains unclear.
The present study comprises brain structure and MA data from 79 healthy, Spanish children. The Behavioural Rating Inventory of Executive Function (BRIEF)12,13 was used to profile each child’s behavior in specific domains of executive function. The BRIEF indices that were of primary interest for the present study were: inhibitory control and impulsivity (INHBIT), ability to switch and alternate attention (SHIFT) and on-line, representational memory (WORKING MEMORY). The BRIEF is regularly used in clinical and education settings, where higher scores indicate greater difficulty, and therefore lower capacity, in each domain. Numerical representation skills were assessed using a number line task, consisting of positioning numbers on an analogue scale (PN)14, mathematical achievement was determined using the Woodcock Johnson III Achievement (WJ)15 and the level of MA was established via the Math Anxiety Scale (Math-AS)16. Voxel-based morphometry (VBM) was used to identify brain-structure correlates of MA.
Method
Participants
Participants were recruited through two state primary schools in Murcia, Spain, as part of a wider study17–19. The primary sample comprised 137 Spanish children, aged 7–12 years (2nd – 6th grade). Written, informed consent was obtained from parents prior to acquiring any data, and verbal consent reobtained immediately prior to data acquisition. Parents were advised that they would be informed by the hospital Radiologist if any clinically relevant abnormalities were detected. T1-weighted structural MRI data were acquired from an initial sample comprising 110 children whose parents gave previous informed consent. Two were not included in the current study as they were reported to be bilingual, which may affect the measurement of math anxiety and numerical achievement. A further 7 were excluded due to learning disabilities. Note that children with learning disabilities were diagnosed before our study and were receiving special education from their schools. We administered tests on all children to ensure that no one felt excluded. We only analysed data of children without learning disabilities. Following exclusion due to unsatisfactory image quality resulting from movement- or other imaging artefacts (n = 21) or neuro-incidental findings (n = 1), the final sample comprised 79 children (age M = 115.20, SD = 14.13; males = 50.6%; right-handed = 88.6%). The study was approved by the University of Murcia Ethics Committee, and it was conducted in accordance with the approved guidelines and the Declaration of Helsinki.
Materials
Measures that were modeled in the current study include math anxiety, mathematical abilities and working memory.
Math Anxiety
MA was assessed using the Math-AS (known also as the EAM, Escala de Ansiedade a Matemática16). It consists of 25 items that describe situations that are commonly experienced by elementary and high school students during their math lessons. This scale measures the variations in the degrees of math anxiety, from absence to extreme math anxiety. This task was translated from Portuguese into European Spanish by a speaker fluent in both languages (FHS) to enable cross-cultural adaptation. Children indicated the intensity of their response to each item on a five-point Likert scale by crossing out one of the following: (1) None (2) Low (3) Moderate (4) High and (5) Very High. The score was the sum of all points from the 25 questions. This measure has been shown to have accurate validity and reliability when used with children20. Note that we could not control for general anxiety in our analyses however, as we did not have normative data for children below 9 years old using the State-Trait Anxiety Inventory for Children (STAI-CH)21. To be confident that we measured MA and not general anxiety trait, we ran a correlation analysis on the standardised scores of the 50 out of 79 children. We found a lack of correlation between general anxiety (STAI-CH) and MA, rp = 0.07, p = 0.63.
Numerical Cognition
Math Achievement
Children’s math abilities were assessed using the Spanish version of the Woodcock-Johnson III (WJ-III) Achievement (ACH) battery15, which has been validated for the use of children aged 6–13 years in Spain22. It comprises 4 subtests: calculation, math fluency, quantitative concepts and applied problems (see Supplementary Information: Method). The raw scores of each subtest were transformed into W scores23 following the Rasch’s measurement model24,25. We used the composite score of all 4 subtests in our analyses.
Numerical Representation
Numerical representation was assessed using a number line task, consisting of positioning numbers on an analogue scale (PN)14. The participants were required to map numbers on a vertical line that was marked with “0” at the bottom and “100” at the top. In half of the trials, the line was further marked with 4 horizontal lines at different locations to assist children with number mapping. Children were required to indicate the position of an Arabic numeral, orally or visually presented by the experimenter, by pointing to a specific location on the line. There were 12 trials in this task.
Executive Function
We used parents’ rating of children’s online memory (WORKING MEMORY), attention (SHIFT) and inhibitory control (INHIBIT) using the Spanish version of the Behavioural Rating Inventory of Executive Function, BRIEFTM 12,13, which assesses the executive functioning of children between 5–18 years old. It contains 86 items in eight non-overlapping clinical scales and two validity scales.
Procedure
Cognitive and Achievement Testing
Children’s performance on a range of behavioural, cognitive and achievement tasks was assessed prior to collecting the structural MRI scans. Testing was conducted during the Autumn term by six trained assistants with children in groups of two.
Analysis of Demographic, Cognitive and Achievement Data
Several of the measures resulted in positively skewed data. Parametric statistics were combined with permutation testing as this approach, in contrast with non-parametric analyses, has been shown to maximally reduce type I and type II errors26. Confidence intervals (CIs) were estimated using the bias-corrected and accelerated (BCa) percentile bootstrap method (10,000 samples).
The cognitive and achievement data were analysed using SPSS, version 22. The mediation analysis was conducted using the Process macro for SPSS (v2.16.3), available from http://www.processmacro.org/index.html following a published analysis pipeline27. Ten-thousand bootstrap resamples were used to generate bias-corrected, 95% confidence intervals.
Neuroimaging Acquisition
The participants were fitted with ear plugs and soft foam padding used to minimize head movement during the scan. Participants were asked to remain as still as possible for the duration of the scan, and a parent sat beside their child throughout. A T1-weighted image was acquired for each participant using a 1.5 T GE HDX scanner with an 8-channel, phased array, transmit-receive head coil. A 3D FSPGR BRAVO sequence was used to achieve whole brain coverage, composed of 142 axially oriented slices with a reconstructed voxel size of 1 × 1 × 1 mm3, where TR = 12.4 ms, TE = 5.2 ms, flip angle = 12°.
Neuroimaging Analysis using Voxel-Based Morphometry
The MRI data were analyzed using the FMRIB Software Library (FSL, version 6.0.0; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Non-brain tissue was removed from the structural images and an initial weak bias field correction applied using FSL’s anatomy pipeline (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/fsl_anat). These brain extracted, bias corrected images were then fed into the second and subsequent stages of FSL-VBM28 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimised VBM protocol29. The images were grey matter-segmented and registered to the MNI-152 standard space using non-linear registration30. The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific grey matter template. All native grey matter images were then non-linearly registered to this study-specific template and “modulated” to correct for local expansion (or contraction) due to the non-linear component of the spatial transformation. The modulated grey matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm, Full-Width-Half-Maximum (FWHM) ~7 mm. Finally, voxelwise general linear modelling was applied using Randomise31 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise), which permits permutation-based non-parametric testing, correcting for multiple comparisons across space. Here, 10,000 permutations of the data were generated to test against the null. Threshold-free cluster enhancement (TFCE)32 was used to identify cluster-like structures, taking family-wise error rate (FWE) corrected p-values < 0.05. To avoid any labelling bias, probabilistic anatomical descriptors were determined using FSL Atlas Query (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlasquery). Anatomical labels were output for voxels that had survived multiple-comparison correction. Cluster peak information was extracted using FSL’s Cluster tool. See Supplementary Information: Method for a detailed description of the GLM analyses.
Data availability
The research meta-data supporting this publication are available on the Open Science Framework repository, see DOI 10.17605/OSF.IO/PDFJE. The senior author, LJF, may be contacted regarding the wider dataset.
Results
Math Anxiety and its Association with Demographic, Cognitive and Numerical Factors
Table 1 outlines the sample’s demographic, numerical and cognitive characteristics (see also Supplementary Information: Table S1 for a detailed breakdown by grade). Typically, the level of MA within the sample was low. An independent-samples t-test determined that there was no difference in the mean level of MA between the sexes (t(65.569) = −1.063, p = 0.292, 95% CI −13.21, 3.569). Age was positively associated with the level of MA (r(79) = 0.237, p = 0.035, CI 0.031, 0.433), consistent with previous studies33 (see Supplementary Information: Fig. S1).
Table 1.
Sample Characteristics.
| Domain | Scale/Index | n | mean | SD | min | max |
|---|---|---|---|---|---|---|
| Demographic | Age (months) | — | 115.20 | 14.13 | 95.00 | 145.00 |
| School grade | — | 4 | — | 2 | 6 | |
| Sex (M/F) | 40/39 | — | — | — | — | |
| Handedness (L/R) | 9/70 | — | — | — | — | |
| Numerical Cognition | Math Anxiety Scale | 79 | 43.52 | 19.35 | 25 | 101 |
| Woodcock-Johnson III Achievement | 79 | 128.15 | 29.64 | 65 | 208 | |
| Number line task | 73 | 7.51 | 2.18 | 2.00 | 10.50 | |
| Executive Function (BRIEF) | Initiate | 71 | 12.92 | 2.99 | 8 | 22 |
| Working Memory* | 71 | 17.49 | 4.62 | 10 | 27 | |
| Plan | 71 | 19.87 | 5.43 | 12 | 33 | |
| Organization | 71 | 10.75 | 3.28 | 6 | 18 | |
| Monitor | 71 | 13.63 | 3.20 | 8 | 22 | |
| Inhibit* | 71 | 15.34 | 3.53 | 10 | 27 | |
| Shift* | 71 | 12.69 | 3.05 | 8 | 21 | |
| Emotional Control | 71 | 16.49 | 4.21 | 10 | 28 | |
| Behavioral Regulation Index | 71 | 43.96 | 9.41 | 18 | 69 | |
| Metacognition Index | 71 | 74.69 | 16.70 | 48 | 118 | |
| Global Executive Composite | 71 | 119.21 | 24.32 | 79 | 184 |
Note. * indicates BRIEF indices of primary interest due to prior published associations with Math Anxiety.
To assess the relationship between MA, numerical- and cognitive-function we conducted a series of partial correlations. Each of the three BRIEF indices of interest (INHIBIT, SHIFT, WORKING MEMORY), plus the PN and WJ were correlated with the Math-AS score (controlling for age and biological sex). SHIFT, WORKING MEMORY and WJ were statistically significant after applying a Bonferroni correction (Table 2).
Table 2.
Association between Math Anxiety, Numerical and Cognitive Indices.
| Domain | Math Anxiety (MA) | Numerical representation (PN) | Math Achievement (WJ) | BRIEF indices | |||
|---|---|---|---|---|---|---|---|
| Inhibitory Control (INHIBIT) | Attentional Control (SHIFT) | Online Memory (WORKING MEMORY) | |||||
| Math Anxiety (MA) | Pearson’s r | — | −0.112 | −0.302 | 0.278 | 0.320 | 0.313 |
| p-value | — | 0.351 | 0.008* | 0.021 | 0.007* | 0.009* | |
| Numerical Representation (PN) | Pearson’s r | — | 0.384 | −0.189 | −0.155 | −0.321 | |
| p-value | — | 0.002 | 0.138 | 0.226 | 0.010 | ||
| Math Achievement (WJ) | Pearson’s r | — | −0.197 | −0.127 | −0.465 | ||
| p-value | — | 0.104 | 0.299 | <0.001 | |||
| Inhibitory Control (INHIBIT) | Pearson’s r | — | 0.452 | 0.483 | |||
| p-value | — | <0.001 | <0.001 | ||||
| Attentional Control (SHIFT) | Pearson’s r | — | 0.602 | ||||
| p-value | — | <0.001 | |||||
| Online Memory (WORKING MEMORY) | Pearson’s r | — | |||||
| p-value | — | ||||||
Note. p-values reflect two-tailed partial correlation analyses, where age and biological sex was held constant. Degrees of freedom (df) = 69 for MA * PN; df = 75 for MA * WJ; df = 67 for all BRIEF indices. * indicates associations that survive Bonferroni corrected alpha for the 5 primary analyses of interest (first row; Bonferroni corrected alpha, p < 0.01). BCa Bootstrapped 95% CI (10,000 samples) for statistically significant, primary MA analyses: MA * WJ lower −0.455 upper −0.133; MA * SHIFT lower 0.074, upper 0.512; MA * WORKING MEMORY lower 0.052, upper 0.529.
Math Anxiety and its Association with the Brain
VBM was used to identify the structural correlates of MA. A general linear model (GLM) comprising Math-AS score and the nuisance variables, age, biological sex, recruitment source and handedness was created. Contrasts for positive and negative associations between grey matter volume (GMV) and MA were computed, where corrections were applied for multiple comparisons across the brain, and adjustment to correct for running two contrasts using a Bonferroni correction. A whole brain analysis did not identify any regions that were positively associated with MA. Four clusters demonstrated a negative association between GMV and MA, which encompassed only cortical grey matter (Table 3 and Fig. 1). The largest cluster spanned both hemispheres across occipital and parietal cortices, including a section running anterior-posterior along the left anterior intraparietal sulcus (IPS), in areas hIP1 and hIP3, as defined by the Juelich Histological Atlas. A second, smaller cluster was identified in the right visual cortices, encompassing extrastriate areas and additionally encompassing the left anterior IPS. Lastly, a small, left lateralized cluster was identified in the inferior parietal lobule. The Bonferroni correction had split this latter cluster, resulting in a further cluster in the lateral occipital cortex. As this comprised a single voxel (MNI: −18, −88, 42), this voxel was excluded from subsequent analyses.
Table 3.
Probabilistic Labels for Brain Regions where Grey Matter Volume is Negatively Associated with Math Anxiety.
| HO Anatomical Region | Hemi (L/R) | Cluster Size (voxels) | p-value | t-value | MNI Coordinates of Cluster Peak | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Lingual Gyrus* | R | 217 | 0.006 | 5.62 | 12 | −66 | −6 |
| Intracalcarine Cortex | R | ||||||
| Occipital Fusiform Gyrus | R | ||||||
| Temporal Occipital Fusiform Cortex | R | ||||||
| Precuneous Cortex | R | ||||||
| Cuneal Cortex* | L | 1446 | 0.002 | 5.11 | −8 | −82 | 20 |
| Cuneal Cortex | R | ||||||
| Lateral Occipital Cortex sup div (inc. anterior intraparietal sulcus hIP1&3a) | L | ||||||
| Precuneous Cortex | L & R | ||||||
| Occipital Pole | L & R | ||||||
| Supracalcarine Cortex | L & R | ||||||
| Intrcalcarine Cortex | L | ||||||
| Superier Parietal Lobule (inc. anterior intraparietal sulcus hIP1&3a) | L | ||||||
| Angular Gyrus | L | ||||||
| Lingual Gyrus | L | ||||||
| Lateral Occipital Cortex* (inc. anterior intraparietal sulcus hIP1&3 a ) | L | 76 | 0.017 | 4.91 | −42 | −64 | 34 |
| Angular Gyrus | L | ||||||
| Supramarginal Gyrus, posterior division | L | ||||||
Note. Anatomical labels taken from the Harvard-Oxford (HO) Atlas bundled with FSL 6.0.0. *Cluster peak. aReflects anatomical label from Jeulich Histological Atlas available with FSL 6.0.0. p- and t-values reflect cluster peak. Labels are only reported from regions which survived correction at the voxel level, and subsequent Bonferroni correction.
Figure 1.
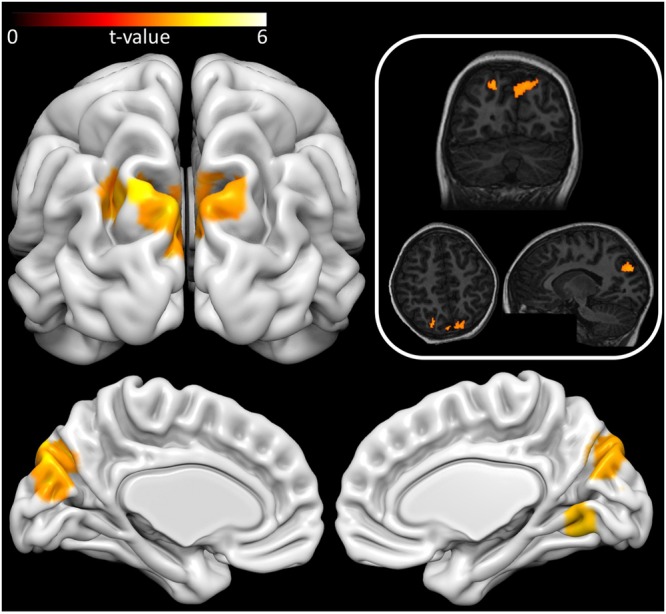
Grey matter correlates of Math Anxiety. Surface rendered image reflects a t-statistic cluster map rendered onto a template brain. All coloured areas reflect those grey matter voxels that were significantly negatively associated with MA after multiple comparison corrections as outlined in the method. The top right panel illustrates the results transformed and rendered onto a single participant’s annoymised T1-structural image. All images are presented in neurological convention, where the left of the image reflects the left of the brain. Surface rendering created using Surf Ice42; Individual subject rendering created using Mango43.
To understand the function of these structural correlates of MA, a further GLM was constructed comprising the Math-AS scores and nuisance variables as outlined previously, plus those variables that had earlier shown the most robust associations with the level of MA: attentional control (SHIFT), online memory (WORKING MEMORY) and mathematical achievement (WJ). This GLM was applied to those voxels that were previously identified as being negatively associated with MA.
When controlling for attention, working memory and mathematical achievement, a large number of those voxels initially found to be negatively associated with MA were no longer statistically significant, particularly within the IPS. Although this result alone is insufficient to determine a difference, the data suggest that the association between MA and GMV in these voxels may be mediated by one or more of the newly modelled variables. To assess this possibility, the linear directional contrasts for each newly modelled variable were computed. These data suggested that, of the additional variables added, attentional control was negatively associated with a large proportion of those voxels that were no longer associated with MA (see Supplementary Information: Fig. S2). However, this result did not survive correction for multiple-comparisons. Working memory and math achievement did not explain the association with MA (see Supplementary Information for detailed modelling procedure).
To further assess this, the mean GMV was extracted for each of the 3 clusters identified in the earlier VBM analysis. Though large, the spatial arrangement of these clusters may demarcate differentiation of function, as each comprised relatively distinct anatomical regions: lingual gyrus, cuneal cortex and the intraparietal sulcus. SHIFT, WORKING MEMORY and WJ were entered into a series of partial correlations to examine their relationship with GMV within each of the 3 clusters. When controlling for age, biological sex and Math-AS score, attentional control (SHIFT) was shown to be negatively associated with GMV across the IPS, although this result did not survive a Bonferroni correction for 9 tests. All other tests were non-significant at the uncorrected level (see Table 4).
Table 4.
Partial Correlation Results for Regional Grey Matter Volume, Numerical and Cognitive Indices.
| Domain | Math Achievement (WJ) | BRIEF INDICES | ||
|---|---|---|---|---|
| Attentional Control (SHIFT) | Online Memory (WORKING MEMORY) | |||
| Lingual Gyrus* | Pearson’s r | −0.086 | −0.070 | 0.051 |
| p-value | 0.460 | 0.572 | 0.678 | |
| Cuneal Cortex* | Pearson’s r | −0.168 | −0.123 | 0.086 |
| p-value | 0.148 | 0.318 | 0.487 | |
| Lateral Occipital Cortex* | Pearson’s r | −0.112 | −0.318 | −0.066 |
| /anterior Intraparietal Sulcus hIP1&3** | p-value | 0.334 | 0.008 | 0.592 |
Note. p-values reflect two-tailed partial correlation analyses, where MAS score, age and biological sex is held constant. Degrees of freedom (df) = 74 for WJ; df = 66 for BRIEF indices. BCa Bootstrapped 95% CI (10,000 samples) for SHIFT * IPS, lower −0.513, upper −0.108. No analyses survive Bonferroni corrected alpha for 9 tests of interest (corrected alpha, p < 0.0056). *Anatomical descriptors reflect VBM cluster peak and are taken from the Harvard-Oxford (HO) Atlas bundled with FSL 6.0.0. **Reflects anatomical label from Jeulich Histological Atlas available with FSL 6.0.0.
The Cognitive Architecture of MA and Resultant Outcomes in Numerical Achievement
Suárez-Pellicioni and colleagues11 outline a model, the processing efficiency theory34 in which intrusive thoughts resultant from MA consume working memory. This, in turn, is argued to expend already limited attentional resources in high MA individuals, leading to diminished performance when complex mathematical operations are performed. Such a relationship provides a causal explanation for the association between MA (Math-AS) and mathematical achievement (WJ). We tested this theoretical model using mediation analysis. When controlling for working memory and the nuisance variables age and biological sex, the relationship between MA and math achievement was no longer significant (c′) (see Table 5 and Fig. 2). The overall mediation model found that MA, working memory, age and biological sex explained approximately 55% of the variance in math achievement, (R2 = 0.5527, F(4, 66) = 20.39, p < 0.0001). Consistent with this model, the mediation analysis suggested that higher levels of MA (Math-AS) were associated with slightly elevated difficulty with holding appropriate information in mind (WORKING MEMORY), which in turn resulted in reduced math achievement (WJ).
Table 5.
Mediation Path Coefficients and Confidence Intervals for Math Anxiety Predicting Math Achievement.
| Path | Estimate | p-value | BCa 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Total effect (c) | −0.3639 | 0.0058 | −0.6188 | −0.1091 |
| Direct effect (c’) | −0.2247 | 0.0743 | −0.4721 | 0.0227 |
| a | 0.0758 | 0.0088 | 0.0197 | 0.1318 |
| b | −1.8378 | 0.0006 | −2.8603 | −0.8154 |
| Indirect effect | ||||
| ab | −0.1392 | — | −0.3074 | −0.0282 |
Note. BCa confidence intervals (CI) reflect 10,000 samples.
Figure 2.
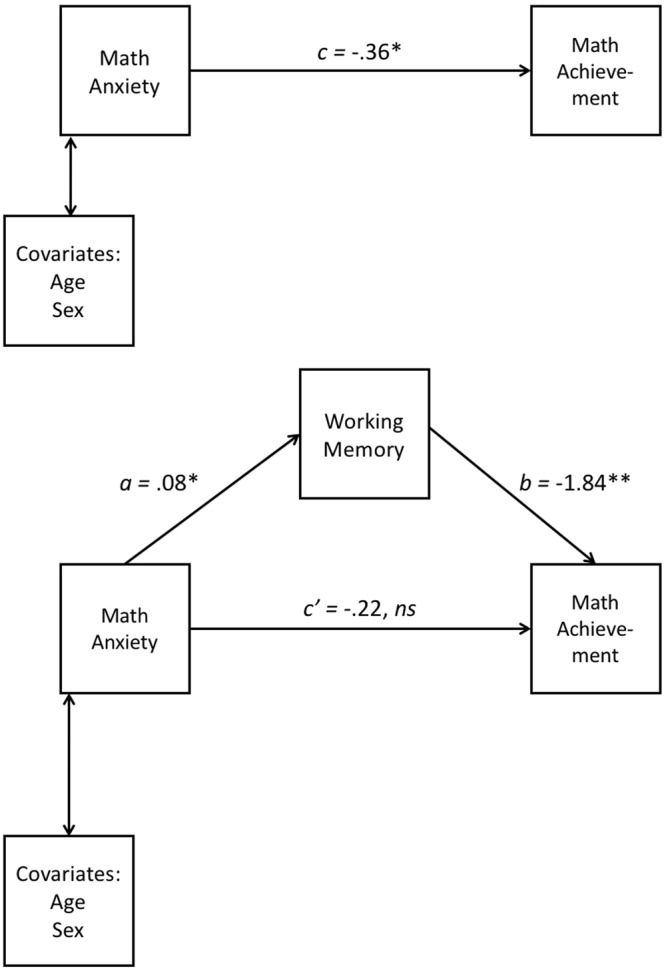
Summary coefficients for mediation model. Note. Path a = unstandardised IV to mediator; path b = unstandardised mediator to DV; path c = unstandardised total effect (IV to DV); path c′ = unstandardised direct effect. Coefficient values rounded to 2 decimal places; full values reported in accompanying table (3). ns = non-significant *p < 0.01; **p < 0.001. Note. Higher working memory values indicate lower working memory.
Discussion
The current study sought to examine the neurocognitive bases of MA in typically developing children. Unlike most prior work, however, the analyses focused on the level of MA, which is a more rigorous approach than making comparisons based on the presence or absence or of it35. This work therefore provides both a description of how MA might manifest itself across a cohort of typical children, as well as a detailed evaluation of theoretically-driven factors that might influence the degree of MA.
Most of the children in the sample demonstrated low-levels of MA. Around 10% of the entire sample reported moderate to high-levels of MA, and prevalence increased linearly with age. Aside from numerical representations, all other cognitive variables that were of theoretical interest were associated with the degree of MA: the more math anxious children demonstrated reduced inhibitory and attentional control, as well as lower working memory capacity and math achievement. Though MA appears to be associated with differences in baseline executive functions in children with MA, working memory was also shown to mediate the relationship between MA and math achievement suggesting that, possibly in addition to baseline differences, capacity issues may be exacerbated ‘online’ when working with mathematics by the physiological response to anxiety, which in turn leads to poorer learning and performance in mathematics.
Contrary to previous studies36,37, we found no association between MA and low-level numerical representations. There has been considerable debate regarding the presence of a low-level deficit in math ability in MA11. It may be that the task used here was not fine-grained enough to highlight any association, although note that the data from the task used did correlate with the measure of math achievement, suggesting that the task was tapping into numerical cognition to some degree. The current pattern of results is also indicative of experiential differences between high and low math anxious individuals, as MA may result in avoidance of exposure to math problems38. Moreover, an experientially-driven reduction in math achievement does not discount a primary, causal deficit in numerical representation; it may be that more extreme levels of MA reflect both causal (representational) and affectual (experiential) mechanisms. Indeed, cross-sectional work strongly suggests the presence of a less precise representation of numerical representation in adults with high MA37.
The present study also identified that reduced grey matter volume in the IPS, lingual gyrus and cuneal cortex was associated with increased MA. The identification of a structural correlate in relatively young children suggests that there may be differences in early brain structure that underpin the development of MA. Thus, whilst prior functional imaging studies demonstrate how network functionality can explain differences in mathematical performance in people with math anxiety8,9, these data provide the first evidence of a possible underlying structural basis. Genetic modelling suggests that around 40% of variance in MA6 can be explained by heredity, thus, a neurodevelopmental precursor is not implausible. Prior work has implicated the IPS in a network of regions showing aberrant activity in young, math anxious children, where these patterns of activation were unrelated to intelligence, general anxiety, reading ability or working memory9. Our data suggest that the IPS region identified in the current study might serve an attentional function, where children with reduced grey matter in this area had lower reported attentional resources, and this reduced attentional capacity was associated with increased MA. This is consistent with the assumption of the processing efficiency theory34 that high MA individuals may already have a limited attentional resource, and MA would further consume working memory, contributing to lower performance on complex mathematical operations. Future studies could investigate whether this reduced grey matter in the IPS is a structural adaptation due to reduced attentional capacity or compensatory strategies associated with MA39, or a potential biomarker on the causal pathway of the development of MA. One model that should be examined in future research is that children with MA, or who go on to develop MA, start off with differences in IPS structure, which translate into a deficit in baseline attentional capacity. According to this view, a limited ability to attend to stimuli, particularly mathematical stimuli – where demands on attentional resources are often high due to the nature of arithmetic problems – could result in general feelings of anxiety, which later become habitually associated with doing math, causing the development of MA.
In addition to providing a rich description of the neurocognitive bases of the level of math anxiety, the current study provides testable hypotheses regarding the emergence and maintenance of MA. Having a more nuanced understanding of the neurocognitive profile of MA, including any impairments that may cause, contribute to, or result from MA, has important implications for the development of targeted, individualized intervention. Longitudinal and cross-sectional work to profile MA against developmental dyscalculia, which may appear qualitatively similar40, is required, however, to assess the validity of these assertions.
Limitations and Suggested Future Directions
The review by Suárez-Pellicioni and colleagues11 outlines three core explanations of MA: (1) Task-related competition for working memory resources (2) A deficit in low-level numerical representation (3) Math anxiety as an inhibition/attentional-control deficit. Using data collected from a wider study, we evaluated each of these, combining paediatric MRI data from typically developing children to advance a neurocognitive model of the level of MA. Whilst our results address some of the core areas of interest in the cognitive literature, the present study does not, however, provide an assessment across all cognitive domains, so cannot be considered an exhaustive evaluation of the neurocognitive architecture of MA. Moreover, our executive function measures were derived from parental report. Although the measure of executive function administered is used extensively in education and clinical settings, a standardized, automated neurocognitive test battery may provide more valid data regarding performance and capacity.
Future work should include measures sampled ‘online’ whilst performing numerical tasks, based on event-related potentials, functional magnetic resonance imaging or autonomic measurement, for example. Whilst such work has been conducted with adults, there has been little progress towards developing a dynamic view of how individual differences in children’s physiological responses to numerical stimuli vary as a function of MA. Importantly, such an approach could identify further potential antecedents of MA; thus, permitting directed early intervention ideally to prevent, or at least reduce, the negative consequences of MA. Similarly, multimodal approaches could achieve deeper evidence regarding the functional properties of MA and, if approached longitudinally, its emergence could be evaluated. Still, though longitudinal approaches permit causal inference, they attract significant technical and practical challenges41.
Electronic supplementary material
Acknowledgements
Authors CEH FS, and RCK were supported by the European Research Council (Learning and Achievement, award number 338065). Authors LJF, FHS, JMGS and CGS were supported by grant PSI2017-84556-P from Ministry of Economy, Industry and Competitiveness (FEDER funding).
Author Contributions
L.J.F. conceived and designed the over-arching study. All authors contributed to the math anxiety study concept. F.H.S. translated the Math-AS into Spanish and organized and interpreted the Math-AS scores. C.G.S. organized and interpreted the BR.IEF questionnaire. L.J.F., A.I. and J.M.G.S. designed and implemented the MRI protocol. L.JF., A.I., F.H.S., C.G.S. and J.M.G.S. oversaw the testing and data collection. C.E.H., C.Y.L., F.S. and R.C.K. were given access to the subset of data described in this article to examine math anxiety, following L.J.F. taking a sabbatical within RCK’s laboratory at the Department of Experimental Psychology, University of Oxford. C.Y.L. contributed to the organization of test measures and raw data. C.E.H., C.Y.L. and F.S. conducted the behavioural analysis. C.E.H. conducted the neuroimaging analyses. C.E.H. and C.Y.L. drafted the manuscript. L.J.F., R.C.K., F.S., A.I., F.H.S., C.G.S. and J.M.G.S. provided critical revisions of the manuscript. All authors approved the final version of the manuscript for submission.
Competing Interests
The authors declare no competing interests.
Footnotes
Charlotte E. Hartwright and Chung Yen Looi contributed equally to this work.
A correction to this article is available online at https://doi.org/10.1038/s41598-018-28762-7.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26912-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/23/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
- 1.Hembree, R. The nature, effects, and relief of mathematics anxiety. Journal for research in mathematics education, 33–46 (1990).
- 2.Devine A, Fawcett K, Szűcs D, Dowker A. Gender differences in mathematics anxiety and the relation to mathematics performance while controlling for test anxiety. Behavioral and brain functions. 2012;8:1–9. doi: 10.1186/1744-9081-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krinzinger H, Kaufmann L, Willmes K. Math Anxiety and Math Ability in Early Primary School Years. J. Psychoeduc. Assess. 2009;27:206–225. doi: 10.1177/0734282908330583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pletzer B, Kronbichler M, Nuerk H-C, Kerschbaum HH. Mathematics anxiety reduces default mode network deactivation in response to numerical tasks. Frontiers in human neuroscience. 2015;9:1–12. doi: 10.3389/fnhum.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown M, Brown P, Bibby T. “I would rather die”: Reasons given by 16-year-olds for not continuing their study of mathematics. Research in Mathematics Education. 2008;10:3–18. doi: 10.1080/14794800801915814. [DOI] [Google Scholar]
- 6.Wang Z, et al. Who is afraid of math? Two sources of genetic variance for mathematical anxiety. Journal of Child Psychology and Psychiatry. 2014;55:1056–1064. doi: 10.1111/jcpp.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyons IM, Beilock SL. When math hurts: math anxiety predicts pain network activation in anticipation of doing math. PloS one. 2012;7:e48076. doi: 10.1371/journal.pone.0048076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons IM, Beilock SL. Mathematics anxiety: separating the math from the anxiety. Cerebral cortex. 2012;22:2102–2110. doi: 10.1093/cercor/bhr289. [DOI] [PubMed] [Google Scholar]
- 9.Young CB, Wu SS, Menon V. The neurodevelopmental basis of math anxiety. Psychological Science. 2012;23:492–501. doi: 10.1177/0956797611429134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar A, Dowker A, Kadosh R. Cognitive Enhancement or Cognitive Cost: Trait-Specific Outcomes of Brain Stimulation in the Case of Mathematics Anxiety. Journal of Neuroscience. 2014;34:16605–16610. doi: 10.1523/JNEUROSCI.3129-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suárez-Pellicioni M, Núñez-Peña MI, Colomé À. Math anxiety: A review of its cognitive consequences, psychophysiological correlates, and brain bases. Cognitive, Affective, & Behavioral Neuroscience. 2016;16:3–22. doi: 10.3758/s13415-015-0370-7. [DOI] [PubMed] [Google Scholar]
- 12.Gioia, G. A., Isquith, P. K., Guy, S. C. & Kenworthy, L. (Psychological Assessment Resources, Inc, 2000).
- 13.Gioia GA, Isquith PK, Guy SC, Kenworthy L. TEST REVIEW Behavior Rating Inventory of Executive Function. Child Neuropsychology. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 14.Dos Santos FH, et al. Number processing and calculation in Brazilian children aged 7–12 years. The Spanish Journal of Psychology. 2012;15:513–525. doi: 10.5209/rev_SJOP.2012.v15.n2.38862. [DOI] [PubMed] [Google Scholar]
- 15.Woodcock, R. W., McGrew, K. S. & Mather, N. Woodcock Johnson III. (The Riverside Publishing Company, 2001).
- 16.Carmo, J. S. et al. In Sobre comportamento e cognição: aspectos teóricos, metodológicos e de formação em análise do comportamento e terapia cognitivista (ed. Silva, W. C. M. P.) 213–221 (SP: Esetec, 2008).
- 17.Sánchez-Pérez, N., Fuentes, L., López-López, J. A., Pina, V. & González-Salinas, C. How do different components of Effortful Control contribute to children’s mathematics achievement? Frontiers in Psychology6, 10.3389/fpsyg.2015.01383 (2015). [DOI] [PMC free article] [PubMed]
- 18.Pina, V., Castillo, A., Cohen Kadosh, R. & Fuentes, L. J. Intentional and automatic numerical processing as predictors of mathematical abilities in primary school children. Frontiers in Psychology6, 10.3389/fpsyg.2015.00375 (2015). [DOI] [PMC free article] [PubMed]
- 19.Sanchez-Perez N, Fuentes LJ, Jolliffe D, Gonzalez-Salinas C. Assessing children’s empathy through a Spanish adaptation of the Basic Empathy Scale: parent’s and child’s report forms. Front Psychol. 2014;5:1438. doi: 10.3389/fpsyg.2014.01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendes, A. C. Identificação de graus de ansiedade à matemática em estudantes do ensino fundamental e médio: contribuições à validação de uma escala de ansiedade à matemática, Universidade Federal de São Carlos, (2012).
- 21.Spielberger, C. D., Gorsuch, R. L., Lushene, R. E., Vagg, P. R. & Jacobs, G. A. Manual for the state-trait anxiety inventory. (Consulting Psychologists Press, 1983).
- 22.Diamantopoulou S, Pina V, Valero-Garcia AV, González-Salinas C, Fuentes LJ. Validation of the spanish version of the woodcock-johnson mathematics achievement tests for children aged 6 to 13. Journal of Psychoeducational Assessment. 2012;30:466–477. doi: 10.1177/0734282912437531. [DOI] [Google Scholar]
- 23.Woodcock, R. W. Development and standardization of the Woodcock-Johnson psycho-educational battery. (Teaching Resources, 1978).
- 24.Rasch, G. Probabilistic Models for Some Intelligent and Attainment Tests Copenhagen. Denmark: Institute of Educational Research (1960).
- 25.Wright, B. D. & Stone, M. H. Best Test Design. Rasch Measurement (1979).
- 26.Bishara AJ, Hittner JB. Testing the significance of a correlation with nonnormal data: comparison of Pearson, Spearman, transformation, and resampling approaches. Psychol Methods. 2012;17:399–417. doi: 10.1037/a0028087. [DOI] [PubMed] [Google Scholar]
- 27.Hayes, A. F. Introduction to mediation, moderation, and conditional process analysis. [electronic resource]: a regression-based approach. (New York: The Guilford Press, [2013], 2013).
- 28.Douaud G, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 29.Good CD, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 30.Andersson, J. L. R., Jenkinson, M. & Smith, S. M. Non-linear registration, aka Spatial normalisationwww.fmrib.ox.ac.uk/analysis/techrep (2007).
- 31.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 33.Dowker, A., Sarkar, A. & Looi, C. Y. MathematicsAnxiety: What Have We Learned in 60 Years? Frontiers in Psychology7, 10.3389/fpsyg.2016.00508 (2016). [DOI] [PMC free article] [PubMed]
- 34.Eysenck M, Calvo M. Anxiety and Performance - the Processing Efficiency Theory. Cognition & Emotion. 1992;6:409–434. doi: 10.1080/02699939208409696. [DOI] [Google Scholar]
- 35.Preacher KJ, Rucker DD, MacCallum RC, Nicewander WA. Use of the extreme groups approach: A critical reexamination and new recommendations. Psychological Methods. 2005;10:178–192. doi: 10.1037/1082-989X.10.2.178. [DOI] [PubMed] [Google Scholar]
- 36.Georges, C., Hoffmann, D. & Schiltz, C. How Math Anxiety Relates to Number–Space Associations. Frontiers in Psychology7, 10.3389/fpsyg.2016.01401 (2016). [DOI] [PMC free article] [PubMed]
- 37.Maloney EA, Ansari D, Fugelsang JA. The effect of mathematics anxiety on the processing of numerical magnitude. The Quarterly Journal of Experimental Psychology. 2011;64:10–16. doi: 10.1080/17470218.2010.533278. [DOI] [PubMed] [Google Scholar]
- 38.Ashcraft MH, Faust MW. Mathematics anxiety and mental arithmetic performance: An exploratory investigation. Cognition and Emotion. 1994;8:97–125. doi: 10.1080/02699939408408931. [DOI] [Google Scholar]
- 39.Eysenck M, Derakshan N, Santos R, Calvo M. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 40.Mammarella IC, Hill F, Devine A, Caviola S, Szűcs D. Math anxiety and developmental dyscalculia: a study on working memory processes. Journal of clinical and experimental neuropsychology. 2015;37:878–887. doi: 10.1080/13803395.2015.1066759. [DOI] [PubMed] [Google Scholar]
- 41.Mills KL, Tamnes CK. Methods and considerations for longitudinal structural brain imaging analysis across development. Developmental Cognitive Neuroscience. 2014;9:172–190. doi: 10.1016/j.dcn.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Surf Ice v. 10.11.16 (McCausland Center for Brain Imaging, University of South Carolina, South Carolina, 2015).
- 43.Mango v. 4.0 (Research Imaging Institute University of Texas Health Science Centre, Texas, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The research meta-data supporting this publication are available on the Open Science Framework repository, see DOI 10.17605/OSF.IO/PDFJE. The senior author, LJF, may be contacted regarding the wider dataset.


