Abstract
Bone morphogenetic proteins (BMPs) activate the canonical Smad1/5/8 and non-canonical Tak1-MAPK pathways via BMP receptors I and II to regulate skeletal development and bone remodeling. Specific ablation of Bmpr1a in immature osteoblasts, osteoblasts, or osteocytes results in an increase in cancellous bone mass, yet opposite results have been reported regarding the underlying mechanisms. Moreover, the role for BMPRIA-mediated signaling in bone marrow mesenchymal stromal cells (BM-MSCs) has not been explored. Here, we specifically ablated Bmpr1a in BM-MSCs in adult mice to study the function of BMPR1A in bone remodeling and found that the mutant mice showed an increase in cancellous and cortical bone mass, which was accompanied by a decrease in bone formation rate and a greater decrease in bone resorption. Decreased bone formation was associated with a defect in BM-MSC osteogenic differentiation whereas decreased bone resorption was associated with a decrease in RANKL production and osteoclastogenesis. However, ablation of Tak1, a critical non-canonical signaling molecule downstream of BMP receptors, in BM-MSCs at adult stage did not affect bone remodeling. These results suggest that BMP signaling through BMPRIA controls BM-MSC osteogenic differentiation/bone formation and RANKL expression/osteoclastogenesis in adult mice independent of Tak1 signaling.
Introduction
The bone undergoes life-long remodeling, which is carried out by bone forming osteoblasts and bone resorbing osteoclasts1–3. Bone mass and quality can be regulated at the level of osteoblastogenesis/bone formation, osteoclastogenesis/bone resorption, and coupling between bone formation and resorption4–6. Several lines of evidence have shown that some osteogenic regulators, e.g., transforming growth factor-β1 (TGF-β1), bone morphogenetic proteins (BMPs), and insulin growth factor-1 (IGF-1), released from bone matrix after resorption or synthesized by osteoclasts are important coupling factors5,7. Besides, osteoblasts produce some of the master regulators of osteoclastogenesis, e.g., macrophage colony stimulating factor (M-CSF), receptor activator of nuclear factor-kappaB ligand (RANKL), and osteoprotegerin (OPG)4,8,9. Disruption of the coupling between bone formation and resorption can cause bone-related diseases10.
BMPs are known as ectopic bone inducers and play crucial roles in skeletal development and bone homeostasis11–15. The BMP signaling pathway commences with binding to the heterodimeric serine/threonine kinase receptors, BMPRI and BMPRII12,16,17. BMPRII is constitutively active and can phosphorylate and activate BMPRI upon ligand binding. All the BMPRI molecules, BMPRIA, BMPRIB and ACVR1, have been shown to contribute to skeletal development15,18–21. It has been reported that Bmpr1a, when overexpressed, successfully rescues the differentiation defect of chondrocytes due to deletion of Bmpr1b22, suggesting that they have redundant functions.
Previous studies have revealed vital roles of BMPs and their receptors in osteoblast and chondrocyte differentiation and bone formation during skeletal development11,16,18,23. Conventional knockout mice for Bmpr1a show early embryonic lethality24. Conditional Bmpr1a ablation in osteoclasts and their progenitors uncovered critical roles for BMP and BMPR in osteoclast differentiation and bone resorption as well25,26. Interestingly, osteoblast-specific Bmpr1a deletion with osteocalcin-Cre (Og2-Cre) results in age-dependent change in bone mass. The young mutant mice show a low bone mass due to reduced bone formation, while the aged mutant mice show a high bone mass due to reduced bone resorption21. Inducible Bmpr1a ablation in osteoblasts using Col1-CreER mice results in a dramatic decrease in osteoclastic activity through the decrease of RANKL/OPG ratio without affecting osteoblastic activity, leading to an increase in bone mass21,27–29. On the other hand, Bmpr1a ablation in mature osteocytes does not affect osteoclastogenesis or bone resorption, yet bone formation rate and bone mass are increased30,31. Thus, the roles played by BMPR1A at different stages from BM-MSC to osteocyte, especially in BM-MSCs, remains discordant and warrants further investigation. In addition, the physiological roles of Tak1-mediated non-canonical pathways of BMPs in BM-MSCs has not yet been elucidated. Expression of Tak1 is essential for embryonic development and germline deletion of Tak1 leads to embryonic lethality32. Earlier studies have reported that Tak1 is critical for normal development of growth plate and joints besides having important regulatory function in innate immunity, hematopoietic stem cell survival, and embryonic angiogenesis as well as in homeostasis of liver, airway smooth muscles, cardiac muscles and cartilages33–39. Moreover, Tak1 has been shown to play important roles in osteoclast differentiation and survival, osteoblast maturation, and chondrocyte differentiation40–44.
In this study, we generated transgenic Prx1-CreERT; Bmpr1af/f and Prx1-CreERT; Tak1f/f mouse lines to delineate the roles of BMPRIA and Tak1 in adult BM-MSCs, when endochondral ossification becomes minimal, using transgenic Prx1-CreERT mice generated in Shunichi Murakami’s lab45 that express tamoxifen inducible CreER under the control of a 2.4 kb Prx1 promoter. The Prx1-CreERT; Bmpr1af/f mice showed increased bone mass due to decreased bone formation and much greater reduction in osteoclastic resorption. On the other hand, Prx1-CreERT; Tak1f/f mice showed no significant change in bone mass. Our results suggest that BMPRIA signaling controls BM-MSC osteogenic differentiation and the coupling to osteoclastogenesis in a Tak1-independent manner.
Materials and Methods
Mice
The Rosa-tdTomato mouse line was purchased from the Jackson Laboratory. The transgenic Prx1-CreERT mice were generated in Shunichi Murakami’s lab45 and the floxed Bmpr1a and Tak1f/f mice were generated in Michael D. Schneider’s lab33,46. Rosa-tdTomato mice were bred with Prx1-CreERT mice to generate Prx1-CreERT; Rosa-tdTomato mice. Bmpr1af/f and Tak1f/f mice were mated with Prx1-CreERT transgenic mice to generate Prx1-CreERT; Bmpr1af/f and Prx1-CreERT; Tak1f/f mice, in which Bmpr1a or Tak1 was deleted within the osteochondral progenitor cells. Tamoxifen was injected to adult mice to delete the genes. Animals were bred and housed under specific pathogen-free (SPF) condition and provided with food and water ad libitum. Animal experimentations in this study were performed following the recommendations of the National Research Council Guide for Care and Use of Laboratory Animals, with all the experimental protocols approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University, China [SYXK (SH) 2011-0112].
Tamoxifen (TAM) administration
TAM (T5648, Sigma-Aldrich, St. Louis, MO, USA) powder was dissolved in corn oil at a concentration of 10 mg/ml. It was foil-wrapped to protect from light. TAM was administered intraperitoneally to 2-month-old mice at a dose of 100 mg/kg body weight every other day in the early morning for a total of 3 doses. Rosa-tdTomato reporter mice were used to evaluate the Cre activity. Femurs, tibiae and lumbar vertebrae were analyzed one month after the last TAM injection by micro-computed tomography (micro-CT), X-ray imaging, and histomorphometry. The same schedule of TAM administration was followed for all the experiments and genotypes.
X-ray and micro-CT analyses
Radiographs of femurs, lumbar vertebrae from adult mice were taken using the Cabinet X-Ray system (LX-60, Faxitron Bioptics) following a standardized setting (45 kV for 8 s).
After dissecting free of soft tissue, femurs and tibiae from adult (n = 8 for all groups) male mice were fixed overnight in 4% paraformaldehyde (PFA) and stored in 70% ethanol. The distal femurs and proximal tibiae were scanned using a high-resolution Skyscan1176 micro-CT scanner system (Bruker, Kontich, Belgium). Briefly, the following settings for scanning were applied: 50 kV source voltage, 0.2 mm aluminium filter, 270 μA source current, 1350 ms exposure time, 8.96 μm image pixel size and rotation step (degree) of 0.500. After scanning, 3D images were reconstructed using NRecon version 1.6.9.8 (Skyscan, Kontich, Belgium), with a ring artifact reduction of 8, 30% beam hardening correction, and smoothing of 2 using a Gaussian kernel. 2D/3D analyses were performed using CTAn version 1.15.4.0+ (Skyscan, Kontich, Belgium). Finally, CTvox version 2.6.0 r908 (Skyscan, Kontich, Belgium) was used for 3D visualisation. Bone morphometric parameters such as bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp) were derived following the manufacturer’s instructions. Nomenclature and abbreviations of micro-CT parameters were followed according to guidelines recommended by American Society for Bone and Mineral Research.
In vivo calcein labeling
For fluorochrome-labeled in vivo bone studies, mice received calcein (C0875, Sigma-Aldrich, Louis, MO, USA) twice intraperitoneally at a dose of 0.2 mg/20 g body weight at 7-day interval and were sacrificed 1 day after the second injection. The dynamic histomorphometric parameters of bone turnover including mineral apposition rate (MAR), bone formation rate per bone volume (BFR/BV), mineralizing surface per bone surface (MS/BS) were quantified from calcein labeling.
Bone Histology
For histologic evaluation of the bone, femurs were fixed in 4% PFA overnight, decalcified in EDTA for 3–4 weeks, dehydrated in gradient ethanol, cleared in xylene, and embedded in paraffin. After that, 4 μm thick sections were cut with microtome (RM2255, Leica Microsystems GmbH). For histological analysis, sections were stained with Villanueva-Goldner’s one-step trichrome and Safranin O/Fast green stain.
Bone histomorphometry
Undecalcified femurs were used as histomorphometric samples. In brief, after anaesthetized mice were euthanized by cervical dislocation, femurs were removed, fixed in 4% PFA overnight, rinsed in 1XPBS for 3 times, dehydrated in an increasing series of alcohol from 95% to 100%, cleared in xylene overnight, vacuumed for 30 min. and then embedded in methyl methacrylate (Merck-Schuchardt). Sections were cut to a thickness of 4 μm using a hard tissue microtome (RM2255, Leica Microsystems GmbH). Dynamic histomorphometry was conducted on unstained bone sections while sections stained with Villanueva-Goldner’s one-step trichrome were used for static histomorphometry. All parameters were measured at the secondary spongiosa of distal femur section where analyses commenced 350 μm distal to the growth plate. Nikon Eclipse 80i microscope (Nikon Instruments Inc.) was used for capturing and analyzing images. All bone-specific parameters were measured using OsteoMeasure software (OsteoMetrics Inc.). Units of measurement and expression of these parameters accord with the guidelines of the American Society for Bone and Mineral Research (ASBMR).
Real-Time Quantitative PCR (qPCR)
Total RNA from cells or femurs were extracted with Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions. cDNA was synthesized using Transcriptor First Strand cDNA synthesis kit (Roche, Basel, Switzerland) with random anchored-oligo (dT) 18 primers and random hexamer. qPCR analyses were performed using FS Universal SYBR Green Master Premix (Roche). Reactions were run in triplicate in three repeated experiments in a total volume of 20 μl with Light Cycler 480 Instrument II (Roche). The relative expression levels of mRNA species were quantified using the delta-delta Ct (∆∆Ct) method. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was used as endogenous control gene to normalize the expression of all target genes. The primer sequences used for real-time PCR are listed below:
Opg F: 5′-CACCCTGTGTGAAGAGGCCT-3′
R: 5′-GCAGGCTCTCCATCAAGGCA-3′
M-Csf F: 5′-CTGACACAGGCCATGTGGAG-3′
R: 5′-GAGAGGGTAGTGGTGGATGT-3′
Rankl F: 5′-GCA CAC CTC ACC ATC AAT GCT-3
R: 5′-GGT ACC AAG AGG ACA GAG TGA CTT TA-3′
Alp F: 5′-TGAGCGACACGGACAAGA-3′
R: 5′-GGCCTGGTAGTTGTTGTGAG-3′
Osx F: 5′-ACTCATCCCTATGGCTCGTG-3′
R: 5′-GGTAGGGAGCTGGGTTAAGG-3′
Ocn F: 5′AGCAGGAGGGCAATAAGGTAGT-3′
R: 5′-ACCGTAGATGCGTTTGTAGGC-3′
Runx2 F: 5′-TTTAGGGCGCATTCCTCATC-3′
R: 5′-TGTCCTTGTGGATTAAAAGGACTTG-3′
Trap F: 5′-TGTCATCTGTGAAAAGGTGGTC-3′
R: 5′-ACTGGAGCAGCGGTGTTATG-3′
Bmpr1a F: 5′-TCATGTTCAAGGGCAGAATCTAGA-3′
R: 5′-GGCAAGGTATCCTCTGGTGCTA-3′
GapdhF: 5′-CCACAGTCCATGCCATCAC-3′
R: 5′-CATACCAGGAAATGAGCTTGAC-3′
Western blot analysis
Cells or bone tissues were homogenized in T-PER reagent (Thermo Fisher Scientific) supplemented with protease and phosphatase inhibitors. Protein concentration was determined using a BCA Protein Assay Kit (Thermo Fisher Scientific). 20 μg of protein lysates of each sample was subjected to SDS-PAGE (8–12%) and transferred onto a nitrocellulose membrane. Immunoblots were incubated with the specific primary antibodies overnight at 4 °C with gentle shaking. After being washed three times for 15 min each with TBST (Tris-buffered saline, 0.1% Tween 20), blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Sigma) for one hour. Next, the protein level was detected with Super Signal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) and visualized by FluorChem E system (Protein Simple, CA, USA). The following antibodies were used: p-Smad1/5/8 (Cell Signaling, 9511), Tak1 (Cell Signaling, 4505, Boston, MA), Bmpr1a (Abcam, ab38560), β-Actin (Santa Cruz, Sc-47778).
Measurement of urine Deoxypyridinoline (DPD)
Urinary DPD level was determined to evaluate the bone resorption rate in vivo, following the procedure recommended by MicroVue (Quidel, CA, USA). For this study, mice were individually housed and urine samples were collected early in the morning using a microcapillary tube. DPD values were normalized to the urine levels of creatinine.
TRAP (tartrate-resistant acid phosphatase) staining
Paraffin sections (4 μm) were stained using the Acid Phosphatase, Leukocyte TRAP Kit (387 A, Sigma-Aldrich) following the manufacturer’s instructions. Images were taken using the Nikon Eclipse 80i microscope (Nikon Instruments Inc.). Cells with ≥3 nuclei were considered TRAP-positive multinuclear osteoclasts.
Isolation and culture of bone marrow BM-MSCs
Briefly, after sacrificing the adult mice, whole body was rinsed in 70% (v/v) ethanol for 2–3 min. Femurs and tibiae were dissected and carefully cleaned from muscle, connective tissue and residual soft tissues. Bones were then transferred to a sterile culture dish containing α-MEM supplemented with 1× penicillin/streptomycin on ice. Two ends of each bone were excised and a syringe needle (27-gauge) was inserted into bone cavity to flush out bone marrow with α-MEM. This process was repeated several times until the color of the bones turned from red to white. To get rid of any bone debris, the cell suspension was filtered through a 70 μm nylon mesh filter (BD Falcon, USA). Cells were cultured in cell culture plate in α-MEM supplemented with 10% fetal bovine serum (FBS; Sigma), 100 μg/ml penicillin and 100 μg/ml streptomycin (Gibco). Plates were then incubated at 37 °C with 5% CO2 in a humidified chamber for 5 days. Non-adherent cells were removed by changing the medium and the BM-MSCs were either directly used or kept frozen for future experiments.
CFU (Colony forming unit) assay
To enumerate the number of MSCs in bone marrow, the CFU assay was performed. Cells were seeded in 12-well plates at the density of 5 × 106 per well. The plates were incubated in a humidified 5% CO2/37 °C environment. Fresh complete α-MEM medium was changed every 3 days. After 10 days of culture, the formation of CFUs was evaluated by washing the plates with sterile phosphate buffered saline (PBS) twice, fixing with 4% PFA for 30 min at room temperature, and staining with ALP (Sigma). Colonies with more than 50 cells were counted using light microscopy.
Osteogenic differentiation of BM-MSCs
For alkaline phosphatase (ALP) activity assay, BM-MSCs were plated at a density of 5 × 104 cells/well in 12-well plates containing complete medium. After 24 hr., culture medium was replaced with osteogenic differentiation medium containing complete medium supplemented with 10 mM β-glycerol phosphate and 50 μg/ml Ascorbic acid for 7–10 days. Medium was changed every 3 days. To evaluate osteogenic differentiation, cells were then washed with sterile 1X PBS, fixed in 4% PFA for 30 min at room temperature, and stained for ALP using an Alkaline Phosphatase Kit (sigma) following the manufacturer’s protocol.
Statistical analysis
Results were expressed as means ± SD. Comparisons between wild-type and transgenic mice were analyzed using two-tailed unpaired Student’s t-test and a p value of less than 0.05 was considered to indicate statistical significance. 3–4 litters were analyzed for every parameter. Each experiment was repeated 3–4 times using materials from different mice to ensure the reproducibility of the experimental findings. For in vitro experiments, 3–4 pairs of mice were used. For bone histomorphometry and micro-CT analyses, 8 mutants and 8 control littermates were used.
Data Availability
Data supporting the findings of this study are available from the corresponding author on request (email: libj@sjtu.edu.cn).
Results
Tracing of Prx1 lineage cells in the adult bone
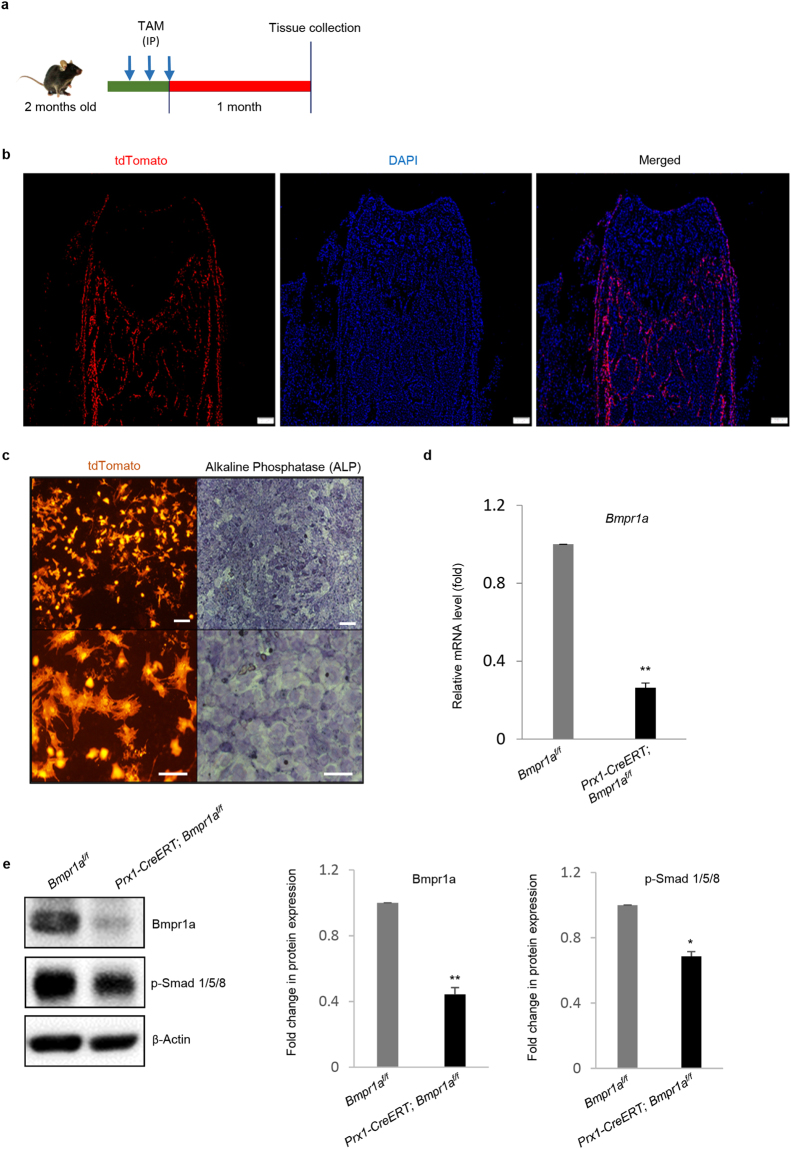
In an attempt to trace Prx1 lineages in adult bone, we generated Prx1-CreERT; Rosa-tdTomato mice. We administrated TAM to adult mice through intraperitoneal route. Similar to previously reported results45, we detected tdTomato fluorescence signals in osteoblasts and osteocytes in cancellous and cortical bone, as well as cells at the endosteal and periosteal surfaces (Fig. 1a,b), yet, the absence of tdTomato signals in growth plate or articular cartilage suggests that Prx1 + BM-MSCs do not generate chondrocytes in adult mice (Fig. 1b). In addition, we isolated bone marrow BM-MSCs from Prx1-CreERT; Rosa-tdTomato mice (injected with 3 doses of TAM) and cultured them in vitro. A large portion of the cells were Tomato positive and they could differentiate into ALP positive osteoblasts (Fig. 1c). These results confirmed that Prx1 + BM-MSCs are present in the bone marrow and can give rise to osteoblasts and osteocytes, but not chondrocytes, in adult mice.
Figure 1.
Prx1-CreERT mediated tracing and Bmpr1a ablation in adult mice. (a) Schedule for TAM administration to 2-month-old mice. TAM was injected to both Prx1-CreERT; Bmpr1af/f and Bmpr1af/f mice (age- and sex-matched littermates) on every other day for 3 doses. One month after the last Tam injection, bone tissues were retrieved and analyzed. (b) Tissue specificity and effectiveness of Cre recombinase in Prx1-CreERT; Rosa-tdTomato mice. Osteoblasts and osteocytes as well as the endosteal and periosteal surfaces of bones were labeled by tdTomato fluorescent signals. N = 3 mice, Scale bar = 200 μm. (c) In vitro analysis revealed tdTomato + bone marrow BM-MSCs (left panel) with the potential to differentiate into ALP-positive osteoblasts (right panel). Lower panel indicates higher magnification of upper panel. Upper panel scale bar = 100 μm and lower panel scale bar = 50 μm. (d) Quantitative real-time RT-PCR analysis of BM-MSCs showed that Bmpr1a was deleted (>70%) in the mutant mice. (e) Immunoblotting of bone extracts also confirmed a reduction in the level of Bmpr1a and phosphorylated-Smad 1/5/8. Left panel: Representative Western blots; Right panel: Quantification of relative protein expressions (from three repeated experiments) plotted in the graph as fold change. N = 4 mice/group; *P < 0.05. For the full-length blots see Supplementary Fig. S1.
Bmpr1a ablation resulted in increased bone mass
To evaluate the functions of BMPR1A in adult BM-MSCs, we generated Prx1-CreERT; Bmpr1af/f mice. TAM was administrated to 2-month-old Prx1-CreERT; Bmpr1af/f mice 3 times and the mouse bones were analyzed one month after the last TAM injection. Quantitative RT-PCR analysis of bone extracts revealed a significant reduction in the levels of Bmpr1a mRNA in the mutant mice (Fig. 1d). Western blot analysis of bone extracts also confirmed a decrease in Bmpr1a and phosphorylated Smad 1/5/8 in the mutant mice (Fig. 1e and Supplementary Fig. S1). These results demonstrated that the TAM administration deleted Bmpr1a in the adult bone.
During the studies, we found that even 3 doses of TAM showed an effect on bone mass in adult wild type mice. TAM appeared to increase bone mass at the cancellous bone (Supplementary Fig. S2a and b). To exclude the possible complication of TAM, we used Bmpr1af/f treated with TAM as a control for Prx1-CreERT; Bmpr1af/f mice treated with TAM. Prx1-CreERT; Bmpr1af/f and Bmpr1af/f mice without TAM treatment were also included as controls. X-Ray analysis of femurs from Prx1-CreERT; Bmpr1af/f and WT littermates showed no difference in the radiodensity without TAM injection (Supplementary Fig. S3a). Similar findings were found when we examined femurs histologically (Supplementary Fig. S3b). Therefore, Prx1-CreERT; Bmpr1af/f mice have no baseline phenotype, validating the effectiveness of tamoxifen-inducible CreERT system.
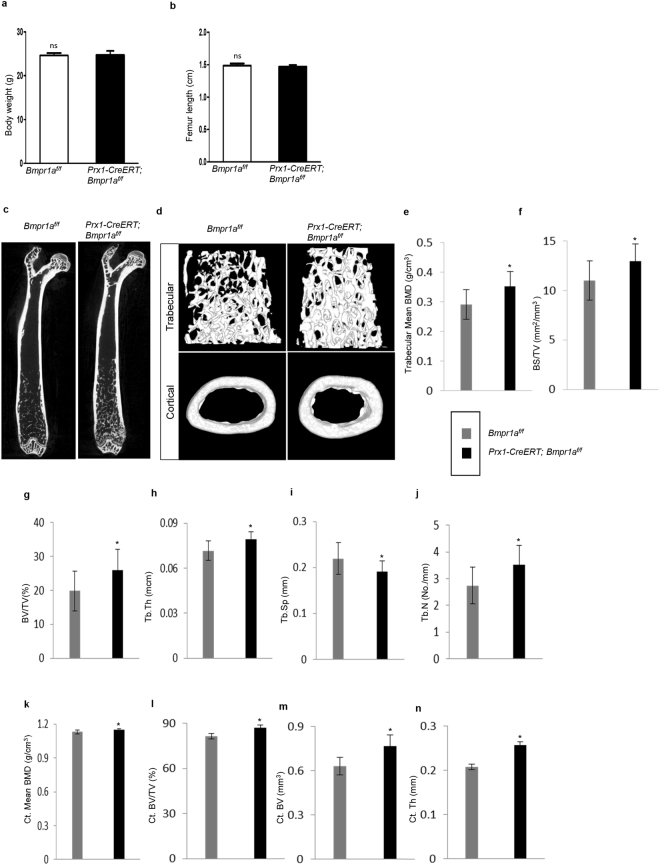
We found that one month after Bmpr1a was deleted in Prx1 + BM-MSCs, the mutant mice looked normal morphologically with no significant difference in body weight or femur length compared with control (Fig. 2a,b). Quantitative data obtained from micro-CT of femurs showed an increase in bone volume and bone mineral density in Prx1-CreERT; Bmpr1af/f mice at the cancellous bone (Fig. 2c–j). Unlike other reported osteoblast-specific Bmpr1a knockout mouse lines, Prx1-CreERT; Bmpr1af/f mice showed an increase in bone volume and bone mineral density at the cortical bone (Fig. 2k–n). Moreover, qualitative analysis of femurs by von-Kossa staining also revealed an increase in the amount of mineralized bone in mutant mice (Supplementary Fig. S4a). On the other hand, the lumbar vertebrae in the mutant mice did not show a significant change compared to control mice (Supplementary Fig. S4b), indicating that Prx1 is not expressed in vertebral bones.
Figure 2.
Micro-CT-derived bone morphometry results revealed an increase in bone volume and bone mineral density in Prx1-CreERT; Bmpr1af/f mice. (a,b) Body weight and femur lengths of mutant and control littermates showed no significant difference. Data are expressed as mean ± SEM from 8 mice per group. (c) Representative micro-CT images of 2D whole femur segital section from adult Prx1-CreERT; Bmpr1af/f and their respective control mice. (d) 3D distal femoral metaphyseal cancellous bone (upper panel) and mid-diaphyseal cortical bone (lower panel). N = 8 mice per group. (e–j) Micro-CT quantification of bone histomorphometry indices revealed high trabecular bone mass in mutant mice. Tb. BMD, trabecular bone mineral density (g/cm3) (e); BS/TV, bone surface/tissue volume (mm2/mm3) (f); BV/TV, bone volume/tissue volume (%) (g); Tb.Th, trabecular thickness (mcm) (h); Tb.Sp, trabecular spacing (mm) (i); and Tb.N, trabecular number (No./mm) (j). (k–n) Micro-CT quantification revealed high cortical bone phenotypes in mutant mice. Ct. BMD, Cortical bone mineral density (g/cm3) (k); Ct, BV/TV, cortical bone mass (%) (l); Ct. BV, cortical bone volume (mm3) (m); and Ct.Th, cortical thickness (mm) (n). N = 8 mice per genotype; *P < 0.05.
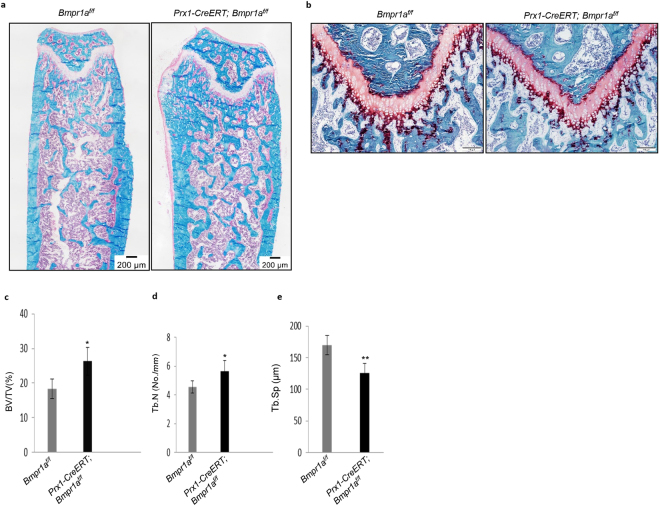
To validate these findings, we carried out bone histomorphometric analysis of Villanueva-Goldner’s one-step trichrome-stained undecalcified femurs. The long bones of the mutant mice showed normal growth plates (Fig. 3a,b), yet, the bone volume (BV/TV, %) and trabeculae number (Tb.N, mm) were markedly increased whereas trabecular spacing (Tb.Sp, mm) was decreased in the mutant compared to control mice (Fig. 3c–e), similar to the micro-CT results. These results collectively indicate that ablation of Bmpr1a in Prx1 + BM-MSCs led to an increase in bone mass.
Figure 3.
Static histomorphometric analysis showed deletion of Bmpr1a resulted high bone mass in adult Prx1-CreERT; Bmpr1af/f mice. (a) Villanueva-Goldner’s one-step trichrome staining on undecalcified femurs demonstrated the micro-architecture of bone. Scale bar = 200 μm. (b) Safranin O staining showed no significant differences in the growth plates of mutants and their respective controls. Scale bar = 100 μm. (c–e) Quantification using osteomeasure software. BV/TV, bone volume/tissue volume (%) (c); and Tb/N, trabecular number (No./mm) (d) were increased but Tb.Sp (μm) (e) was decreased in Prx1-CreERT; Bmpr1af/f mice compared with Bmpr1af/f control littermates. Data are mean ± SEM (N = 8 bones/group; 4 fields/bone); *P < 0.05; **P < 0.01.
Disruption of Bmpr1a led to a decrease in bone formation and resorption
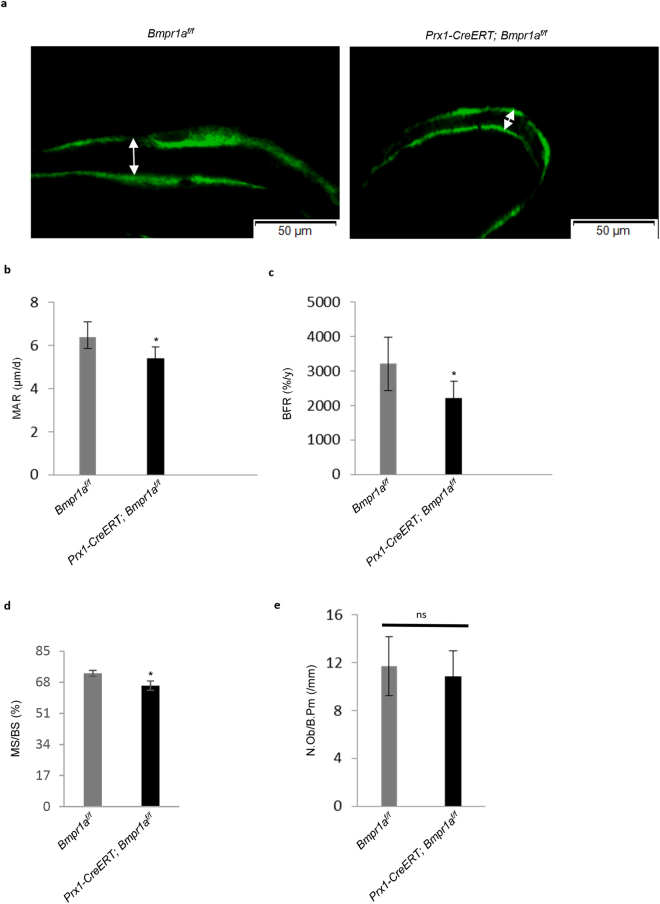
A harmonized balance between bone formation and resorption is crucial to maintain bone mass. We then analyzed bone formation and resorption rates in Bmpr1a deficient bones. Bone formation parameters, MAR, BFR, and MS/BS of the cancellous bone of femurs were assessed by dynamic histomorphometry (Fig. 4a). We found a significant reduction in MAR, BFR, and MS/BS parameters in bone sections of the mutant mice compared to control mice, indicating an overall decrease in bone formation rate (Fig. 4b–d). However, no significant change in the numbers of osteoblasts at the cancellous bone was observed in the mutant mice compared to control mice (Fig. 4e), suggesting that the decrease in the bone formation rate is likely caused by reduced activities of the mutant osteoblasts.
Figure 4.
Dynamic morphometric markers for bone formation parameters in adult Prx1-CreERT; Bmpr1af/f mice. (a) Representative calcein-labeled images of the distal femurs. The distance between two layers of calcein labels visualized by fluorescence represented bone formation that occurred during the 5-d period between calcein injections. Scale bar = 50 μm. (b–d) Adult Prx1-CreERT; Bmpr1af/f mice showed a significant decrease in MAR, mineral apposition rate (μm/d) (b), BFR, bone formation rate (%/y) (c) and MS/BS, mineralizing surface per bone surface (%) (d) values compared with age-matched control mice. Data are mean ± SEM (N = 8 bones/group; 5 fields/bone); *P < 0.05. (e) Prx1-CreERT; Bmpr1af/f mice revealed no change in the number of osteoblasts when measured the value of one histomorphometric index of bone formation, N.Ob/B.Pm, osteoblast number per bone perimeter (/mm). N = 8 mice. ns = Non-significant.
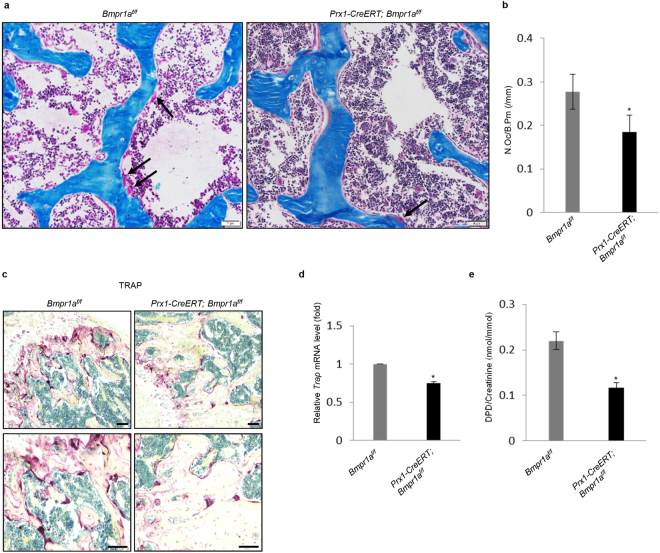
In addition, the number of osteoclasts per bone surface calculated from static histomorphometric analysis on Villanueva-Goldner’s one-step trichrome-stained femurs was reduced in the mutant mice compared to control mice (Fig. 5a,b). To validate this finding, we stained the femur sections for tartrate-resistant acid phosphatase (TRAP), an osteoclast marker. The mutant mice showed a decrease in TRAP staining (Fig. 5c), yet we found that it was hard to count TRAP-positive cells on these bone sections. We then measured the mRNA levels of Trap of the bone homogenates and found that the mutant mice showed a decrease in the levels of Trap (Fig. 5d). Furthermore, we examined the levels of urinary deoxypyridinoline (DPD), an in vivo bone resorption marker and found that the mutant mice showed decreased DPD levels (Fig. 5e). Taken together, these results suggest that both bone formation and resorption in adult mice were declined by the deficiency of Bmpr1a gene in BM-MSCs, yet the decrease in bone resorption was greater than the decrease in bone formation rate.
Figure 5.
Bmpr1a ablation resulted in significant reduction in the bone resorption parameters in adult Prx1-CreERT; Bmpr1af/f mice. (a) Histological sections of the femurs of adult mice where arrows identified osteoclasts lining cancellous bone surfaces. N = 8 bones/group, Scale Bar = 50 μm. (b) Measurements of N.Oc/B.Pm (Number of osteoclast/bone perimeter, /mm) on Villanueva-Goldner’s Trichrome-stained femurs. Prx1-CreERT; Bmpr1af/f mice showed a reduction in the number of osteoclasts. *P < 0.05. (c) Representative images of TRAP-stained bones of mutant and control littermates exhibited a decrease in the TRAP staining in Prx1-CreERT; Bmpr1af/f mice compared to control mice. Scale bar = left panel 100 μm and right panel 50 μm. (d) Quantitative RT-PCR analysis of Trap as bone resorption marker. mRNA was isolated from bone extract and realtime quantitative RT-PCR was carried out to determine the level of Trap. N = 4 mice; *P < 0.05. (e) Prx1-CreERT; Bmpr1af/f mice showed a decrease in DPD (deoxypyridinoline) level. The ELISA assay was performed using urine samples from mutant and control mice. N = 4 mice per group; *P < 0.05.
Bmpr1a−/− BM-MSCs showed impaired osteoblast differentiation
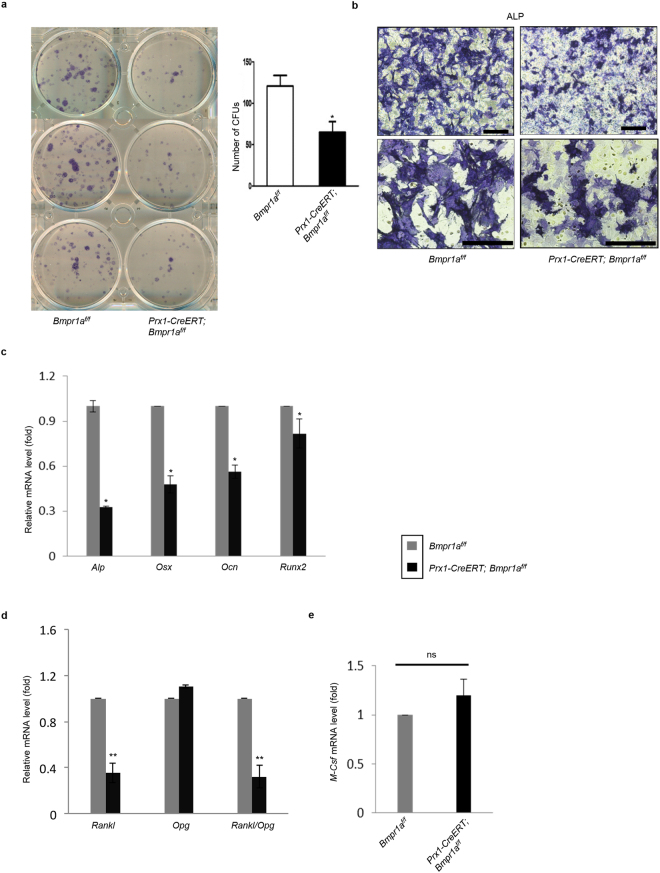
To determine whether low bone formation rate in the mutant mice was related to osteoblasts defects, we examined the role of Bmpr1a in BM-MSC osteogenic differentiation. We harvested bone marrow BM-MSCs to carry out in vitro CFU assay and found that deletion of Bmpr1a led to significantly reduced number of ALP-positive CFUs, consistent with histomorphometric results in Prx1-CreERT; Bmpr1af/f mice (Fig. 6a). We also cultured BM-MSCs in osteoblast differentiation medium and found that inactivation of the Bmpr1a impaired osteoblast differentiation, manifested by a decrease in ALP staining and the mRNA levels of Alp, Osx, Runx2, and Ocn (Fig. 6b,c). These results, together with the findings that Col1-CreERT-mediated Bmpr1a ablation did not affect osteoblast differentiation whereas Bmpr1a ablation in mature osteocytes increased bone formation29,31, suggest that BMPR1A is required for MSC osteogenic differentiation at an early stage, which is likely through regulating the expression of Runx2 and Osx23.
Figure 6.
Deletion of Bmpr1a in BM-MSCs resulted in impaired osteoblast differentiation and decreased RANKL expression. (a) Comparison of bone marrow cell cultures isolated from Bmpr1a deficient mice and their respective littermates to evaluate ALP-positive CFU. Prx1-CreERT; Bmpr1af/f mice showed a decrease in the number of CFUs. Left panel: ALP staining. Right panel: quantitation data. N = 3 mice; *P < 0.05. (b) Alkaline phosphatase (ALP) activity in osteogenic medium showed a decrease in osteoblast differentiation in Bmpr1a ablated mice. N = 3. Scale bar = Upper panel 200 μm, lower panel 100 μm. (c) Quantitative RT-PCR analysis showed that the expression levels of some osteoblast marker genes, alkaline phosphatase (Alp), osterix (Osx), osteocalcin (Ocn) and runt-related transcription factor 2 (Runx2) were decreased in mutant mice. N = 3 mice. (d-e) Quantitative RT-PCR analysis of Rankl, Opg and M-Csf mRNA. Results showed that the levels of Rankl was down-regulated, while the expression of Opg was not affected, resulting in a significant reduction of the ratio of Rankl to Opg in Prx1-CreERT; Bmpr1af/f mice at adult stage. N = 4 mice; *P < 0.05, **P < 0.01 (d). Bmpr1a deleted BM-MSCs showed no significant alteration in the expression level of M-Csf. N = 4 mice. ns = Non-significant (e).
Furthermore, we checked mRNA levels of Rankl, Opg, and M-Csf in BM-MSC cultures by quantitative RT-PCR. These factors are synthesized by BM-MSCs, osteoblasts, and osteocytes to regulate osteoclastogenesis. It was found that the levels of Rankl was down-regulated (Fig. 6d), while the levels of Opg and M-Csf were not significantly affected by BMPRIA deficiency (Fig. 6d,e). This resulted in a significant decrease in the ratio of Rankl to Opg, consistent with the decrease in the number of osteoclasts and in bone resorption observed in Prx1-CreERT; Bmpr1af/f mice.
Ablation of Tak1 in Prx1 + BM-MSCs showed no significant bone phenotype
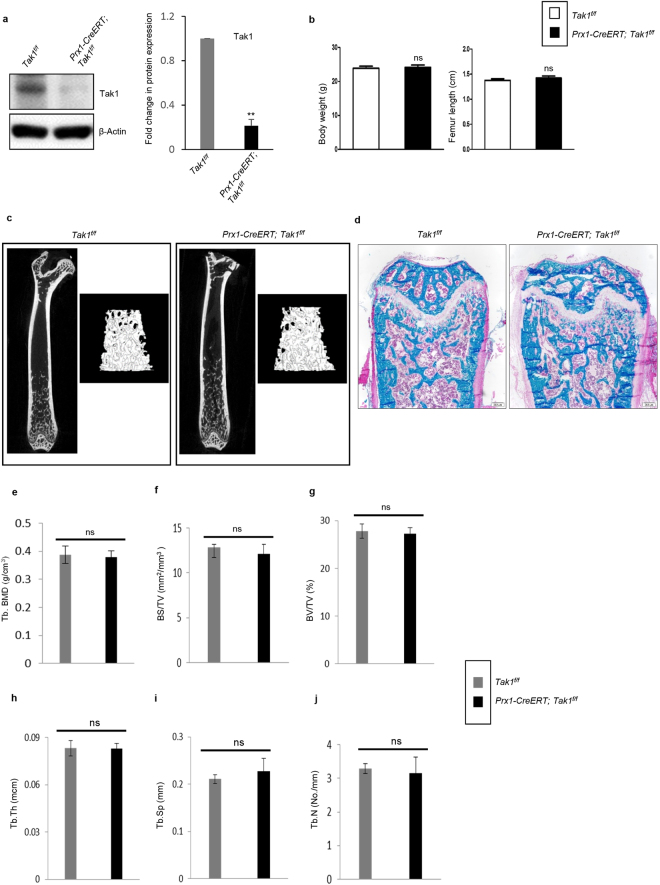
To determine the roles of the non-canonical pathways of BMPs in adult BM-MSCs, we chose to ablate Tak1, which encodes a kinase that acts upstream the MAPKs and NF-κB pathways in response to BMPs and TGFβ47–49. We generated Prx1-CreERT; Tak1f/f mice and induced Tak1 deletion in adult mice by injection of 3 doses of TAM (Fig. 7a and Supplementary Fig. S5). Body weight and femur length in these mice were unchanged (Fig. 7b). The mice were sacrificed one month after the last TAM injection. Micro-CT and X-ray analysis of long bones were performed and no significant change in bone mass at the cancellous and cortical bone was observed between mutant mice and WT littermates (Fig. 7c and e–j and Supplementary Fig. S6a–d). The growth plate of Prx1-CreERT; Tak1f/f mice was not altered either (Fig. 7d).
Figure 7.
Tak1 deletion in adult BM-MSCs showed no significant bone phenotype. (a) Western blot analysis of bone extracts confirmed a significant reduction in the level of Tak1 in Prx1CreERT; Tak1f/f mice. Left panel: Representative Western blots; Right panel: Quantification of relative protein expressions (from three repeated experiments) plotted in the graph as fold change. N = 4 mice/group; *P < 0.05; **P < 0.01. For the full-length blots see Supplementary Fig. S5. (b) Graph showing no significant difference in body weight, and femur length of mutant and control (age- and sex-matched) mice at adult age. ns = Non-significant. N = 8 per group. (c) Micro-CT scanning was performed to analyze cancellous and cortical bone architecture and mineralization on femurs of mice. No significant change between Prx1CreERT; Tak1f/f and Tak1f/f was observed. N = 6 mice per group. (d) Villanueva-Goldner’s one-step trichrome staining on undecalcified femurs showed the micro-architecture of bone. Scale bar = 200 μm. N = 6 mice per group. (e–j) Micro-CT results of the femurs. Tb. BMD, trabecular bone mineral density (g/cm3) (e); BS/TV, bone surface/tissue volume (mm2/mm3) (f); BV/TV, bone volume/tissue volume (%) (g); Tb.Th, trabecular thickness (mcm) (h); Tb.Sp, trabecular spacing (mm) (i); and Tb.N, trabecular number (No./mm) (j). ns = Non-significant.
To validate the in vivo findings, we carried out in vitro experiments using BM-MSC from Prx1-CreERT; Tak1f/f and Tak1f/f mice. We isolated BM-MSCs to perform CFU assay and found no significant difference in the number of ALP-positive CFUs between mutant mice and control littermates, consistent with micro-CT and X-ray results (Supplementary Fig. S7a). Next, we checked mRNA levels of Rankl and Opg in BM-MSC cultures by quantitative RT-PCR and found that the levels of Rankl and Opg and the ratio of Rankl to Opg were not significantly affected by TAK1 deficiency (Supplementary Fig. S7b and data not shown). These results indicate that Tak1 in BM-MSCs may not play an essential role in adult mice.
Discussion
In this study, we generated BM-MSC-specific Bmpr1a knockout mice in an attempt to gain an understanding of the effects of BMPRIA-mediated signaling on bone remodeling in adult mice. Note that Prx1-CreERT-mediated Bmpr1a ablation only occurs in BM-MSCs and their descendent cells in adult mice, when endochondral ossification is completed. This is in contrast to Dmp1-Cre and Osteocalcin-Cre mediated Bmpr1a ablation, which occurs in early embryonic development, and Col1-CreERT mediated Bmpr1a ablation, which occurs in differentiated osteoblasts45. Our results show that although the numbers of osteoblasts are not altered in Prx1-CreERT; Bmpr1af/f mice, their function is compromised. This could be attributable to the differentiation defect and the status of maturity of osteoblasts. We cannot exclude the possibility that BMP-BMPR signaling plays a role in the activity of osteoblasts. Our genetic results also show that BMPRIA plays a role in BM-MSC production of RANKL and coupling to osteoclastogenesis. Moreover, the cortical bone phenotypes observed in Prx1-CreERT; Bmpr1af/f mice suggest that Prx1 may be a better marker to label periosteal and endosteal BM-MSCs or osteoblasts.
Comparison of our BM-MSC-specific Bmpr1a and previously reported osteoblast/osteocyte-specific Bmpr1a knockout mouse lines reveals the following similarities and differences. First, adult deletion of Bmpr1a in Prx1 + BM-MSCs (Prx1-CreERT; Bmpr1af/f mice) results in an increase in bone mass, this is similar to aged Osteocalcin-Cre; Bmpr1af/f mice, adult Col1-CreERT; Bmpr1af/f mice, and Dmp1-Cre; Bmpr1af/f mice21,29,31. Prx1-CreERT; Bmpr1af/f mice also showed an increase in cortical bone mass, whereas Dmp1-Cre; Bmpr1af/f mice showed a reduction in cortical bone. Secondly, Prx1-CreERT; Bmpr1af/f mice showed an increase in bone resorption, this is similar to aged Osteocalcin-Cre; Bmpr1af/f mice and Col1-CreERT; Bmpr1af/f mice, but different from Dmp1-Cre; Bmpr1af/f mice, which showed no alterations in osteoclastogenesis and bone resorption. Thirdly, Prx1-CreERT; Bmpr1af/f mice showed a decrease in bone formation, this is similar to Osteocalcin-Cre; Bmpr1af/f mice and Col1CreERT; Bmpr1af/f mice, but opposite to Dmp1-Cre; Bmpr1af/f mice (Supplementary Table 1). In general, ablation of Bmpr1a in BM-MSCs or osteoprogenitor/osteoblasts gives rise to similar phenotypes in bone mass, bone formation, and bone resorption, which are different from ablation of Bmpr1a in terminally differentiated osteocytes.
The discrepancy among these mouse models can be explained in several ways. First, while Cre-mediated Bmpr1a ablation affects bone development in embryos, postnatal bone growth, and bone remodeling in adult mice, TAM-induced Bmpr1a ablation in adult Prx1 + BM-MSCs affects only bone remodeling. Moreover, due to the closure of the growth plates, TAM-induced Bmpr1a ablation in adult Prx1 + BM-MSCs does not go through endochondral ossification, instead, it reflects a direct BM-MSC to osteoblast differentiation. The increase in cortical bone mass, which does not require endochondral ossification, also support that BMPRIA plays a positive role in BM-MSC to osteoblast differentiation. Secondly, production of osteocytes from BM-MSCs is a multi-step process. Cells at various stages are different in their proliferation and differentiation potentials, and their contact with osteoclasts. Thus, their behaviors in response to BMPs may be stage-specific. Indeed, our previous studies have shown that Tsc1 and p38 MAPK have stage-specific effects on osteoblastogenesis and coupling to osteoclastogenesis50,51. Lastly, a recent study showed that Dmp1 could label cell types other than osteocytes including neurons and gut stromal cells52. It cannot be excluded that BMPRIA signaling in these cells may have an effect on bone remodeling.
Previous studies have demonstrated the importance of the canonical BMP-Smad1/5/8 pathway in osteoblastogenesis and bone formation. Ablation of Smad1 in osteoblasts using Col1-Cre led to osteopenia53. Tak1 is an important signaling molecule of the non-canonical pathway of BMPs. Downstream of Tak1, there are NF-κB and MAPKs pathways, which have been shown to affect osteoblastogenesis40. Previous studies have shown that Tak1 is required for chondrocyte differentiation and survival and for osteoblast differentiation54–56. However, TAM-induced Tak1 ablation in adult Prx1 + BM-MSCs appears not to affect bone mass. One possible explanation is that Tak1 deficiency may simultaneously impair both NF-κB signaling and MAPK signaling, which have been shown to have opposite functions in regulating osteoblast differentiation57–61. Nevertheless, these results suggest that the coordination or even integration of other signals to the BMP-signaling pathways may be crucial in regulating the activity of BM-MSCs, as previously reported62.
BMPs and BMPRs are targets for treatment of bone-related diseases15,63. Our genetic studies based on BM-MSC-specific Bmpr1a and Tak1 knockout models revealed critical roles of BMPRIA but not Tak1 in adult BM-MSC biology and reinforced the coupling between BM-MSCs and osteoclastogenesis.
Electronic supplementary material
Acknowledgements
We would like to thank Lina Gao and Haiyang Xie for technical assistance. The work was supported by the National Key Scientific Program (2012CB966901 and 2014CB942902) and National Natural Science Foundation of China (81130039 and 81421061).
Author Contributions
B.L. and J.L. designed research; S.B., P.L., H.W. and M.S. performed research; S.B., M.S., J.L. analyzed data; S.M. provided the Prx1-CreERT mice. M.D.S. and Y.M. provided the Bmpr1af/f and Tak1f/f mice. S.B. and B.L. wrote the paper. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26820-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Baojie Li, Email: libj@sjtu.edu.cn.
Jing Li, Email: lijing@xinhuamed.com.cn.
References
- 1.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaidi M, Buettner C, Sun L, Iqbal J. Minireview: The link between fat and bone: does mass beget mass? Endocrinology. 2012;153:2070–2075. doi: 10.1210/en.2012-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldridge D, Shchelochkov O, Kelley B, Lee B. Signaling pathways in human skeletal dysplasias. Annu Rev Genomics Hum Genet. 2010;11:189–217. doi: 10.1146/annurev-genom-082908-150158. [DOI] [PubMed] [Google Scholar]
- 4.Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- 5.Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-beta signaling in bone remodeling. J Clin Invest. 2014;124:466–472. doi: 10.1172/JCI70050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engin F, Lee B. NOTCHing the bone: insights into multi-functionality. Bone. 2010;46:274–280. doi: 10.1016/j.bone.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA. 2008;105:20764–20769. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards JR, Mundy GR. Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol. 2011;7:235–243. doi: 10.1038/nrrheum.2011.23. [DOI] [PubMed] [Google Scholar]
- 9.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–192. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michael H, Harkonen PL, Vaananen HK, Hentunen TA. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res. 2005;20:2224–2232. doi: 10.1359/JBMR.050803. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grafe, I., et al. TGF-beta Family Signaling in Mesenchymal Differentiation. Cold Spring Harb Perspect Biol. (2017). [DOI] [PMC free article] [PubMed]
- 13.Yang W, et al. Bmp2 in osteoblasts of periosteum and trabecular bone links bone formation to vascularization and mesenchymal stem cells. J Cell Sci. 2013;126:4085–4098. doi: 10.1242/jcs.118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shu B, et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124:3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu PB, et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaki-Yumoto M, Katsuno Y, Derynck R. TGF-beta family signaling in stem cells. Biochim Biophys Acta. 2013;1830:2280–2296. doi: 10.1016/j.bbagen.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Rigueur D, Lyons KM. TGFbeta signaling in cartilage development and maintenance. Birth Defects Res C Embryo Today. 2014;102:37–51. doi: 10.1002/bdrc.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan H, et al. BmpR1A is a major type 1 BMP receptor for BMP-Smad signaling during skull development. Dev Biol. 2017;429:260–270. doi: 10.1016/j.ydbio.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi C, et al. Deletion of BMP receptor type IB decreased bone mass in association with compromised osteoblastic differentiation of bone marrow mesenchymal progenitors. Sci Rep. 2016;6:24256. doi: 10.1038/srep24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowery JW, et al. Loss of BMPR2 leads to high bone mass due to increased osteoblast activity. J Cell Sci. 2015;128:1308–1315. doi: 10.1242/jcs.156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishina Y, et al. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem. 2004;279:27560–27566. doi: 10.1074/jbc.M404222200. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Lyons KM, McMahon AP, Kronenberg HM. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc Natl Acad Sci USA. 2005;102:18023–18027. doi: 10.1073/pnas.0503617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu M, Chen G, Li YP. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto M, et al. Conditional deletion of Bmpr1a in differentiated osteoclasts increases osteoblastic bone formation, increasing volume of remodeling bone in mice. J Bone Miner Res. 2011;26:2511–2522. doi: 10.1002/jbmr.477. [DOI] [PubMed] [Google Scholar]
- 26.Li A, et al. Pharmacologic Calcitriol Inhibits Osteoclast Lineage Commitment via the BMP-Smad1 and IkappaB-NF-kappaB Pathways. J Bone Miner Res. 2017;32:1406–1420. doi: 10.1002/jbmr.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamiya N, et al. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res. 2010;25:200–210. doi: 10.1359/jbmr.090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamiya N, et al. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res. 2008;23:2007–2017. doi: 10.1359/jbmr.080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamiya N, et al. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008;135:3801–3811. doi: 10.1242/dev.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He G, et al. Differential involvement of Wnt signaling in Bmp regulation of cancellous versus periosteal bone growth. Bone Res. 2017;5:17016. doi: 10.1038/boneres.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim J, et al. Dual function of Bmpr1a signaling in restricting preosteoblast proliferation and stimulating osteoblast activity in mouse. Development. 2016;143:339–347. doi: 10.1242/dev.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jadrich JL, O’Connor MB, Coucouvanis E. The TGF beta activated kinase TAK1 regulates vascular development in vivo. Development. 2006;133:1529–1541. doi: 10.1242/dev.02333. [DOI] [PubMed] [Google Scholar]
- 33.Xie M, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ajibade AA, Wang HY, Wang RF. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 2013;34:307–316. doi: 10.1016/j.it.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Inokuchi S, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci USA. 2010;107:844–849. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morioka S, et al. TAK1 kinase signaling regulates embryonic angiogenesis by modulating endothelial cell survival and migration. Blood. 2012;120:3846–3857. doi: 10.1182/blood-2012-03-416198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pera T, Sami R, Zaagsma J, Meurs H. TAK1 plays a major role in growth factor-induced phenotypic modulation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2011;301:L822–828. doi: 10.1152/ajplung.00017.2011. [DOI] [PubMed] [Google Scholar]
- 38.Tang M, et al. TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice. J Exp Med. 2008;205:1611–1619. doi: 10.1084/jem.20080297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang D, et al. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med. 2000;6:556–563. doi: 10.1038/75037. [DOI] [PubMed] [Google Scholar]
- 40.Yu B, et al. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-kappaB. Nat Med. 2014;20:1009–1017. doi: 10.1038/nm.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swarnkar G, Karuppaiah K, Mbalaviele G, Chen TH, Abu-Amer Y. Osteopetrosis in TAK1-deficient mice owing to defective NF-kappaB and NOTCH signaling. Proc Natl Acad Sci USA. 2015;112:154–159. doi: 10.1073/pnas.1415213112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi B, et al. Ablation of Tak1 in osteoclast progenitor leads to defects in skeletal growth and bone remodeling in mice. Sci Rep. 2014;4:7158. doi: 10.1038/srep07158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamothe B, Lai Y, Xie M, Schneider MD, Darnay BG. TAK1 is essential for osteoclast differentiation and is an important modulator of cell death by apoptosis and necroptosis. Mol Cell Biol. 2013;33:582–595. doi: 10.1128/MCB.01225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao L, et al. TAK1 regulates SOX9 expression in chondrocytes and is essential for postnatal development of the growth plate and articular cartilages. J Cell Sci. 2013;126:5704–5713. doi: 10.1242/jcs.135483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawanami A, Matsushita T, Chan YY, Murakami S. Mice expressing GFP and CreER in osteochondro progenitor cells in the periosteum. Biochem Biophys Res Commun. 2009;386:477–482. doi: 10.1016/j.bbrc.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- 47.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 48.Shim JH, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi K, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 50.Cong Q, et al. p38alpha MAPK Regulates Lineage Commitment and OPG Synthesis of Bone Marrow Stromal Cells to Prevent Bone Loss under Physiological and Pathological Conditions. Stem Cell Reports. 2016;6:566–578. doi: 10.1016/j.stemcr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H, et al. Bone Size and Quality Regulation: Concerted Actions of mTOR in Mesenchymal Stromal Cells and Osteoclasts. Stem Cell Reports. 2017;8:1600–1616. doi: 10.1016/j.stemcr.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim J, Burclaff J, He G, Mills JC, Long F. Unintended targeting of Dmp1-Cre reveals a critical role for Bmpr1a signaling in the gastrointestinal mesenchyme of adult mice. Bone Res. 2017;5:16049. doi: 10.1038/boneres.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang M, et al. Smad1 plays an essential role in bone development and postnatal bone formation. Osteoarthritis Cartilage. 2011;19:751–762. doi: 10.1016/j.joca.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shim JH, et al. TAK1 is an essential regulator of BMP signalling in cartilage. EMBO J. 2009;28:2028–2041. doi: 10.1038/emboj.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gunnell LM, et al. TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. J Bone Miner Res. 2010;25:1784–1797. doi: 10.1002/jbmr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yumoto K, et al. TGF-beta-activated kinase 1 (Tak1) mediates agonist-induced Smad activation and linker region phosphorylation in embryonic craniofacial neural crest-derived cells. J Biol Chem. 2013;288:13467–13480. doi: 10.1074/jbc.M112.431775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang J, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenblatt MB, Shim JH, Glimcher LH. Mitogen-activated protein kinase pathways in osteoblasts. Annu Rev Cell Dev Biol. 2013;29:63–79. doi: 10.1146/annurev-cellbio-101512-122347. [DOI] [PubMed] [Google Scholar]
- 59.Novack DV. Role of NF-kappaB in the skeleton. Cell Res. 2011;21:169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez-Carballo E, Gamez B, Ventura F. p38 MAPK Signaling in Osteoblast Differentiation. Front Cell Dev Biol. 2016;4:40. doi: 10.3389/fcell.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao Z, et al. NF-kappaB RelB negatively regulates osteoblast differentiation and bone formation. J Bone Miner Res. 2014;29:866–877. doi: 10.1002/jbmr.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kua HY, et al. c-Abl promotes osteoblast expansion by differentially regulating canonical and non-canonical BMP pathways and p16INK4a expression. Nat Cell Biol. 2012;14:727–737. doi: 10.1038/ncb2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baud’huin M, et al. A soluble bone morphogenetic protein type IA receptor increases bone mass and bone strength. Proc Natl Acad Sci USA. 2012;109:12207–12212. doi: 10.1073/pnas.1204929109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author on request (email: libj@sjtu.edu.cn).