Abstract
Background
Non-cardiac chest pain (NCCP) is recurrent angina pectoris-like pain without evidence of coronary heart disease in conventional diagnostic evaluation. The prevalence of NCCP is up to 70% and may be detected (in this order) at all levels of the medical health care system (general practitioner, emergency department, chest pain unit, coronary care). Reduction of quality of life due to NCCP is comparable, and partially even higher, to that caused by cardiac chest pain. Reasons for psychological strain are symptom recurrence in approximately 50%, nonspecific diagnosis with resulting uncertainty, and insufficient integration of other medical disciplines in the diagnostic workup.
Methods and Results
The management of patients with chest pain has to be multidisciplinary because non-cardiac causes may be frequently encountered. Especially gastroenterological expertise is required since the cause of chest pain is gastroesophageal reflux disease (GERD) in 50–60%, hypercontractile esophageal motility disorders with nutcracker/jackhammer esophagus or diffuse esophageal spasm or achalasia in 15–18%, and other esophageal alterations (e.g., infectious esophageal inflammation, drug-induced ulcers, rings, webs, eosinophilic esophagitis) in 32–35%.
Conclusion
This review highlights the importance of regular interdisciplinary ward rounds and management of chest pain units.
Keywords: Non-cardiac chest pain, Gastroesophageal reflux, GERD, Esophageal motility disorders, Distal esophageal spasm, Nutcracker esophagus, Jackhammer esophagus, Achalasia
Definition
Non-cardiac chest pain (NCCP) is recurrent angina pectoris-like pain without evidence of coronary heart disease in conventional diagnostic evaluation, such as coronary angiography and/or troponin assay [1]. Since its first description [2, 3], NCCP has been given several names such as ‘Syndrome X’ [4, 5] or ‘microvascular angina’ [6].
Epidemiology
Epidemiologic studies indicate that the proportion of NCCP among patients with chest pain is reported between 20 and 40%. This proportion is robust and worldwide comparable in different countries, such as Germany Europe, USA, China, or Australia [7]. A significant proportion of NCCP may be detected (in this order) at all levels of the medical health care system, including general practitioner, emergency department, chest pain unit (CPU), and coronary care. For example, in a large study in Germany with over 190,000 patients [8], approximately 0.7% of the patients consulted a general practitioner because of chest pain. However, among these patients, ischemic heart disease or acute coronary heart syndrome were detected in only 15%. In addition, the handbook of the Disease-Management-Programm (DMP) Coronary Heart Disease of the Deutscher Hausärzteverband (German General Practitioner Association) and a general insurance company (AOK) indicates that only 16–22% of the differential diagnoses in primary care involve chest pain [9]. Furthermore, the proportion of NCCP in emergency departments and CPUs is even higher and has been reported as 60–80% [9, 10, 11, 12, 13]. This was also illustrated by an analysis of 38 German CPUs including 11,656 patients [14]. In this study, acute coronary syndrome or perimyocarditis as the cause of the chest pain were detected in only 45 and 1%, respectively; however, NCCP was found in at least 15%. Even in cardiac catheter labs, NCCP may be found in a significant proportion of cases as shown by the register of the Geschäftsstelle Qualitätssicherung NRW Regionalvertretung Nordrhein (National Quality Assurance Center North Rhine Westphalia, North Rhine region) [15] for the years 2011–2013. Here, approximately 30% of the coronary catheter investigations revealed a diagnosis of ‘exclusion of coronary heart disease’ or found coronary heart disease with stenosis of the coronary lumen below 50%.
Quality of Life and Socioeconomic Burden
Patients with NCCP cause a significant socioeconomic burden, because quality of life is reduced to a similar magnitude, or even higher in certain quality of life parameters, compared to patients with cardiac chest pain (CCP) [16]. This leads to frequent doctor's visits and significant direct and indirect costs to the health insurance system [17, 18]. 80% of patients with NCCP visit their GP more than once for their complaint, and 30–60% experience interruptions in their professional activities or require sick leave. According to a large study in Germany [19], reasons for psychological strain are persistence of symptoms in 50% of cases with inadequate medical care, uncertainty regarding the origin of the pain, and insufficient integration of medical disciplines other than cardiology to clarify NCCP in 10% of cases. Even after exclusion of cardiac reasons for the chest pain, patients with NCCP very often retain the stigma of an undetected cardiologic disorder [20]. This discrepancy is pronounced by inadequate interdisciplinary cooperation and insufficient referral to the other medical disciplines. This is evidenced by the fact that only half of the patients with NCCP will be referred to other medical disciplines after exclusion of cardiac causes [21]. In line with this, patients with NCCP and underlying psychogenic causes show frequent doctor's visits but are rarely referred to a psychologist [19].
Patient Characteristics
Several studies have shown that patients with NCCP show certain specific characteristics. For example, NCCP patients visiting a doctor have more complaints, develop a greater magnitude of social detraction, and suffer more from gastroesophageal reflux disease (GERD) compared to NCCP patients not visiting a doctor [12, 22]. This finding was confirmed by other studies indicating more frequent gastrointestinal complaints in NCCP patients such as sore throat, dysphagia, and regurgitation [12, 18]. However, patients with NCCP are not generally different to patients with cardiac chest pain (CCP) with regard to demography and long-term follow-up [8, 12, 23, 24]. Risk factors for NCCP are overweight (odds ratio (OR) 3.0, 95% confidence interval (CI) 1.64–5.50), reflux (OR 2.8, 95% CI 1.73–4.32), smoking (OR 2.0, 95% CI 1.27–3.18), aspirin (OR 1.5, 95% CI 1.00–2.31), non-steroidal anti-inflammatory drugs (OR 2.0, 95% CI 1.27–3.16), neuroticism (OR 1.14, 95% CI 1.08–1.21), and anxiety (OR 1.12, 95% CI 1.08–1.17) [24, 25, 26]. NCCP is found in all age groups, but the prevalence decreases with age. There is no gender difference [8, 12, 18]. The clinical management of NCCP is ambiguous because no clinically significant characteristics, such as location or quality of pain or response to nitroglycerine, exist to differentiate between NCCP and CCP. In addition, transmission of pain and associated symptoms show low sensitivity and specificity [18, 27, 28, 29]. One cause of this dilemma is that sensory stimulation of the heart and adjacent organs, e.g., the esophagus, is transferred via overlapping sensory pathways to the brain. In this context, the esophagus is an important sensory organ, because 90% of the vagal fibers are afferent, mediating chemical (e.g., gastroesophageal) reflux and mechanical (e.g., motility disorders) stimulation to the brain. These characteristics imply that the diagnosis of NCCP can be confirmed only after exclusion of CCP.
Disorders Leading to NCCP
Many disorders may lead to NCCP [1, 30] (table 1). Among these, musculoskeletal (36–49%), gastrointestinal (2–19%), psychiatric (5–11%), and pulmonary or mediastinal (3–6%) causes are detected [8, 9, 19]. However, especially gastroenterological expertise is required in the differential diagnosis of NCCP, because gastrointestinal diseases are frequently found in NCCP. For example, gastroesophageal reflux disease is present in 50–60%, esophageal motility disorders in 15–18% (including diffuse esophageal spasm, nutcracker esophagus, and achalasia), and abnormalities suspicious for being of esophageal origin in 32–35% of NCCP cases [31] (table 1, fig. 1). It is worth mentioning that drug-induced esophageal mucosal lesions may be a cause of NCCP, especially in elderly patients. Risk factors are intake of pills just before bedtime and with insufficient amounts of fluid as well as infectious esophageal mucosal inflammation. Especially esophageal webs or rings leading to NCCP, dysphagia, bolus obstruction, or regurgitation are frequently overlooked (fig. 2). In addition, esophageal esophagitis is increasingly detected in NCCP (fig. 3). Therefore, esophageal biopsies should be taken routinely during upper gastrointestinal endoscopy.
Table 1.
| Gastrointestinal |
| Gastroesophageal (GERD), erosive (ERD), non-erosive (NERD) reflux disease |
| Barrett's esophagus |
| Esophageal motility disorder (diffuse esophageal spasm, hypercontractile esophagus, achalasia) |
| Hypersensitive esophagus |
| Schatzki ring, webs |
| Eosinophilic esophagitis |
| Mallory-Weiss syndrome, Boerhaave syndrome |
| Drug-induced esophageal ulcer |
| Infections (viral/mycotic esophagitis) |
| Gastroduodenal ulcer |
| Pancreatitis, biliary colic pain, cholangitis |
| Pneumological |
| Lung embolism |
| Pneumonia |
| Pneumothorax |
| Musculoskeletal syndrome (‘chest wall syndrome’) |
| Neurological |
| Nerve compression |
| Zoster infection, neuralgia |
| Orthopedic/rheumatologic |
| Degenerative spinal disorders |
| Rheumatologic disease |
| Psychiatric |
| Pain disorders |
| Depression |
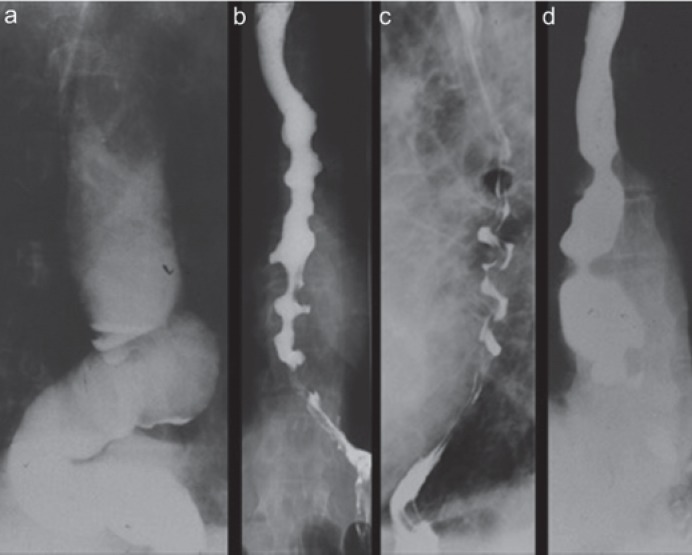
Fig. 1.
Esophageal motility disorder as a cause of non-cardiac chest pain (NCCP). From left to right: achalasia type I, achalasia type III, hypercontractile (‘jackhammer’) esophagus, distal diffuse esophageal spasm.
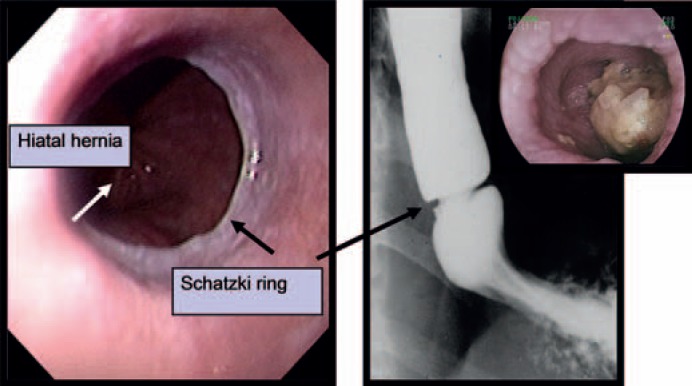
Fig. 2.
Esophageal ring (‘Schatzki ring’) at the cardiac region. Left: endoscopy, right: barium swallow, insert: bolus obstruction.
Fig. 3.
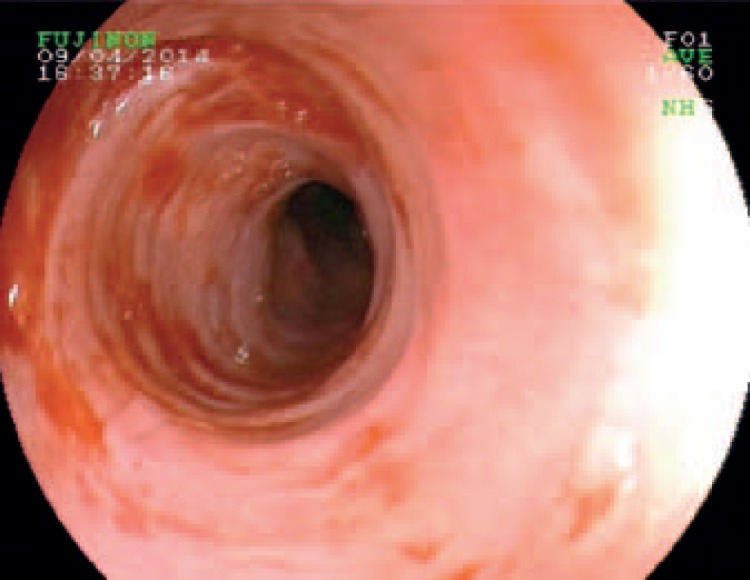
Eosinophilic esophagitis with mucosal edema, Tatami pattern, and irregular mucosa.
Esophageal Differential Diagnoses - Diagnostic and Therapeutic Algorithms
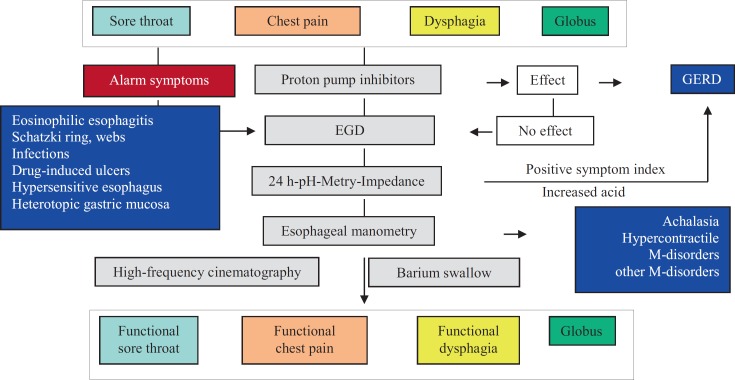
Table 1 illustrates the esophageal differential diagnoses that may cause NCCP. Among these, gastroesophageal reflux plays a predominant role, because 50–60% of patients with NCCP suffer from elevated gastroesophageal reflux. Therefore, after exclusion of alarm symptoms, therapy with proton pump inhibitors (PPI) on a trial basis is recommended [31]. However, in the case of insufficient efficacy or in the presence of associated symptoms such as dysphagia, an endoscopic evaluation is mandatory. It is recommended that during esophagogastroduodenoscopy (EGD), esophageal mucosal biopsies should be taken to detect and confirm eosinophilic, viral, or mycotic esophagitis. It is essential to carefully inspect the whole esophagus to detect reflux esophagitis, Barrett's esophagus, Schatzki ring, webs, or heterotopic gastric mucosa. Mucosal erosions or ulcers within the tubular esophagus proximal to the gastroesophageal junction are always suspicious for drug-induced mucosal damage. In contrast, Mallory-Weiss syndrome and Boerhaave syndrome may be detected easily by their characteristic clinical development. EGD is also helpful to detect gastrointestinal diseases outside of the esophagus, such as gastroduodenal ulcer, pancreatitis, biliary colic pain, or cholangitis. Figure 4 shows a diagnostic algorithm that is practicable for clinical use. After exclusion of differential diagnoses by EGD and ineffective PPI therapy (table 1), esophageal function tests are mandatory to further elucidate the cause of the chest pain. In this context, 24 h combined pH-metry/impedance is the gold standard to detect acid and non-acid reflux or non-response to PPI therapy. In addition, high-resolution manometry is gold standard for the detection of esophageal motility disorders which are differentiated by the Chicago classification [32, 33, 34].
Fig. 4.
Diagnostic algorithm for the diagnosis of non-cardiac chest pain (NCCP) from the gastroenterological point of view (modified from [35]).
Multidisciplinary Approach in Diagnosis and Therapy
Multidisciplinary cooperation between the different disciplines is essential in the diagnosis and therapy of NCCP. In this context, it is worth mentioning that the CPUs in Germany provide defined structure and process guidelines for the acute diagnosis and therapeutic management of patients with CCP and are partly integrated within an operational rescue service. However, a structured workup after exclusion of CCP is still missing. This is significant, because NCCP is frequently found in CPUs. Regular common rounds with participation of different disciplines could solve this dilemma. Table 2 and figure 4 illustrate a diagnostic and therapeutic workup from the gastroenterologist's point of view [35]. This includes treatment with PPI (PPI test); however, one should be aware that the PPI test encompasses only acid reflux, limiting its diagnostic value. In addition, it is recommended that in NCCP, esophageal biopsies should be taken routinely during EGD to detect eosinophilic esophagitis. In individual cases, further differential gastroenterological diagnostic workup through endoscopy, radiology, 24-h pH-metry/impedance, or high-resolution esophageal manometry is mandatory.
Table 2.
Therapeutic options for non-cardiac chest pain (NCCP) from the gastroenterological point of view
| GERD/Barrett's esophagus | PPI, alginate, OP |
| Esophageal motility disorders | nitrogylcerine, calcium channel blockers, bouginage, dilation, cardiomyotomy, OP, POEM |
| Hypersensitive esophagus | PPI, tricyclic antidepressant |
| Schatzki ring, webs | bouginage, dilation |
| Eosinophilic esophagitis | PPI, diet (‘6-food diet’), local corticoid therapy, bouginage, dilation |
| Mallory-Weiss syndrome / Boerhaave syndrome | PPI, endoscopic injection, clipping, OP |
| Drug-induced esophageal ulcer | information, training, drug modification |
| Infections (viral/Candida esophagitis) | antiviral drugs, antimycotic drugs |
| Gastroduodenal ulcer | PPI, Helicobacter pylori eradication |
| Pancreatitis, bile colic, cholangitis | intravenous fluids, spasmolytics, antibiotics, OP |
OP = Surgery; POEM = peroral endoscopic myotomy;GERD = gastroesophageal reflux disease; PPI = proton pump inhibitor.
Disclosure Statement
The author of this manuscript has no conflict of interest.
References
- 1.Lenfant C. Chest pain of cardiac and noncardiac origin. Metabolism. 2010;59((suppl 1)):S41–S46. doi: 10.1016/j.metabol.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med. 1967;276:1063–1066. doi: 10.1056/NEJM196705112761904. [DOI] [PubMed] [Google Scholar]
- 3.Kemp HG, Elliot WC, Gorlin R. The anginal syndrome with normal coronary arteriography. Trans Assoc Am Physicians. 1967;80:59–70. [PubMed] [Google Scholar]
- 4.Arbogast R, Bourassa MG. Myocardial function during atrial pacing in patients with angina pectoris and normal coronary arteriograms: comparison with patients having significant coronary artery disease. Am J Cardiol. 1973;32:257–263. doi: 10.1016/s0002-9149(73)80130-4. [DOI] [PubMed] [Google Scholar]
- 5.Kemp HG., Jr Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol. 1973;32:375–337. doi: 10.1016/s0002-9149(73)80150-x. [DOI] [PubMed] [Google Scholar]
- 6.Cannon RO III, Epstein SE. ‘Microvascular angina’ as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61:1338–1343. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 7.Eslick GD. Classification, natural history, epidemiology, and risk factors of noncardiac chest pain. Dis Mon. 2008;54:593–603. doi: 10.1016/j.disamonth.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Bösner S, Becker A, Haasenritter J, et al. Chest pain in primary care: epidemiology and pre-work-up probabilities. Eur J Gen Pract. 2009;15:141–146. doi: 10.3109/13814780903329528. [DOI] [PubMed] [Google Scholar]
- 9.Donner-Banzhoff N, Popert U, Muth C, Beyer M, Gerlach FM. Differentialdiagnostik des akuten Brustschmerzes in der Hausarztpraxis; in Deutscher Hausärzteverband, AOK Bundesverband (Hrsg): Hausarzt Handbuch DMP Koronare Herzkrankheit (KHK) München, MED. KOMM. 2004:34–42. [Google Scholar]
- 10.Mayou R, Bryant B, Clark D. Non-cardiac chest pain and benign palpitations in the cardiac clinic. Br Heart J. 1994;72:548–553. doi: 10.1136/hrt.72.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain D, Fluck D, Sayer JW, Ray S, Paul EA, Timmis AD. One-stop chest pain clinic can identify high cardiac risk. J R Coll Physicians Lond. 1997;31:401–414. [PMC free article] [PubMed] [Google Scholar]
- 12.Wong WM, Lam KF, Cheng C, et al. Population based study of noncardiac chest pain in southern Chinese: prevalence, psychosocial factors and health care utilization. World J Gastroenterol. 2004;10:707–712. doi: 10.3748/wjg.v10.i5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stallone F, Twerenbold R, Wildi K, et al. Prevalence, characteristics and outcome of non-cardiac chest pain and elevated copeptin levels. Heart. 2014;100:1708–1714. doi: 10.1136/heartjnl-2014-305583. [DOI] [PubMed] [Google Scholar]
- 14.Maier LS, Darius H, Giannitsis E, et al. The German CPU Registry: comparison of troponin positive to troponin negative patients. Int J Cardiol. 2013;168:1651–1653. doi: 10.1016/j.ijcard.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Geschäftsstelle Qualitätssicherung NRW: Jahresauswertung 2013 Koronarangiographie und PCI 21/3. www.qs-nrw.org/service.
- 16.Eslick GD, Talley NJ. Non-cardiac chest pain: predictors of health care seeking, the types of health care professional consulted, work absenteeism, and interruption of daily activities. Aliment Pharmacol Ther. 2004;20:909–915. doi: 10.1111/j.1365-2036.2004.02175.x. [DOI] [PubMed] [Google Scholar]
- 17.Eslick GD, Coulshed DS, Talley NJ. Review article: the burden of illness of non-cardiac chest pain. Aliment Pharmacol Ther. 2002;16:1217–1223. doi: 10.1046/j.1365-2036.2002.01296.x. [DOI] [PubMed] [Google Scholar]
- 18.Eslick GD, Jones MP, Talley NJ. Non-cardiac chest pain: prevalence, risk factors, impact and consulting-a population-based study. Aliment Pharmacol Ther. 2003;17:1115–1124. doi: 10.1046/j.1365-2036.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- 19.Glombiewski JA. The course of nonspecific chest pain in primary care symptom persistence and health care usage. Arch Intern Med. 2010;170:251–255. doi: 10.1001/archinternmed.2009.474. [DOI] [PubMed] [Google Scholar]
- 20.Chambers J, Bass C, Mayou R. Non-cardiac chest pain: assessment and management. Heart. 1999;82:656–657. doi: 10.1136/hrt.82.6.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong WM, Risner-Adler S, Beeler J, et al. Noncardiac chest pain: the role of the cardiologist-a national survey. J Clin Gastroenterol. 2005;39:858–862. doi: 10.1097/01.mcg.0000180635.92313.3b. [DOI] [PubMed] [Google Scholar]
- 22.Dumvillea JC, MacPherson H, Griffith K, Miles JN, Lewin RJ. Non-cardiac chest pain: a retrospective cohort study of patients who attended a Rapid Access Chest Pain Clinic. Fam Pract. 2007;24:152–157. doi: 10.1093/fampra/cmm002. [DOI] [PubMed] [Google Scholar]
- 23.Williams JF, Sontag SJ, Schnell T, Leya J. Non-cardiac chest pain: the long-term natural history and comparison with gastroesophageal reflux disease. Am J Gastroenterol. 2008;104:2145–2152. doi: 10.1038/ajg.2009.279. [DOI] [PubMed] [Google Scholar]
- 24.Ruddox V, Mathisen M, Otterstad JE. Prevalence and prognosis of non-specific chest pain among patients hospitalized for suspected acute coronary syndrome - a systematic literature search. BMC Med. 2012;10:58–64. doi: 10.1186/1741-7015-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wise JL, Locke GR, Zinsmeister AR, et al. Risk factors for non-cardiac chest pain in the community. Aliment Pharmacol Ther. 2005;22:1023–1031. doi: 10.1111/j.1365-2036.2005.02666.x. [DOI] [PubMed] [Google Scholar]
- 26.Eslick GD. Health care seeking behaviors, psychological factors, and quality of life of noncardiac chest pain. Dis Mon. 2008;54:604–612. doi: 10.1016/j.disamonth.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Cooke RA, Smeeton N, Chambers JB. Comparative study of chest pain characteristics in patients with normal and abnormal coronary angiograms. Heart. 1997;78:142–146. doi: 10.1136/hrt.78.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodacre S, Locker T, Morris F, Campbell S. How useful are clinical features in the diagnosis of acute, undifferentiated chest pain? Acad Emerg Med. 2002;9:203–208. doi: 10.1111/j.1553-2712.2002.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 29.Panju AA, Hemmelgarn BR, Guyatt GH, Simel DL. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256–1263. doi: 10.1001/jama.280.14.1256. [DOI] [PubMed] [Google Scholar]
- 30.Weingart W, Allescher H-D. Nicht-kardialer Thoraxschmerz. Dtsch Med Wochenschr. 2010;135:2135–2146. doi: 10.1055/s-0030-1267493. [DOI] [PubMed] [Google Scholar]
- 31.Fass R, Navarro-Rodriguez T. Noncardiac chest pain. J Clin Gastroenterol. 2008;42:636–646. doi: 10.1097/MCG.0b013e3181684c6b. [DOI] [PubMed] [Google Scholar]
- 32.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frieling T. Differential diagnosis ‘non-cardiac chest pain’. Dtsch Med Wochenschr. 2015;140:1166–1172. doi: 10.1055/s-0041-103305. [DOI] [PubMed] [Google Scholar]
- 34.Bruno RR, Donner-Banzhoff N, Söllner W, et al. The interdisciplinary management of acute chest pain. Dtsch Arztebl Int. 2015;112:768–779. doi: 10.3238/arztebl.2015.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar AR, Kath PO. Functional esophageal disorders: a review of diagnosis and management. Expert Rev Gastroenterol Hepatol. 2013;7:453–461. doi: 10.1586/17474124.2013.811028. [DOI] [PubMed] [Google Scholar]