Abstract
Background
Estrogen receptor (ER)α and ERβ are ligand-activated transcription factors that regulate gene expression by binding to estrogen-responsive elements and interacting with several coregulators through protein-protein interactions. Usually, these coregulators bind to the various conserved and functional domains of the receptor through a consensus LXXLL sequence, although variations can be found. The interaction of receptor domains and the consensus motif can be a possible target for nuclear receptor (NR) pharmacology, since modifications in these are responsible for possible pathogenesis of various diseases.
Purpose
The present study focuses on the secondary structure and conserved domains of the ERα and ERβ interacting proteins, using bioinformatics tools and their relation to the function of the coregulators.
Methods
Bioinformatics-based prediction tools like STRING, PSIPRED, PROTPARAM and Conserved Domain Database (CDD) were used. The prediction tools utilized in this study basically determines the characteristics of a possible coregulator by using an already existing protein as a template and determines the presence of any conserved consensus sequence. Coregulators have been enlisted with the help of NCBI, STRING and iHOP. The secondary structures were analyzed using PSIPRED and conserved domains were determined using CDD.
Results
The analysis of the structure has shown the presence of conserved domains and homology between the various coregulators. Each interacting protein contains conserved domains like the nuclear coactivators' domain, the helix-loop-helix domain and the SRC domain.
Conclusion
Such studies give the characteristic features of ERα and ERβ interacting proteins and maybe useful to determine their family and uses in NR pharmacology in health and diseases.
Keywords: Estrogen receptor α, Estrogen receptor β, Estrogen receptor coregulators, Secondary structure, Homology, Insilico study
Introduction
Estrogens are steroid molecules and primarily secreted by the female gonads. Apart from playing an important role in the regulation of the female reproductive systems, they have important functions in bone development, in lipid metabolism and in maintaining the cardiovascular system and the neuronal systems [1, 2]. Estrogens are known to exert their functions through ER, which are ligand-activated transcription factors belonging to the nuclear receptor (NR) superfamily. Like the other members of the NR superfamily, ER contains 6 functional domains [3, 4]. The DNA-binding domain shares the highest homology with the other NR superfamily members and is the most conserved domain. The ligand binding domain (LBD) is relatively less conserved in nature and contains the ligand-dependent transcription activation function activation function (AF)-2. The most variable portion of the ER is the A/B domain that also contains a ligand independent, constitutively active transcription activation factor AF-1.
Biochemical analysis has shown the presence of 2 isoforms of the ER, namely, ERα and ERβ. Although ERα an ERβ share the DBD, they differ to some extent in their LBD and thus their ligand-binding affinity and expression patterns. ERβ modulated the function of ERα when they are co-expressed and supplements the activity of ERα. Most effects of estrogen are cell-specific, which can be attributed to the differential expression of the ER in different tissues. Studies have shown the presence of ERα in the uterus, liver, kidney and heart, whereas ERβ is shown to be present in the gastrointestinal tract, ovary, prostate, lung, bladder and the central nervous system. The co-expression of the 2 isoforms is seen in the mammary gland, epididymis, adrenal gland and regions of brain like the hypothalamus and the amygdala [5].
Usually, the ERs are bound to the heat shock proteins 90 present in the cytoplasm [6]. On interaction with the ligand, phosphorylation takes place at the serine and the threonine residues, which causes a conformational change in the receptor. This releases the receptor from the heat shock proteins complex and interaction with the respective response elements (ERE) is facilitated. This acts as a scaffold for the recruitment for a number of other factors termed as coregulators. The coregulators facilitate estrogen-mediated chromatin reorganization. By altering the chromatin structure, the coregulators provide interaction with receptor and the transcription machinery, thereby controlling the transcription.
Coregulators exist as multi-protein complexes and can be broadly classified into 2 categories. The first class contains the interacting proteins that have histone-acetylating or -deacetylating activities. The most widely studied coregulators family under this category is the p160 family of coregulators. As the name suggests, the family contains molecules that are 160kD in size and basically comprise SRC1, GRIP1/TIF2 and AIB1/ACTR/pCIP [7, 8, 9]. They function by binding histone acetyl transferases such as p300/CBP and pCAF, which in turn regulated the chromatin remodeling. The second class contains the interacting proteins that facilitate chromatin remodeling through ATP hydrolysis. This class includes the SWI/SNF family of coregulators. They have homology similar to the helicases [10, 11]. The coregulators can be coactivators like SRC1, ERAP140 [12], GRIP1, TRAP220 and so on or can be corepressors like NCoR and SMRT (Table 1). The variation in the expression and interaction of ER coregulators and their dysregulation is shown to have severe consequences for cellular homeostatsis and often contributes to pathogenesis.
Table 1.
ERα interacting proteins
| S. No. | Name of the protein | Tissue specificity | Disease correlation |
|---|---|---|---|
| 1 | SRC-1 | Brain (hypothalamus, hippocampus, cerebellum, PVN) | Breast cancer, endometrial cancer |
| 2 | TIF2 | Pancreas, skeletal muscle, liver, lung, placenta, brain, heart | Colorectal cancer, acute mylemonocytic leukemia |
| 3 | GRIP1 | Muscle, neural tissue, mammary gland | Breast cancer |
| 4 | AIB1 | Heart, skeletal muscle, pancreas and placenta | Polycystic ovarian syndrome, ovarian cancer, breast cancer |
| 5 | ARA70 | Adipose tissue, testis | Polycystic ovarian syndrome, prostate cancer |
| 6 | NCoA3 | Heart, skeletal muscle, pancreas, and placenta | Breast cancer |
| 7 | CBP | Brain, skeletal muscles | Rubinstein-Taybi syndrome, Huntington's disease |
| 8 | pCAF | Intestines | Colorectal cancer |
| 9 | TRAP220 (MED1) | Ubiquitously present | Lung adenocarcinoma, crohn's disease |
| 10 | SRA | Mammary gland, ovaries, placenta, adipose tissue | Breast cancer, prostate cancer, ovarian cancer |
| 11 | p68 | Mammary gland, adipose tissue | Breast cancer |
| 12 | ASC-2 | Mammary gland, spleen, liver | Breast cancer |
| 13 | PNRC | Liver, lung, adipose tissue, NK/T cells | Hepatitis B, breast Cancer |
| 14 | SPBP (TCF20) | Brain, heart, testis, kidney, liver, lungs | Arrhythmia, cardiac problems |
| 15 | DHX9 | Neuronal cells, pituitary, ovary | Werner syndrome |
| 16 | STAU1 | Brain, pancreas, heart, skeletal muscles, liver, lung, kidney | Cardiac diseases, lung carcinomas |
| 17 | EIF4G | Heart, skeletal muscles | Encephalomyocarditis virus infection |
| 18 | PELP1 | Breast cancer cells | Endometrial cancer, breast cancer |
| 19 | PGC1 | Heart, brain, skeletal muscles | Obesity, type II diabetes |
| 20 | NRIP1 | Placenta, ovary, brain, lung, stomach, kidney | Post-menopausal osteoporosis, infertility |
| 21 | N-COR | Lung, spleen, brain, mammary gland | Breast cancer, myeloid leukemia |
| 22 | SMRT (NCOR2) | Ubiquitous, high levels in embryo | B-cell lymphoma, myeloid leukemia |
| 23 | RIP140 | Placenta, ovary, liver | Post-menopausal osteoporosis, infertility |
| 24 | CARM1 | High expression in prostate, epithelial cells | Prostate cancer, vascular diseases |
| 25 | SHP | Liver, heart, pancreas, placenta | Obesity, endometrial cancer, insulin resistance. |
| 26 | DAX | Adrenal gland, pituitary | Hypogonadism, congenital adrenal hyperplasia |
| 27 | HET-SAFB | Mammary gland, stomach, liver | Breast cancer, duodenal ulcer |
In addition, these coregulators have been shown to interact with the receptor through a consensus LXXLL sequence, also called NR boxes [13], although variation is reported. These structural motifs are found as helices. The leucines create a hydrophobic surface that interacts with the hydrophobic groove of the receptor [14]. The interaction between the receptor motifs and the NR boxes is used as a possible target for NR pharmacology studies [15]. The structure and properties of the interacting proteins per se coregulators play an important role in determining their interaction with ERα and ERβ and function. There exists a great deal of homology among various coregulators due to the presence of conserved domains and responsible for specific functions. The present study is focused to determine the secondary structure of ERα and ERβ interacting proteins and their conserved domains using various bioinformatics tools. Such a study may be useful to determine the family of the coregulators, newly identified, and helpful to understand the etiology of different diseases and treatment by NR pharmacology.
Methods
Enlisting ERα and ERβ Interacting Proteins
ERα and ERβ interacting proteins were enlisted using the NCBI, STRING and iHOP databases. STRING is a database that maintains a list of known or predicted protein interactions that are derived from 4 basic sources: genomic analysis, high throughput experiments, conserved coexpression and previously known literature. iHOP provides information about the proteins and their interacting members in a concise manner with access to related literature. NCBI is a database maintained by the National Institutes of Health, USA. Amino acid sequences of the protein can be obtained in the FASTA format from the NCBI database.
Prediction of Secondary Structure of ERα and ERβ Interacting Proteins
The secondary structure was predicted using prediction tools such as PSIPRED. PSIPRED is a tool that predicts the secondary structure by using the information gained from PSI-BLAST. The amino acid sequence of the interacting domain was input in the FASTA format, derived from NCBI database and the secondary structure is determined in the form of helices denoted by cylindrical figures and strands denoted by arrow marks.
Prediction of the Physical Parameters of the ERα and ERβ Interacting Proteins
The physical parameters were determined using ExPasy tool and PROTPARAM. The protein sequence was input in the FASTA format from the NCBI database. PROTPARAM gives information about molecular weight, stability index, extinction coefficients, half-life and the amino acid composition of the protein.
Prediction of Conserved Sequences among ERα and ERβ Interacting Proteins
Homology modeling was done using the bioinformatics tool CD Search, which is a part of the Conserved Domain Database (CDD). The amino acid sequence of the target protein is input in the FASTA format or as the PDB ID. The software performs alignment with the help of BLAST and CLUSTAL and provides the homology model based on the entries. It displays a schematic structure of the conserved domains and gives the gi number of the proteins that share homology with these domains.
Results
List of ERα and ERβ Interacting Proteins Using NCBI, STRING and iHOP
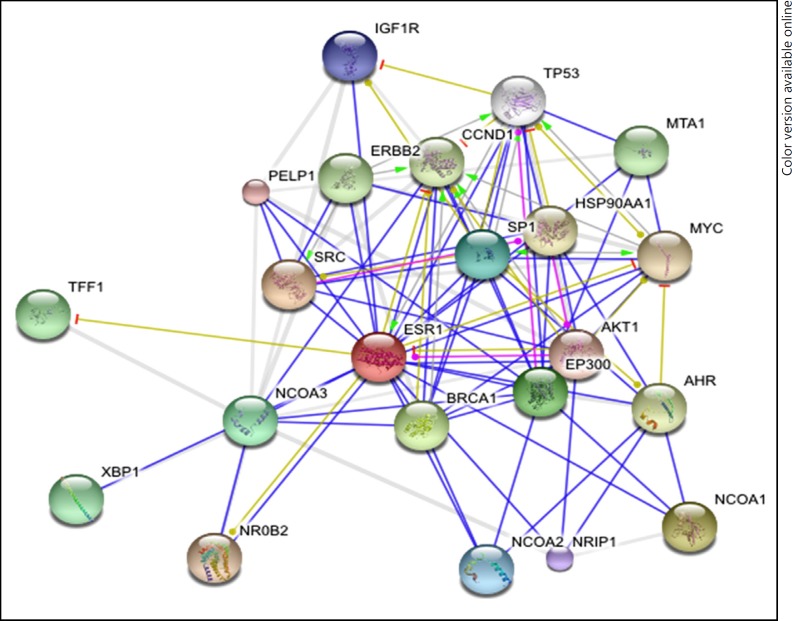
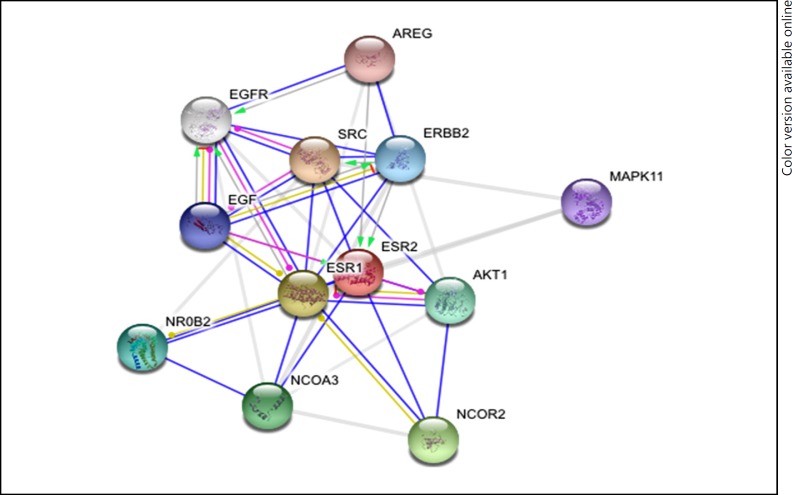
A list of the interacting proteins was generated (Tables 1, 2; Fig. 1, 2), based on the literature of the predicted as well as experimentally derived proteins. NCBI, STRING and iHOP maintain a pool of information on the proteins found in the primary protein databases such as the PDB and SWISS PROT and so on.
Table 2.
ERβ interacting proteins
| S. No. | Name of the protein | Tissue specificity | Disease correlation |
|---|---|---|---|
| 1 | NCoA3 | Heart, skeletal muscle, pancreas, and placenta | Breast cancer |
| 2 | PELP1 | Breast cancer cells | Endometrial cancer, breast cancer |
| 3 | TRIM24 | Thyroid, mammary gland | Breast cancer, myeloid leukemia |
| 4 | ERAP140 | Brain, mammary gland, ovary, uterus, stomach, bladder | Breast cancer, ovarian cancer |
| 5 | AIB-1 | Heart, skeletal muscle, pancreas, and placenta | Polycystic ovarian syndrome, ovarian cancer, breast cancer |
| 6 | TRKA | Neuronal cells | Congenital insensitivity to pain with anhidrosis |
| 7 | CREB | Brain | Spina bifida, neuroblastoma |
| 8 | GRIP1 | Muscle, neural tissue, mammary gland | Breast cancer |
| 9 | MED1 | Ubiquitously present | Lung adenocarcinoma, crohn's disease |
Fig. 1.
Interacting proteins of ERα by STRING tool.
Fig. 2.
Interacting proteins of ERβ by STRING tool.
Secondary Structure of the Interacting Proteins Contain Alpha Helices
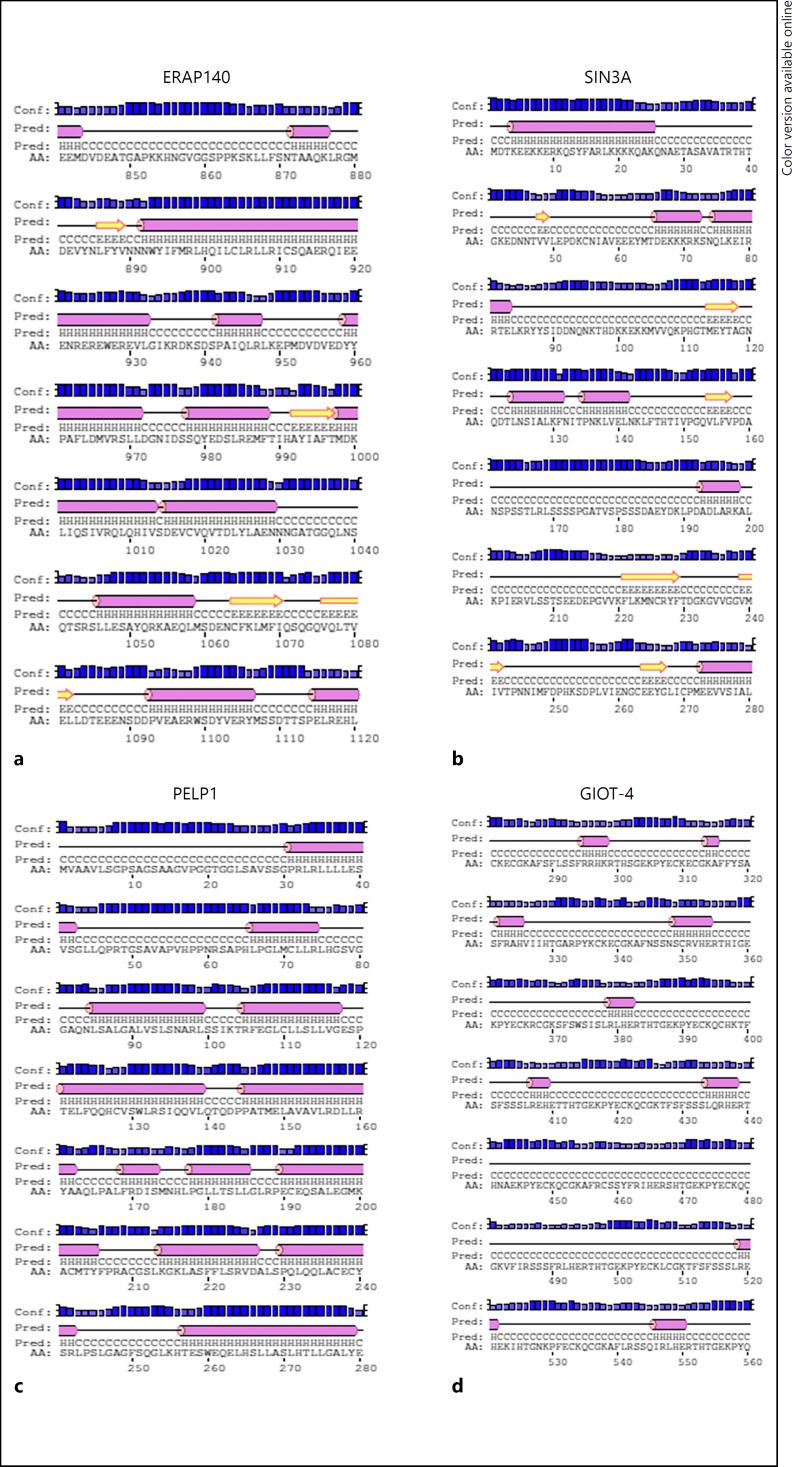
The secondary structures predicted using PSIPRED showed the presence of alpha helices in the AF1- and AF2-binding regions of the protein. These alpha helices were found to be rich in leucine residues. The leucine residues are known to form a hydrophobic region that fits into the hydrophobic groove of the receptor (Fig. 3, 4). Biochemical and structural studies clearly showed that the LBDs of the NRs recognize a short alpha helix present in the co-activators. Apart from alpha helices, random coils were also predicted. In addition, the secondary structures of AREG and TRKA showed the presence of beta turns in their interacting regions (Fig. 3). TRKA is widely reported in neuronal cell cultures and have been shown to be downregulated in case of anhidrosis and congenital insensitivity to pain [16]. AREG, on the other hand, has been found to be an essential regulator of ERα in the mammary gland and its dysregulation has been known to cause breast cancer [17].
Fig. 3.
Secondary structures of ERα interacting proteins; PNRC (a), RIP140 (b), SMRT (c), AREG (d) and TRKA (e).
Fig. 4.
Secondary structures of ERβ interacting proteins; ERAP140 (a), SIN3A (b), PELP1 (c) and GIOT-4 (d).
Homology Prediction Using CDD
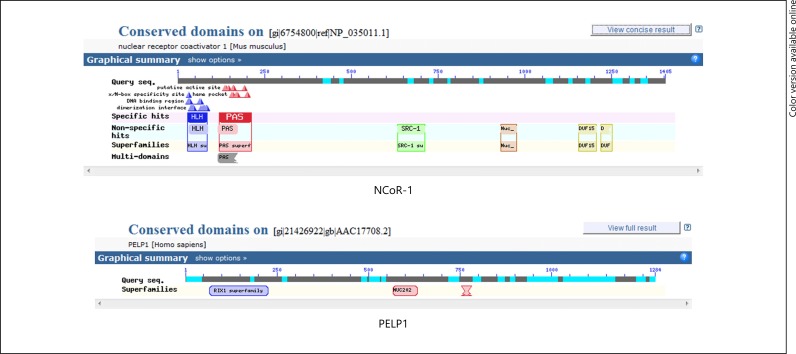
The conserved domains were determined using CD SEARCH. The conserved domain of the NCoR-1 and PELP1 molecule was shown in Figure 5. The NCoR-1 conserved domain structure acts as a template for a wide variety of coregulators. PELP1 was chosen as the other template, since it contains the RIX1 superfamily conserved domain, which is absent in the SRC-1 domain structure but is a part of many co-regulators like SCAF. The characteristic feature of any co-activator viz SRC domain (basically contains the NR box), NR coactivators domain (forms an alpha helical structure) and the helix-loop-helix domain that are specifically found in transcription factors were present. Further, proteins were found, which share a similar homology pattern with the NCOR1. Some of the proteins that were determined were NCoA3 (gi 82112094), SCAF14542 (gi 82267094), novel protein similar to vertebrate NCoA1 (gi 82196443) and the others (Fig. 6, 7). Proteins that were found to be homologous with PELP1 were modulators of non-genomic activity of ER (gi 82072956), At1g30240 (gi 122236349), Acetyltransferase catalyses diacyl glycerol esterification (gi 254569024; Fig. 7). There exists a great amount of homology between the interacting proteins. Some of the proteins having homology are enlisted in Table 3.
Fig. 5.
Conserved domains of NCoR-1 and PELP1.
Fig. 6.
Homologous proteins of NCoR-1.
Fig. 7.
Homologous proteins of PELP1.
Table 3.
ERα and ERβ interacting proteins by STRING (low confidence level)
| ERα | ||||||
| SRC | NRIP 1 | ERBB2 | SMAD4 | HDAC4 | CREBBT | UBC |
| NCoA1 | PELP1 | MTA1 | ARNT | TP53 | CDH1 | TRIM24 |
| BRCA1 | AKT 1 | TFF1 | STAT54 | JUN | FOX03 | |
| EP300 | NROB2 | XBP1 | CEBPB | MED1 | FOXA1 | |
| NCOA3 | MYC | IGF1 | HDAC9 | ESR2 | NCOA6 | |
| SP1 | HSP90AA1 | BCAR1 | SAFB | CTSD | SMARCA4 | |
| NCOA2 | AHR | EGFR | NR2F1 | NCOR2 | PRDM2 | |
| IGF1R | CCND1 | PIK3R1 | GRIP1 | CAV1 | KAT2B | |
| ERβ | ||||||
| SRC | MAPK11 | THRB | RARA | TCF19 | RORC | |
| ESR1 | AREG | NR1D1 | NR4A2 | HNF4G | NR2F1 | |
| NCOR2 | AHR | VDR | PPARA | RDRB | PPARD | |
| NCOA3 | IGF1 | PELP1 | NR2 C2 | GNA14 | NRID2 | |
| AKT1 | NCOA1 | ESRRB | RARG | NR2E1 | GNA15 | |
| NROB2 | THRA | PPARG | RARB | GNG2 | ||
| ERBB2 | OXT | ESRRA | RXRG | GNAL | ||
| EGF | NR5A1 | ESRRG | NR2C1 | NR2C2AP | ||
Discussion
Usually, bioinformatics prediction tools, such as the CDD, PSIPRED and STRING, help in utilizing information from different proteins as templates and derive the structure and function of the newly discovered protein. In the present study, the classical example of the coregulators, that is, the members of the p160 family of coregulators were used as template to compare with predicted coregulators enlisted using NCBI, STRING and iHOP. The secondary structure of the enlisted proteins share structural homology with the p160 family of proteins. The AF1 and AF2 interacting domains of the coregulators show the presence of alpha helices, which are conserved domains. Some of the interacting proteins, such as TRKA and AREG, possessing beta turn in their interacting domains suggesting that, apart from alpha helices, other secondary structures play an important role in gene regulation.
ERs recruit a host of coregulators for regulating estrogen-dependent gene expression [2]. The level of estradiol decreases during aging in both genders and a sharp decline is reported in females after their menopause [18]. Thus, the optimum level of estrogen, receptors in the normal form and binding of ERs with coregulators are vital factors to decrease risk factors of neurodegenerative diseases.
Here, our study shows that majority of the interacting proteins contain α helices. Their presence may induce the aggregation properties of proteins as shown in earlier studies. The study shows that the intrinsic stabilization of α helix proteins can trigger the formation of α helical aggregates [19]. Further, higher concentration helix-promoting trifluoroethanol induces stabilization of tau protein aggregates [20], a known factor in case of Alzheimer disease. In addition, the microtubule-binding domain of tau, which constitutes the core of paired helical filaments, has significant α helix propensity [21]. Taking together, the present work may provide an insight into the structural abnormalities leading to functional dysregulation and can be an excellent target for NR pharmacology studies.
Disclosure Statement
The source of funding is Department of Biotechnology, Government of India to MKT. There are no conflicts of interest among authors.
Authors Contribution
V.P. designed, performed the work and analyzed the data. M.K.T. designed the work and analyzed the data. H.K. performed the work.
Acknowledgements
M.K.T. acknowledged the Department of Biotechnology, Government of India for financial support. H.K. acknowledged the Indian Academy of Sciences for providing Summer Research Fellowship.
References
- 1.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakur MK, Paramanik V. Role of steroid hormone co-regulators in health and disease. Horm Res. 2009;71:194–200. doi: 10.1159/000201107. [DOI] [PubMed] [Google Scholar]
- 3.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G., Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang P, Chandra V, Rastinejad F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu Rev Physiol. 2010;72:247–272. doi: 10.1146/annurev-physiol-021909-135917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 6.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 7.Meijer OC, Steenbergen PJ, De Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141:2192–2199. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- 8.Tetel MJ. Nuclear receptor coactivators in neuroendocrine function. J Neuroendocrinol. 2000;12:927–932. doi: 10.1046/j.1365-2826.2000.00557.x. [DOI] [PubMed] [Google Scholar]
- 9.Paramanik V, Thakur MK. AIB1 shows variation in interaction with ERβTAD and expression as a function of age in mouse brain. Biogerontology. 2011;12:321–328. doi: 10.1007/s10522-011-9330-y. [DOI] [PubMed] [Google Scholar]
- 10.Kadam S, Everson BM. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol Cell. 2003;11:377–389. doi: 10.1016/s1097-2765(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh S, Thakur MK. Interaction of estrogen receptor-alpha transactivation domain with nuclear proteins of mouse brain: p68 RNA helicase shows age- and sex-specific change. J Neurosci Res. 2009;87:1323–1328. doi: 10.1002/jnr.21948. [DOI] [PubMed] [Google Scholar]
- 12.Paramanik V, Thakur MK. Interaction of estrogen receptor associated protein (ERAP) 140 with ER beta decreases but its expression increases in aging mouse cerebral cortex. Cell Mol Neurobiol. 2010;6:961–966. doi: 10.1007/s10571-010-9526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lionder K, Söderström M. Functional analyses of an LXXLL motif in nuclear receptor corepressor (N-CoR) J Steroid Biochem Mol Biol. 2004;91:191–196. doi: 10.1016/j.jsbmb.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 15.Hall JM, Mcdonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Molecular Interv. 2005;5:343–357. doi: 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- 16.Greco A, Villa R, Romano L, Penso D, Pierotti MA. A novel NTRK1 mutation associated with congenital insensitivity to pain with anhidrosis. Am J Hum Genet. 1999;64:1207–1210. doi: 10.1086/302319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci U S A. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma PK, Thakur MK. Expression of estrogen receptor (ER) alpha and beta in mouse cerebral cortex: effect of age, sex and gonadal steroids. Neurobiol Aging. 2006;27:880–887. doi: 10.1016/j.neurobiolaging.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Kunjithapatham R, Oliva FY, Doshi U, Perez M, Avila J, Munoz V. Role for the alpha-helix in aberrant protein aggregation. Biochemistry. 2005;44:149–156. doi: 10.1021/bi048564t. [DOI] [PubMed] [Google Scholar]
- 20.Luo PZ, Baldwin RL. Mechanism of helix induction by trifluoroethanol: a framework for extrapolating the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry. 1997;36:8413–8421. doi: 10.1021/bi9707133. [DOI] [PubMed] [Google Scholar]
- 21.Mandelkow EV, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]