Abstract
This case represents the challenge and creativity necessary when treating patients with metastatic renal cell carcinoma who have been exposed to multiple lines of therapy. At present, treatment with immune checkpoint inhibition has stabilized and improved the metastatic disease of this patient with the exception of hepatic lesions. This isolated progression within the liver led the employment of radioembolization, which successfully treated those metastases. This is the first documented case of metastatic renal cell carcinoma controlled with concurrent use of immune checkpoint inhibition and radioembolization for both extrahepatic and hepatic metastases, respectively. This case can be construed as a potential example of the abscopal effect and may provide the basis for understanding this type of response in select patients.
Keywords: Radioembolization, Metastatic renal cell carcinoma, Immune checkpoint inhibition, Internal radiotherapy, Immunotherapy
Introduction
Targeted therapy and immune checkpoint inhibition (ICI) represent the mainstay of treatment for metastatic renal cell carcinoma (mRCC) [1]. Radiotherapy is typically used for palliation, although there is increasing interest in potential synergy between this modality and systemic therapy. Herein, we describe a patient who was treated simultaneously with radioembolization and ICI. In contrast to conventional external beam radiotherapy, radioembolization is a directed technique utilizing microspheres loaded with a radioactive compound (in the current case, yttrium-90 [Y-90]). Currently, radioembolization is most commonly applied in primary hepatocellular tumors or colorectal and neuroendocrine hepatic metastases [2, 3, 4]. Our case suggests a potential role in select patients with mRCC.
Case Report
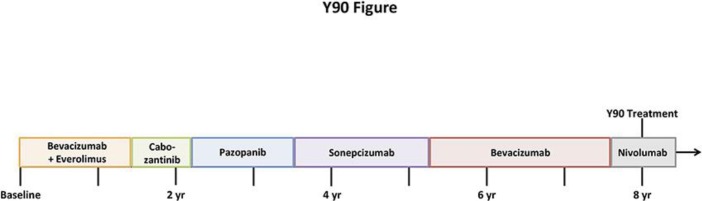
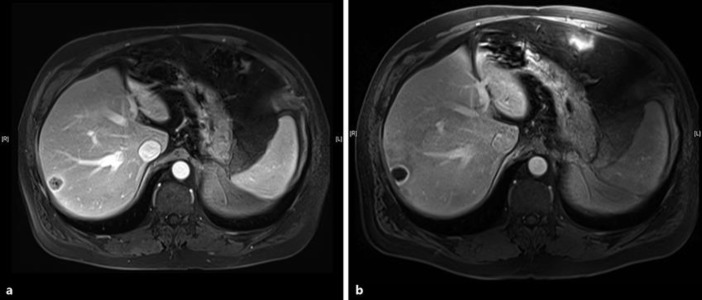
A 58-year-old male initially presented with back pain and imaging showed a left-sided renal mass with multiple skeletal, lung, bone, and liver metastases. The patient underwent nephrectomy with pathology indicating clear cell RCC. The patient was initially enrolled on the RECORD-2 protocol, and received bevacizumab and everolimus for a total of 17 months [5]. At the time of progression, he was enrolled on a phase I protocol of cabozantinib for 9 months [6]. Ultimately, this treatment was discontinued because of toxicity; the patient was then transitioned to pazopanib, which he received for 16 months with ensuing progression. He then received sonepcizumab on a phase II protocol, a monoclonal antibody directed at sphingosine-1-phosphate receptor-1, for 21 months [7]. Given a lack of distinct options at the time, the patient received bevacizumab at the time of progression, which stabilized his disease for an additional 28 months. He had gradual progression and was then transitioned to nivolumab shortly after the drug was granted FDA approval (Fig. 1). Imaging completed 5 months after the initiation of nivolumab demonstrated stable and improving disease in all of the metastatic sites with the exception of the liver lesions (Fig. 2a). Given his excellent tolerance of the drug and asymptomatic progression at this site alone, he was amenable to exploring local therapy. The patient underwent transarterial radioembolization therapy using 19.5 mCi of intra-arterial Y-90 resin microspheres to right lobe and segment 4 liver metastases. Postprocedural imaging at 2 months showed a significant decrease in enhancement (Fig. 2b).
Fig. 1.
Succession of treatments from diagnosis to current treatment (yttrium-90 [Y-90]).
Fig. 2.
a Preradioembolization MRI demonstrating a solid metastasis in the right hepatic lobe. b Postradioembolization MRI demonstrating cavitation of the lesion with a thin rim of residual enhancement.
Discussion
The use of Y-90 radioembolization has been described throughout the literature beyond hepatocellular, cholangiocarcinoma, and colorectal carcinoma; however, there is a paucity of information for its use in mRCC. A retrospective review of 17 patients with liver-dominant mRCC treated with Y-90 radioembolization identified a complete response in 14 patients, partial response in 1 patient, and progression of disease in 2 patients. The median overall survival (OS) following Y-90 radioembolization was 22.8 months (95% CI 13.2–32.3) [8]. Another study investigated the use of Y-90 radioembolization in 6 patients with liver-dominant mRCC refractory to targeted therapy and conventional immunotherapy (IL-2 and IFN-α). Of the 6 patients treated, 1 patient was treated with IFN-α alone with subsequent disease progression following Y-90 treatment and a different patient was treated with both IL-2 and IFN-α with a partial response to Y-90 therapy. The median OS following Y-90 radioembolization in this patient cohort was 35.8 months (95% CI 5.9–65.6) [9]. In both studies, the use of Y-90 radioembolization was well tolerated and showed a sustained durable response in the majority of patients. In both of these studies, the cohorts did not have extrahepatic disease and their treatment of hepatic lesions was subsequent to conventional immunotherapy or targeted therapy. In the current case, our patient has extensive extrahepatic disease and is receiving ICI concurrently. To our knowledge, this is the first checkpoint inhibitor and Y-90 concomitantly or sequentially.
Our case can be construed as a potential demonstration of the abscopal effect – a phenomenon when treating metastatic cancer with localized radiotherapy to one metastatic site triggers tumor shrinkage in other sites [10]. The abscopal effect is dependent on activation of the immune system. Both proinflammatory mediators and danger signals are released as a result of radiation therapy, which promote the stimulation of circulating dendritic cells. The dendritic cells uptake apoptotic cancer cells and cluster within lymph nodes where they present various tumor antigens to T cells. The presentation of tumor antigens to T cells is the mechanism by which antitumor responses are produced [11]. There have been multiple cases on the abscopal effect of radiotherapy with ICI published across a wide variety of tumor subtypes [12]. The basis for employing Y-90 for mRCC was a case published on metastatic melanoma pretreated with immunotherapy and subsequent local radiotherapy describes the synergy of radiation and immunostimulatory feedback in enhancing the abscopal effect [13]. Additionally, a phase I study assessed the safety of combining stereotactic body radiotherapy (SBRT) with ICI in patients with solid tumors. In this study including 79 patients, SBRT was delivered followed by pembrolizumab. This trial reported an overall objective response rate of 13.2% with a median progression-free survival of 3.1 months (95% CI 2.9–3.4) and a median OS of 9.6 months (95% CI 6.5–undetermined) [14]. This trial shares similarities to our case with the use of ICI and radiotherapy; however, 2 important distinctions are that first, this was SBRT and not radioembolization and second, the ICI was post-SBRT, further suggesting a unique strategy.
With both of these studies in mind, the initiation of Y-90 to locally treat isolated hepatic progression with concurrent use of ICI allows for the assessment of the abscopal effect for internal radiotherapy with ICI, which has yet to be described in the literature [15]. Although the authors cannot conclude that this is a case of abscopal effect, it is noteworthy that on imaging, the extrahepatic lesions all remained stable. The strategy of combining of ICI and internal radiotherapy employed in our patient is an example of the creativity necessary to successfully treat mRCC patients to improve outcomes and prolong survival. The authors are interested in further collaboration and updated cases or trials on the concomitant use of radioembolization and ICI.
Statement of Ethics
Verbal consent was obtained from the patient to use his case for this paper.
Disclosure Statement
The authors have no disclosures to report.
verified
References
- 1.Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017 Jun;15(6):804–34. doi: 10.6004/jnccn.2017.0100. [DOI] [PubMed] [Google Scholar]
- 2.Levi S, ri GB, Ettorre GM, Giannelli V, Colasanti M, Sciuto R, Pizzi G, et al. Trans-arterial radio-embolization: a new chance for patients with hepatocellular cancer to access liver transplantation, a world review. Transl Gastroenterol Hepatol. 2017 Nov;2(11):98. doi: 10.21037/tgh.2017.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filippi L, Schillaci O, Cianni R, et al. Yttrium-90 resin microspheres and their use in the treatment of intrahepatic cholangiocarcinoma. Future Oncol. 2017 doi: 10.2217/fon-2017-0443. [DOI] [PubMed] [Google Scholar]
- 4.Atay K, Akkoc FN, Samanci C, Gülsen F, Kepil N, Hatemi I. Gastric Ulcers Related to The Transarterial Radioembolization of Yittrium-90 in A Patient with Paraganglioma. Acta Gastroenterol Belg. 2017 Jan-Mar;80(1):85–6. [PubMed] [Google Scholar]
- 5.Ravaud A, Barrios CH, Alekseev B, Tay MH, Agarwala SS, Yalcin S, et al. RECORD-2: phase II randomized study of everolimus and bevacizumab versus interferon α-2a and bevacizumab as first-line therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2015 Jul;26(7):1378–84. doi: 10.1093/annonc/mdv170. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri TK, Pal SK, McDermott DF, Morrissey S, Ferguson KC, Holland J, et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol. 2014 Aug;25(8):1603–8. doi: 10.1093/annonc/mdu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal SK, Drabkin HA, Reeves JA, Hainsworth JD, Hazel SE, Paggiarino DA, et al. A phase 2 study of the sphingosine-1-phosphate antibody sonepcizumab in patients with metastatic renal cell carcinoma. Cancer. 2017 Feb;123(4):576–82. doi: 10.1002/cncr.30393. [DOI] [PubMed] [Google Scholar]
- 8.Kis B, Shah J, Choi J, El-Haddad G, Sweeney J, Biebel B, et al. Transarterial Yttrium-90 Radioembolization Treatment of Patients with Liver-Dominant Metastatic Renal Cell Carcinoma. J Vasc Interv Radiol. 2017 Feb;28(2):254–9. doi: 10.1016/j.jvir.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdelmaksoud MH, Louie JD, Hwang GL, et al. Yttrium-90 radioembolization of renal cell carcinoma metastatic to the liver. J Vasc Interv Radiol. 2012 Mar;23:323–3.e10. doi: 10.1016/j.jvir.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Ng J, Dai T. Radiation therapy and the abscopal effect: a concept comes of age. Ann Transl Med. 2016 Mar;4(6):118. doi: 10.21037/atm.2016.01.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghodadra A, Bhatt S, Camacho JC, Kim HS. Abscopal Effects and Yttrium-90 Radioembolization. Cardiovasc Intervent Radiol. 2016 Jul;39(7):1076–80. doi: 10.1007/s00270-015-1259-0. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro Gomes J, Schmerling RA, Haddad CK, Racy DJ, Ferrigno R, Gil E, et al. Analysis of the Abscopal Effect With Anti-PD1 Therapy in Patients With Metastatic Solid Tumors. J Immunother. 2016 Nov/Dec;39(9):367–72. doi: 10.1097/CJI.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 13.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012 Mar;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol. 2018 Feb doi: 10.1200/JCO.2017.76.2229. JCO2017762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson CB, Jagsi R. The Promise of the Abscopal Effect and the Future of Trials Combining Immunotherapy and Radiation Therapy. Int J Radiat Oncol Biol Phys. 2016 Jul;95(4):1254–6. doi: 10.1016/j.ijrobp.2016.02.067. [DOI] [PubMed] [Google Scholar]