Abstract
The diagnosis of osteoporosis is mainly based on clinical examination and bone mineral density assessments. The present pilot study compares the plasma lipid and polar metabolite profiles in blood plasma of 95 Singaporean-Chinese (SC) menopausal women with normal and low bone mineral density (BMD) using an untargeted metabolomic approach. The primary finding of this study was the association between lipids and femoral neck BMD in SC menopausal women. Twelve lipids were identified to be associated with low BMD by the orthogonal partial least squares (OPLS) model. Plasma concentrations of eight glycerophospholipid, glycerolipid, and sphingolipid species were significantly lower in menopausal women with low BMD but higher in two glycerophospholipid species (phosphatidylinositol and phosphatidic acid). Further, this study found no significant differences in plasma amino acid metabolites. However, trends for lower 4-aminobutyric acid, turanose, proline, aminopropionitrile, threonine, and methionine were found in women with low BMD. This pilot study identified associations between lipid metabolism and femoral neck BMD in SC women. Further studies are required on larger populations for evaluating the bone health effect of these compounds and their usefulness as clinical biomarkers for osteoporosis prediction in women.
Keywords: metabolomics, lipidomics, osteoporosis, menopause, biomarkers
1. Introduction
Menopausal women have a greater risk of bone loss and developing osteoporosis. Osteoporosis affects above 200 million people worldwide, and about 9 million osteoporotic fractures, of which 1.6 million are at the hip, are registered per year [1]. In Asian populations, hip fractures account for around 30% of those worldwide, and in Singapore, hip fracture rates have increased by 1.2% annually in Chinese women [2].
In women, low oestrogen level is a risk factor for osteoporosis. Oestrogen withdrawal promotes the activation of bone remodelling at the basic multicellular units (BMUs). Bone formation decreases because there is reduction of osteoblastic cell lifespan, and bone resorption increases as a result of increased differentiation and lifespan of osteoclasts [3]. The impact of oestrogen loss in bone metabolism, including several biochemical and physiological alterations, is characterized by high levels of oxidative stress, inflammation, and altered metabolism in the bone microenvironment [4,5]. Changes in lipid and polar metabolites have been associated with oxidative stress and inflammation affecting bone metabolism. Previous studies demonstrated that oxidative stress, caused by mitochondrial alterations, induces reactive oxygen species (ROS) generation, and leads to osteoblast cell death by increasing the oxidized bone microenvironment [6,7]. In addition, oestrogen withdrawal upregulates bone microenvironment pro-inflammatory cytokines like interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), granulocyte macrophage colony-stimulating factor, macrophage colony-stimulating factor (M-CSF), and prostaglandin-E2 (PGE2), which regulate osteoclast differentiation and function and therefore bone loss [8,9]. Osteoporosis may be present before diagnosis; despite current serum biochemical analysis and radiological examination methods for screening of osteoporosis and fracture risk in women [10], none of these methods are suitable for prediction of early bone loss in women. The elucidation of the cellular and biochemical events on bone metabolism after oestrogen withdrawal may lead to a better understanding of the molecular mechanisms involved in osteoporosis and bone cell signalling pathways in women, and subsequently identify early predictors of bone loss that can be used as prognostic markers.
Metabolomics offers the potential for analyzing the biochemical changes in the pathology of diseases. Metabolomic studies are conducted by using several analytical platforms; however, nuclear magnetic resonance (NMR) and mass spectrometry (MS) are the most widely reported techniques [11]. A small number of plasma metabolomic studies have reported that the metabolite concentration shifts under menopausal conditions in ovariectomized (OVX) animals and humans [12,13,14,15], while numerous studies on the relationship between bone loss and low oestrogen levels in both OVX animals and humans have been reported [16,17,18,19,20,21,22].
Because of the limited information reported on the association between the plasma metabolome and bone mineral density (BMD) in Singaporean-Chinese menopausal women (SC), research in this area is required to allow the identification of potential metabolites that can be used to understand the causal pathways involved in menopausal osteoporosis. We hypothesized that novel metabolomic markers will improve the prediction of SC menopausal women at increased osteoporotic risk. Therefore, this study aimed to analyze the lipid and polar metabolite profiles of blood plasma SC menopausal women using a liquid chromatography–mass spectrometry (LC–MS) untargeted metabolomic approach. Moreover, to maximize the identification of biomarkers associated with bone loss in menopausal women, we also performed statistical tests on a restricted subset of the SC women with either osteoporosis (T-score < −2.5) or normal BMD (T-score > −1). We hypothesized that lipids and polar metabolites of women with osteoporosis differed from the whole population. Further, correlation analyses were conducted between metabolites and femoral neck bone mineral density (BMD) in menopausal women.
2. Materials and Methods
2.1. Standards and Reagents
Formic acid, d2-tyrosine, sucrose, alanine, arginine, asparagine, aspartic acid, glutamic acid, glutamine, histidine, homoserine, isoleucine, leucine, lysine, phenylalanine, proline, serine, theanine, threonine, tyrosine, valine, amino acid standard mixture (physiological mixture), 16:0 d31-18:1 PE (phosphatidylethanolamine), and ammonium formate were purchased from Sigma–Aldrich Chemicals Co. (St. Louis, MO, USA). Ultrapure water was obtained from a Milli-Q® system (Millipore, Bedford, MA, USA). Acetonitrile (ACN), methanol and isopropanol were optima LC–MS grade, chloroform was high-performance liquid chromatography (HPLC) grade, and all were purchased from Thermo Fisher Scientific (Auckland, New Zealand).
2.2. Study Population, Inclusion and Exclusion Criteria
All subjects were informed about the objective of the study and gave their informed consent for the participation in the present study. The study was approved by the Ethics Committee for Research Involving Human Subjects, Singapore (Approval No. 2014/01066). The study was done in accordance with the Declaration of Helsinki (2000) of the World Medical Association.
Ninety-seven SC menopausal women aged between 55 and 70 years were included in the study. The key inclusion criterion was women who were at least five years menopausal (based on a history of cessation of menstruation). Exclusion criteria included prior diagnosis with osteoporosis, diabetes mellitus or any condition that affects bone and liver function, and to not be taking any medication that will affect the study.
2.3. Blood Collection
Blood samples were taken only once between 8 and 10 a.m., after an overnight fast (10–12 h). Blood was collected in ethylenediamine tetraacetic acid (EDTA) tubes (BD VacutainerTM K3E 15%, Becton, Dickinson and Company, Plymouth, UK) and plasma samples transferred into separate 1 mL tubes for extractions for metabolomics analysis; C-terminal telopeptide of type I collagen (CTx-1), parathyroid hormone (PTH) and 25(OH) vitamin D3 were stored frozen at −80 °C and thawed on the day of the analysis.
2.4. Analysis of Blood Parameters
Blood samples were taken to measure plasma markers of CTx-1 as well as PTH and 25(OH) vitamin D3. CTx-1 and PTH concentrations were analyzed by electrochemiluminescence immunoassay using the Roche COBAS® e411 system (Roche Diagnostics, Indianapolis, IN, USA). Serum 25(OH) vitamin D3 concentration was analyzed using isotope-dilution liquid chromatography–tandem mass spectrometry (IDLC–MS–MS) [23] by Canterbury Health Laboratories, Christchurch, New Zealand.
2.5. Bone Mineral Density
BMD was measured using dual X-ray absorptiometry (DXA). DXA scans of hip (femoral neck) were carried out using a Hologic QDR-Discovery A densitometer (Hologic Discovery QDR 4500A densitometer, Hologic Inc., Bedford, MA, USA). BMD was determined and women were classified into normal or low BMD according to the World Health Organization (WHO) classification. WHO provides an operational definition of osteoporosis based on T-score, where the T-score is the number of the standard deviation below of the mean peak BMD for young-adults. Women with a BMD 2.5 standard deviations below are classified as osteoporotic. In this study, participants in the entire cohort were classified into two groups according to bone status: (1) low BMD = women with a T-score < −1, and (2) normal BMD = women with a T-score > −1.0. Further, a selected subset of participants were also classified into two groups: (1) osteoporosis = women with a T-score < −2.5, and (2) normal BMD: women with T-score > −0.1 [24].
2.6. Metabolomic Analysis
2.6.1. Sample Preparation
Lipids and polar metabolites were extracted from plasma using a biphasic solvent. Lipids were analyzed by reverse-phase liquid chromatography–mass spectrometry (RP LC–MS) with positive- and negative-mode electrospray ionization. Polar metabolites were analyzed by hydrophilic interaction liquid chromatography–mass spectrometry (HILIC LC–MS) with only positive-mode electrospray ionization. Briefly, plasma samples were thawed in fridge at 4 °C. Then, the samples were vortexed for 1 min. 100 µL of plasma was transferred to a microcentrifuge tube and 800 µL of cold chloroform:methanol (1:1 v/v) were added to the tubes. The tubes were agitated by hand for 1 min and the samples were stored for 30 min at -20 °C. 400 µL of water were added to each sample and vortexed for 30 s. The samples were centrifuged for 15 min at 13,913 g at 4 °C. 200 µL of the lower phase (organic phase) were taken for lipid analysis and transferred to a new microcentrifuge tube to evaporate to dryness under a stream of nitrogen. Two hundred and fifty µL of the upper phase (polar aqueous) were collected for polar metabolite analysis and transferred in to new microcentrifuge tube to evaporate to dryness under stream of nitrogen. The lower-phase tubes were reconstituted in 100 µL of Folch solvent mixture (choroform:methanol 2:1 v/v) containing 16:0 d31-18:1-PE internal standard at 10 µg/mL concentration. The upper-phase tubes were reconstituted in 300 µL of ACN:water (1:1 v/v) containing formic acid (0.1%). All the samples were vortexed for 1 min, centrifuged for 10 min 13,913 g at 4 °C and 100 µL were transferred to a vial containing an insert and stored at 4 °C for immediate polar metabolite analysis. Polar metabolite quality control (QC) samples were prepared with 60 µL of each sample and placed in a tube to be pooled. The pooled QC samples were transferred into multiple pooled QC vials. Lipid QC samples were prepared with 20 µL of each sample. The pooled lipid QC samples were aliquoted into multiple pooled quality control vials [25].
2.6.2. LC-MS Conditions and Metabolite Identification
Untargeted metabolomic approach was carried out on the Thermo LC–MS system (Thermo Fisher Scientific, Waltham, MA, USA). This system consisted of an Accela 1250 quaternary pump, a Thermo-PAL auto-sampler fitted with a 15,000 psi injection valve (CTC Analytics AG., Zwingen, Switzerland).
Lipid chromatographic separation was conducted by using a C18 column (100 × 2.1 mm; 1.7 μm particle size, Waters, Milford, MA, USA). The samples were separated with a gradient elution program and flow rate of 600 μL/min. The mobile phase was a mixture of 0.1% formic acid in ACN–isopropanol (50:50, v/v) (Solvent A), 0.1% formic acid in ACN-water (60:40 v/v) with 10 mM ammonium formate (Solvent B) and a mixture of 0.1% formic acid in isopropanol-ACN (90:10 v/v) with 10 mM ammonium formate (Solvent C). The gradient elution was conducted starting with 85% solvent B (0–1 min), decreased at 70–18% (2–11 min) and held at 1% (11.50–14 min), returned to 85% (22–27 min), while solvent A was held at 100% (14.1–22 min) and allowed to equilibrate for 5 min prior to the next sample injection. Column effluent was connected to a 2 μL injection loop and a Q Exactive Orbitrap mass spectrometer using positive and negative electrospray ionization. Data were collected in profile data acquisition mode over a mass range of m/z 200–2000 at a mass resolution setting of 70,000 with a maximum trap fill time of 100 ms using the Xcalibur software package ver. 2.2 SP1.48 provided by the manufacturer (Thermo Fisher Scientific, San Jose, CA, USA). Lipid positive ion mode parameters were as follows: spray voltage, 3.2 kV; capillary temperature, 275 °C; capillary voltage, −0.2 V, tube lens 120 V. Negative ion mode parameters were as follows: spray voltage, −3.2 kV; capillary temperature, 275 °C; capillary voltage, −0.1 V, tube lens −100 V.
Polar metabolites chromatographic separation was conducted by using a ZIC-pHILIC column (100 mm × 2.1 mm, 5 μm; Merck, Darmstadt, Germany). The samples were separated with a gradient elution program and a flow rate of 250 μL/min. The mobile phase was a mixture of ACN-formic acid (99.9:0.1, v/v) (solvent A) and water–ammonium formate (16 mM, pH 6.3) (solvent B). The gradient elution programme was: held at 97% A (0–1 min), 97–70% A (1–12 min), 70–10% A (12–14.5 min), held at 10% A (14.5–17 min), returned to 97% A (17–18.5 min) and allowed to equilibrate for a further 5.5 min prior to the next injection. Column effluent was connected to a 20 μL injection loop and an Exactive Orbitrap mass spectrometer using positive and negative electrospray ionization. Data were collected in profile data acquisition mode over a mass range of m/z 55–1100 at a mass resolution setting of 25,000 with a maximum trap fill time of 100 ms using the Xcalibur software package provided by the manufacturer. Samples were run in positive ionization mode. HILIC positive ion mode parameters were as follows: spray voltage, 2.7 kV; capillary temperature, 275 °C; capillary voltage, 29.1 V, tube lens 108.8 V.
2.6.3. Metabolite Identification and Processing
Raw LC–MS data were acquired using Xcalibur v2.1 software (Thermo Scientific, Hemel Hemstead, UK). The raw data for each sample analysis consisted LC-MS signals identified by m/z, retention time and ion signal intensity. The full datasets were imported and converted to .mzXML files by MSconvert (ProteoWizard; Software Foundation, San Diego, CA, USA). Then, XCMS was used to extract the ions of the spectra (determines mass-to charge ratios m/z), detect chromatographic peaks (retention time), assign them to molecular ions or adducts, align peaks across samples and quantify their relative abundance, which is calculated based on peak area ratios from sample peaks. Finally, peak detection, grouping, and retention time correction were recorded into peak tables [26]. Data from lipids and polar metabolites were unavailable for two participants. In total, the full datasets for 95 participants were included in the analysis.
Peak intensities of each sample were corrected for run-order signal correction by using a locally quadratic (loess) regression model to fit the QC values. Metabolites with a coefficient of variation of their QC values superior to 30% after run-order correction were removed from the peak table [27]. Missing values in the peak tables were replaced by using k-nearest neighbour (KNN) method. Tentative identification of key metabolites was achieved by using an in-house library, as well as in the appropriate metabolite databases: LipidSearch software (Thermo Scientific, San Jose, CA, USA), the Human Metabolome data-base (HMDB) [28] and METLIN [29].
2.7. Statistical Analyses
2.7.1. Univariate Analysis
The characteristic of the study population was compared using t-test. Normality of the data was tested using the Shapiro–Wilk normality test in. Age, BMI, CTx-1, PTH, vitamin D3, and BMD parameters were expressed as mean and standard deviation (SD). Analysis of covariance was performed to adjust for possible confounders using age and BMI. In addition, our objective was to assess whether lipids and amino acids were associated to BMD. Age-adjusted correlation coefficients were calculated for each lipid and polar metabolites. Age and BMI adjusted mean concentrations of each lipid and polar metabolite were calculated in both groups. To assess whether those associations were independent, we evaluated the pairwise correlations between identified lipids and polar metabolites. p values were calculated using Benjamini–Hochberg false discovery rate (FDR), where FDR p-value < 0.05 was considered as statistically significant. The fold changes in lipids and polar metabolites between BMD groups were performed by parametric test using Metaboanalyst 4.0 web server [30]. Statistical data analysis was performed using R (R 3.3.3, R Foundation for Statistical Computing, Vienna, Austria).
2.7.2. Multivariate Analysis
Prior to multivariate analysis (MVA), different data sets were created (1) an entire cohort containing all samples (n = 95) and (2) a subset (n = 30), in which only 15 women with osteoporosis and 15 women with normal BMD were included. Both the entire cohort and subset were used for modelling the correlations between BMD and metabolites in SC menopausal women. For MVA, each variable was mean centered and univariate scaled over all the samples and imported to SIMCA-P+ v14.1 software (Umetrics, Umea, Sweden). SIMCA was used to construct an orthogonal partial least squares (OPLS) regression model for analyzing BMD. The supervised method analyzes the linear relationship between BMD and metabolite profiles. In OPLS, the R2X, R2Y, and Q2 (cum) parameters were used for the model evaluation, representing the explanation, fitness and prediction power respectively. R2X is the percentage of all LC-MS response variables explained by the model. R2Y is the percentage of all sample variables explained by the model. Q2 is the percentage of all sample variables predicted by the model [31]. Only one component was extracted to predict membership probability in each group. Then, from the coefficient score plot, a normal probability plot was created and 95% of features were excluded to fit a new model. The objective was to select the most relevant variables from the interaction between the metabolites and femoral neck BMD relative to bone status (step 1, OPLS methods) and to add these variables into a new model to display the contribution of each metabolite to the modulation of osteoporosis (step 2).
3. Results
This study analyzed lipid and polar metabolites in blood plasma of SC menopausal women. Overall, after analyzing lipid and polar metabolites, two data sets were created for further multivariate analysis. The correlations between those compounds and femoral neck BMD were investigated by using univariate and OPLS regression statistical analyses. The lipid profiles revealed differences in plasma of women with normal BMD and lower BMD/ osteoporosis. Polar metabolites exhibited no differences; however, a trend was observed in several metabolites such as a peptide, amino acids, and amines.
3.1. Characteristics of the Menopausal Women Bone Status
Women with low BMD were older and had lower BMI when compared with women in the normal group. PTH concentrations were lower in the menopausal groups with low BMD/osteoporosis compared to normal BMD groups. (Table 1). Serum 25(OH) vitamin D3 concentrations were adequate (equal to or >50 nmol/L) for all the groups. Bone resorption marker (CTx-1) concentrations were significant higher in the menopausal group with low BMD/ osteoporosis compared to normal BMD groups.
Table 1.
Characteristics of the SC menopausal women according to bone status, entire cohort (n = 95) and subset (n = 30) analyses.
| Parameters | Entire Cohort (n = 95) | Subset (n = 30) | ||||
|---|---|---|---|---|---|---|
| Normal BMD (n = 23) | Low BMD (n = 72) | p-Value | Normal BMD (n = 15) | Osteoporosis (n = 15) | p-Value | |
| Age (years) | 59.4 (4.19) | 61.3 (4.19) | 0.06 | 58 (3.42) | 61 (3.42) | 0.02 * |
| BMI (kg/m2) | 23.8 (2.61) | 22.5 (2.61) | 0.04 * | 23.8 (2.25) | 20.7 (2.25) | <0.001 * |
| PTH (pmol/L) | 4.7 (1.35) | 4.5 (1.32) | 0.29 | 4.8 (1.59) | 4.3 (1.59) | 0.08 |
| CTx-1 (ug/L) | 0.44 (0.21) | 0.55 (0.20) | 0.02 * | 0.41 (0.26) | 0.64 (0.26) | 0.04 * |
| Vitamin D (nmol/L) | 57.4 (15.24) | 60.1 (14.87) | 0.23 | 56.5 (16.84) | 54.8 (16.84) | 0.81 |
| Femoral neck BMD (g/cm2) | 0.75 (0.05) | 0.60 (0.05) | <0.001 * | 0.78 (0.04) | 0.51 (0.04) | <0.001 * |
Data are presented as mean (SD). The resulting p-values obtained by ANOVA are given. * p < 0.05 was considered as statistically significant.
3.1.1. Entire Cohort
Table 1 shows the population characteristics for the menopausal women. The mean ages for these groups were 59.4 and 61.3 for the normal and low BMD groups, respectively. Women with low BMD had lower BMI than those with normal BMD (23.8 vs. 22.5 kg/m2, p = 0.04) There were no significant differences between the groups for PTH and 25(OH) vitamin D3 concentrations. Mean PTH was 4.7 and 4.5 pmol/L (p = 0.29) and mean 25(OH) vitamin D3 concentrations were 57.4 and 60.1 nmol/L (p = 0.23) for the normal and low BMD groups, respectively. However, CTx-1 concentrations and femoral neck BMD were significant different. Mean CTx-1 concentrations were 0.44 and 0.55 ug/L for the normal versus low BMD group. Femoral neck BMD was 0.75 g/cm2 and 0.60 g/cm2 (p ≤ 0.001) for the normal and low BMD groups, respectively.
3.1.2. Subset
Women with osteoporosis were older than those with normal BMD (58 vs. 61, p = 0.02), and had lower BMI (23.8 vs. 20.7 kg/m2, p = 0.008). Similarly, there were no significant differences between the groups for PTH, CTx-1, and 25(OH) vitamin D3 concentrations. Mean PTH was 4.8 and 4.3 pmol/L (p = 0.08) and mean 25(OH) vitamin D3 concentrations were 56.1 and 54.8 nmol/L (p = 0.81) for the normal BMD and osteoporosis groups, respectively. Serum CTx-1 concentrations were 0.41 and 0.64 ug/L (p = 0.04) and femoral neck BMD was 0.78 g/cm2 and 0.51 g/cm2 (p < 0.001) for the normal BMD and osteoporosis groups, respectively.
3.2. Untargeted Analysis Metabolomic Approach
3.2.1. Lipids
Entire Cohort
Overall, 7082 features (positive and negative ionization) were detected by lipidomics and after filtration and removal of noise and unstable compounds, the remaining 1662 lipid features were fitted within the OPLS model. The OPLS model was fitted and the cumulative R2Y and Q2 values were 0.302 and 0.042, respectively (Table 2). However, the predictive ability for the model Q2 was low, which indicated that it was not a good model. Therefore, the OPLS model with 1662 features was not able to explain the association of the explanatory variables and femoral neck BMD.
Table 2.
Parameters of orthogonal partial least-squares regression models based on the data from lipids separation for the entire cohort and the selected subset of SC menopausal women.
| Dataset | Step | Component | R2X | R2Y | Q2 |
|---|---|---|---|---|---|
| Entire cohort (n = 95) | 1 | 1P and O1 | 0.395 | 0.302 | 0.042 |
| Entire cohort (n = 95) | 2 | 1P and O2 | 0.678 | 0.469 | 0.233 |
| Subset (n = 30) | 1 | 1P and O1 | 0.434 | 0.601 | 0.209 |
| Subset (n = 30) | 2 | 1P and O1 | 0.540 | 0.773 | 0.540 |
P predictive. O orthogonal in X. Component: number of significant component calculated by cross validation. R2X value is the predictive and orthogonal variation in model samples (X) explained by the model. R2Y value is the amount of variation in X which is correlated to Y (response matrix). Q2 value describes the predictive ability of the model.
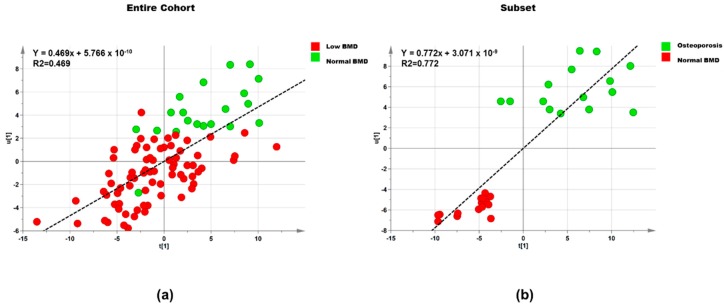
Subsequently, a new model was created by selecting those features based on the exclusion of un-correlated lipid features on the normal probability coefficient score plot (between >0.05 and <0.95%), from model 1 resulting in 98 lipids being selected. The new model yielded R2Y and Q2 values of 0.469 and 0.233, respectively (Table 2). The new OPLS model parameters for fitness and the predictive capability Q2 were better than in the previous model (step 1) and significant based on the cross-validated analysis of variance (CV-ANOVA) (p ≤ 0.001). Figure 1a shows the OPLS predictive model for femoral neck BMD of the lipidomic plasma extracts from normal and low BMD menopausal women. A lineal clustering of the low BMD menopausal women occurred on the left hand of the plot, while normal BMD were clustered on the right top hand side. The results indicated that lipids change linearly with femoral neck BMD (R2Y = 0.469). Further, positive correlations values (upper portion of the plot) indicated increased serum lipid concentrations in normal BMD versus low BMD.
Figure 1.
Orthogonal partial least squares (OPLS). OPLS scatter plot of SC menopausal women with normal (green circles) and low BMD and osteoporosis (red circles) values based on orthogonally filtered partial square (OPLS) regression model 2. Graph (a): 98 lipids among 95 women, entire cohort. Graph (b): 149 lipids among 30 women, subset. t[1]/u[1] correlation plot; t1 refers to scores of the first component in independent variables; u1 refers to scores of the first component in responses.
We found negative associations between femoral neck BMD and the lipids of menopausal women. Based on univariate analysis, the top 50 features were significant by t-tests. However, only 5 features resulted to be known lipids and these were associated to femoral neck BMD (Table 3). The 5 known lipids include 3 phosphatidylserine (PS), 1 diacylglycerol (DG) and 1 plasmenylphosphatidylethanolamine (plasmenyl-PE). The PS species detected comprised 36:1, 33:6 and 29:6, where a decreasing common trend was observed in low BMD group. A correlation matrix revealed negative correlations among the five lipids and femoral neck BMD, PS 36:1 (r = −0.027), PS 33:6 (−0.070), PS 29:6(−0.086), DG 42:4 (r = −0.075), and plasmenyl-PE 38:4 (r = −0.01).
Table 3.
Means and 95% confidence interval of lipids associated to the entire cohort of SC menopausal women (n = 95) with normal and low femoral neck BMD in univariate and multivariate approaches.
| Lipid | Normal BMD a (n = 23) |
Low BMD a (n = 72) |
p-Value | log2 (FC) | Correlation b |
|---|---|---|---|---|---|
| PS 31:6; [M + H]+ | 0.893 (0.427–1.871) |
0.867 (0.675–1.114) |
0.939 | −0.455 | −0.027 |
| PS 33:6; [M + H]+ | 0.857 (0.408–1.800) |
0.876 (0.681–1.127) |
0.955 | −0.373 | −0.070 |
| PS 29:6; [M + H]+ | 0.906 (0.428–1.918) |
0.921 (0.714–1.188) |
0.967 | −0.296 | −0.086 |
| DG 42:4; [M + NH4]+ | 0.850 (0.395–1.828) |
0.947 (0.725–1.219) |
0.804 | −0.176 | −0.075 |
| Plasmenyl-PE 38:4; [M + H]+ | 0.745 (0.353–1.571) |
0.841 (0.653–1.083) |
0.760 | −0.174 | −0.01 |
Lipids are expressed as mean and 95% confidence interval (CI). ANOVA was used to compare lipid mean concentrations. a Covariates: age and BMI; b Age-adjusted correlation coefficient. Statistical p-value calculated using Benjamini–Hochberg false discovery rate (FDR). p-value < 0.05 was considered as statistically significant. Fold of change (FC) expressed as the relation between the lipid mean of the normal BMD group to the lipid mean of the low BMD group. + Adducts in positive ionisation. – Adducts in negative ionisation. PS (phosphatidylserine), DG (diacylglycerol), plasmenyl-PE (plasmenylphosphatidylethanolamines).
Subset
Similarly, an additional OPLS regression model was fitted to the subset of samples (n = 30) with the 1662 lipids to analyze the relationship of femoral neck BMD and lipids.
This OPLS model showed the relationship between the predictive values equals to the observed responses and this was described by high values of R2Y and Q2, 0.601 and 0.209 (Table 2). After excluding 95% of the lipids, a new OPLS model (step 2) was fitted with 149 lipids. The scatter plot for this statistical analysis showed a straight line indicating a relationship between the predictive values equal to the observed responses (Figure 1b). This new model showed higher values of R2Y and Q2 0.773 and 0.54 and was significant based on the CV-ANOVA (p ≤ 0.001), and also showed a strong linear correlation of R2Y = 0.772. Lipids that were highly associated to the normal and osteoporosis groups through both approaches are listed in Table 4. Twelve known lipids were significant and included 1 phosphatidic acid (PA), 2 ceramide-1phosphates (CerP), 4 phosphatidylserine (PS) species, 3 diacylglycerol (DG) species, 1 phosphatidylethanolamine (PE), and 1 phosphatidylinositol (PI) (Table 4). A correlation matrix revealed negative correlations among eight lipids and femoral neck BMD, while a positive correlation was observed with PS (20:4), PI, CerP (24:0), and PE (Table 4).
Table 4.
Means and 95% confidence intervals of lipids associated to the subset of selected SC menopausal women (n = 30) with normal BMD and osteoporosis in univariate and multivariate approaches.
| Lipid | Normal BMD a (n = 15) |
Osteoporosis a (n = 15) |
p-Value | log2 (FC) | Correlation b |
|---|---|---|---|---|---|
| PA 34:4; [M − H]− | 0.531 (0.321–0.878) |
1.617 (0.822–3.182) |
0.005 * | 0.412 | −0.403 |
| CerP 38:1; [M + H]+ | 1.871 (1.196–2.927) |
0.605 (0.3319–1.105) |
0.002 * | −0.637 | −0.384 |
| PS 20:4; [M − H]− | 0.547 (0.330–0.906) |
1.585 (0.804–3.124) |
0.008 * | 0.395 | 0.274 |
| DG 40:0; [M + NH4]+ | 1.545 (0.907–2.630) |
0.425 (0.207–0.870) |
0.0029 * | 0.729 | −0.270 |
| PS 33:6; [M + H]+ | 1.435 (0.908–2.267) |
0.504 (0.277–0.933) |
0.0046 * | −0.560 | −0.363 |
| PS 31:6; [M + H]+ | 1.423 (0.886–2.286) |
0.506 (0.267–0.958) |
0.0065 * | −0.656 | −0.377 |
| PS 32:6; [M + H]+ | 1.414 (0.885–2.259) |
0.515 (0.274–0.967) |
0.007 * | −0.573 | −0.359 |
| PI 14:0; [M − H]− | 0.608 (0.357–1.036) |
1.572 (0.767–3.220) |
0.022 * | 0.327 | 0.165 |
| DG 42:4; [M + NH4]+ | 1.510 (0.976–2.336) |
0.574 (0.319–1.033) |
0.005 * | −0.327 | −0.374 |
| CerP 24:0; [M − H]− | 1.254 (0.728–2.160) |
0.418 (0.201–0.869) |
0.010 * | −0.265 | 0.008 |
| DG 47:5; [M + NH4]+ | 1.670 (0.920–3.032) |
0.854 (0.382–1.905) |
0.135 | −0.351 | −0.014 |
| PE 42:1; [M − H]− | 0.592 (0.346–1.013) |
1.246 (0.605–2.568) |
0.069 | 0.585 | 0.034 |
Lipids are expressed as mean and 95% confidence interval (CI). ANOVA was used to compare lipid mean concentrations. a Covariates: age and BMI; b Age-adjusted correlation coefficient; Statistical p-value calculated using Benjamini–Hochberg false discovery rate (FDR). * p-value < 0.05 was considered as statistically significant. Fold of change (FC) expressed as the relation between the lipid mean of the normal BMD group to the lipid mean of the osteoporosis group. + Adducts in positive ionisation. − Adducts in negative ionisation. PS (phosphatidylserine), PA (phosphatidic acid), DG (diacylglycerol), PE (phosphatidylethanolamine), CerP (ceramide-1-phosphate), PE (phosphatidylethanolamine), and PI (phosphatidylinositol).
3.2.2. Polar Metabolites
Entire Cohort
574 features (positive ionisation) were detected by metabolomics. After filtration and removal of unwanted background and unstable compounds, the remaining 127 polar metabolites were fitted in an OPLS regression model. However, cumulative R2Y and Q2 values were 0.223 and −0.371, respectively (Table 5), and the predictive ability for the model Q2 was very low, which indicated that it was not a suitable model.
Table 5.
Parameters of orthogonal partial least squares regression models based on the data from polar metabolites separation for the entire cohort and the selected subset of SC menopausal women.
| Dataset | Step | Component | R2X | R2Y | Q2 |
|---|---|---|---|---|---|
| Entire cohort (n = 95) | 1 | 1P and O1 | 0.483 | 0.223 | −0.371 |
| Entire cohort (n = 95) | 2 | 1P and O1 | 0.512 | 0.205 | 0.035 |
| Subset (n = 30) | 1 | 1P and O1 | 0.437 | 0.749 | −0.399 |
| Subset (n = 30) | 2 | 1P and O1 | 0.514 | 0.526 | 0.247 |
P: predictive. O: orthogonal in X. Component: number of significant component calculated by cross-validation. R2X value is the predictive and orthogonal variation in model samples (X) explained by the model. R2Y value is the amount of variation in X which is correlated to Y (response matrix). Q2 value describes the predictive ability of the model.
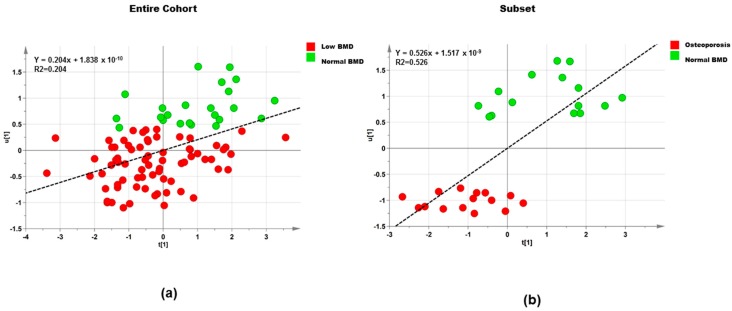
Features scoring between >0.05 and <0.95% on the normal probability coefficient score plot were taken out to fit a new model (step 2), which had only 12 polar metabolites. Values of cumulative R2Y and Q2 values of for this OPLS model were 0.205 and 0.035, respectively (Figure 2a). Both step 1 and step 2 for polar metabolites showed little prediction for the two OPLS regression models. Therefore, the OPLS model with 127 features was not able to explain the association of the explanatory variables and femoral neck BMD. However, this analysis provided evidence for the association between four polar metabolites and femoral neck BMD (Table 6), 4 aminobutyric acid (r = −0.013), threonine (r = −0.172), a tripeptide formed by asparagine-glycine-cystine (asn-gly-cys) (r = −0.059), and turanose (r = −0.039).
Figure 2.
Orthogonal partial least squares (OPLS) scatter plot of polar metabolites, showing relationship between polar metabolites and femoral neck BMD in SC menopausal women with normal (green circles) and low BMD and osteoporosis (red circles) values based on orthogonally filtered partial square (OPLS) regression model 2 with 12 polar metabolites among 95 women, entire cohort (a) and 30 women, subset (b). t[1]/u[1] correlation plot; t1 refers to scores of the first component in independent variables; u1 refers to scores of the first component in responses.
Table 6.
Means and 95% confidence intervals of polar metabolites associated to the entire cohort of SC menopausal women (n = 95) with normal and low femoral neck BMD in univariate and multivariate approaches.
| Polar Metabolite | Normal BMD a (n = 23) |
Low BMD a (n = 72) |
p-Value | Log2 (FC) | Correlation b |
|---|---|---|---|---|---|
| 4-Aminobutyric acid | 1.400 (0.909–2.155) |
0.874 (0.679–1.125) |
0.062 | −0.185 | −0.013 |
| Threonine | 1.315 (0.806–2.011) |
0.843 (0.658–1.081) |
0.073 | −0.159 | −0.172 |
| Asn–Gly–Cys | 0.794 (0.512–1.230) |
1.014 (0.512–1.230) |
0.337 | 0.058 | 0.059 |
| Turanose | 1.319 (0.858–2.028) |
0.845 (0.657–1.087) |
0.076 | −0.174 | −0.039 |
Polar metabolites are expressed as mean and 95% confidence interval (CI). ANOVA was used to compare polar metabolites mean concentrations. a Covariates: age and BMI; b Age-adjusted correlation coefficient. Statistical p-value calculated using Benjamini–Hochberg false discovery rate (FDR). p-value < 0.05 was considered as statistically significant. Fold change expressed as the relation between the metabolite mean of the normal BMD group to the metabolite mean of the low BMD group.
Subset
In addition, the OPLS regression (step 1) was fitted to the subset of samples (n = 30) with the 127 polar metabolites to analyze the relationship of femoral neck BMD and polar metabolites but this model did not show prediction Q2 = −0.399 (Figure 2b). After filtering using coefficient selection section (as described above) the OPLS model 2 showed a higher cross validation value Q2 = 0.247 when compared with the model from step 1 (Table 5). The correlation matrix revealed negative correlations among lipids and femoral neck BMD, proline (r = −0.295), aminopropionitrile (r = −0.315), threonine (r = −0.170), and asn-gly-cys (r = −0.038); while positive correlation was observed with methionine (r = −0.494) (Table 7).
Table 7.
Means and 95% confidence intervals of polar metabolites associated to a subset of selected SC menopausal women (n = 30) with normal BMD and osteoporosis in univariate and multivariate approaches.
| Polar Metabolite | Normal BMD a (n = 15) |
Osteoporosis a (n = 15) |
p-Value | Log2 (FC) | Correlation b |
|---|---|---|---|---|---|
| Proline | 1.581 (0.928–2.692) |
0.786 (0.384–1.609) |
0.084 | −0.234 | −0.295 |
| Aminopropionitrile | 0.729 (0.418–1.272) |
1.848 (0.875–3.905) |
0.03 * | −0.270 | −0.315 |
| Threonine | 1.561 (0.911–2.674) |
0.765 (0.371–1.579) |
0.081 | −0.219 | −0.170 |
| Methionine | 1.534 (0.894–2.632) |
0.758 (0.366–1.567) |
0.085 | 0.141 | 0.494 |
| Asn-Gly-Cys | 1.428 (0.782–2.607) |
0.593 (0.264–1.334) |
0.056 | 0.142 | −0.038 |
Polar metabolites are expressed as mean and 95% confidence interval (CI). ANOVA was used to compare polar metabolites mean concentrations. a Covariates: age and BMI; b Age-adjusted correlation. Statistical p-value calculated using Benjamini–Hochberg false discovery rate (FDR). * p-value < 0.05 was considered as statistically significant. Fold change expressed as the relation between the metabolite mean of the normal BMD group to the metabolite mean of the osteoporosis group.
4. Discussion
The potential of metabolomics was explored for the discovery of molecules associated with BMD loss in SC menopausal women. It is well established that bone loss is linked to age and low levels of oestrogen in women in both Caucasian and Asian populations. After menopause, there are several molecular changes affecting bone metabolism, where those alterations result in an increased bone resorption and a declined BMD [32,33]. Thus, the identification of novel potential biomarkers may be useful for understanding the connections of the lipids and polar metabolites associated with osteoporosis and their capacity to predict bone loss in SC menopausal women.
In order to optimize the prediction of biomarkers for bone loss, we created a second data set (subset) to analyze the relationship between the plasma metabolome of SC women with osteoporosis (T-score < −2.5) and normal BMD (T score > −1). Therefore, in this study, we presented results from univariate and multivariate analysis using two different datasets: the entire cohort and a selected subset of samples. The findings were similar in both datasets; however, with the subset analysis, more lipids were identified as being of significance. This statistical filtering using OPLS regression modelling facilitated the removal of un-correlated lipid features and allowed several lipids to be detected as predictors of menopausal osteoporosis in women.
4.1. Lipids
In this study, we compared changes of the lipid profiles in SC menopausal women and the relationship of those lipids with femoral neck BMD. Glycerophospholipid species PS (20:4/29:6/31:36/32:6/33:6), PE (42:1), PA (34:4), and PI (14:0) were found to be different between normal BMD and osteoporosis groups.
Lipid metabolism disorders have been linked to pathological conditions including obesity, metabolic syndrome, cardiovascular diseases, and bone loss, where cells and signaling pathways may be affected [34]. Adipocytes and osteoblasts are derived from the mesenchymal stem cells (MSC) and the balance of osteoblast versus adipocyte requires interactions between extracellular signaling stimuli. Changes in any of those factors enhances fat bone deposition and promote bone loss [35]. Oestrogens plays a key role on the cell fate of MSCs to differentiate into either osteoblasts or adipocytes; oestrogen also regulates inflammation [36,37]. Further, peroxisome proliferator-activated receptor (PPARy) transcription factor is essential for adipogenesis. Thus, altered lipid metabolism causes oxidative stress and increased expression of PPARy, which reduces osteoblast number in the skeleton, and oestrogren contributes to the regulation of this PPARy signaling pathway [38,39].
Oestrogen deficiency induces bone loss and changes in lipid profiles, BMD and cytokines; however, little information exists on the application of plasma lipidomics for studying menopausal osteoporosis. In vivo and in vitro studies have reported that lipid metabolism disorders promote bone loss by inhibiting osteoblast differentiation and promoting adipogenic differentiation through MSCs stimuli. Phosphatidylinositol (PI) metabolism is essential for signaling by receptor activator of nuclear factor κB (RANK), a local regulator of osteoclastogenesis and bone resorption [40,41]. Our study showed elevated concentrations of PI species (14:0) in SC women with low BMD/ osteoporosis compared with normal BMD group, suggesting that PI species may lead to chronic inflammatory processes and this might induce bone loss in menopausal osteoporosis. Circulating levels of several lipid classes also have been associated with physiological processes including the regulation of inflammation. In an in vitro study, lipid metabolism of PE, PS, and LysoPC changed during MSCs activation due to pro-inflammatory stimuli with TNF-α and IFN-γ [42]. This suggests that changes in lipid profiles promote production of cytokines and differentiation of osteoclasts and may be attributed to oestrogen withdrawal.
Diacylglycerols (DG) are cellular mediators released from membrane lipids that play a key role in the regulation of inflammation and diseases [43]. Our study showed a decrease in the concentrations of glycerolipid species identified as DG (40:0/42:4) in the osteoporosis group compared to the normal BMD menopausal group. While it is not clear whether DG levels are associated with bone mass there is evidence suggesting a relationship between circulating DG profiles and oestrogen loss. A previous study reported plasma DG (33:2) decreased with ageing [44]. Additionally, an animal study reported serum monoacylglycerol and triacylglycerol concentrations decreased in OVX rats as a model of oestrogen deficiency [45], suggesting that lipid profiles and oestrogen loss upregulate bone-proinflammatory cytokines, which control osteoclast differentiation and promote bone loss.
However, information on the association between plasma lipids and femoral neck BMD in menopausal women is limited. Previous studies in menopausal women have reported conflicting associations between triacylglycerol and hip BMD [34,46,47]. Our findings showed a positive association between DG species and hip femoral neck BMD. This result indicates that lipid profile changes may be involved in MSCs functional, anti-inflammatory activities and cytokine production as a result of oestrogen withdrawal, which enhances MSC signaling and inhibits osteoblast differentiation [48,49,50]. However, further studies are needed to clarify the links between plasma lipid concentrations and femoral neck bone loss in menopausal women.
Sphingolipids also play a structural role in cellular membranes and act as bioactive signalling molecules. Ceramide is one of the simple sphingolipids and is involved in the control of many cellular processes including proliferation, differentiation, and apoptosis [51,52]. It has shown that phosphorylated ceramide (ceramide 1-phosphate) stimulates cell survival and proliferation in bone marrow-derived macrophages through molecules such as NF-κB, RANK, and its ligand RANKL [53,54]. However, in our study two sphingolipid species, CerP (24:0/38:1), were significantly reduced in the osteoporosis group compared with the normal BMD group. Our results contradict a previous animal study of oestrogen loss, where CerP concentrations were upregulated in OVX rats [45]. Furthermore, in a human study, Lee et al. [55] found that higher sphingosine-1-phosphate (S1P) concentrations were associated with low BMD in menopausal women. This suggests that the increased levels of CerP promotes differentiation of bone marrow-derived macrophages with biological effects on bone metabolism and could be attributed to oestrogen deficiency.
Taken together, our findings suggest that altered lipid metabolism could be a regulator of bone cell differentiation and bone loss in SC menopausal women. Further studies are required to investigate the potential role of these lipids as biomarkers for early diagnosis of bone loss in SC menopausal women.
4.2. Polar Metabolites
This approach enabled the identification of amino acids, amines, and other polar metabolites. Our study found no significant associations between polar metabolites and femoral neck BMD in SC menopausal groups based on OPLS analysis. However, proline, threonine, and aminopropinitrile concentrations were found to be lower in SC menopausal women with low BMD and osteoporosis based on univariate analysis.
Amino acids play a key role in bone health and are involved in bone remodeling. During osteoporosis, alterations in amino acids may affect bone mass, suggesting that lower levels of circulating amino acids are associated with low BMD. Previous metabolomic studies in menopausal women have reported tryptophan, lysine, homoserine, and 3-hydroxy-l-proline concentrations decreased in the osteoporosis group compared with pre/menopausal women with normal BMD using GC-MS [56]. Miyamoto et al. [20] reported serum concentrations of a dipeptide formed with glycine and glycine (Gly-Gly) and cysteine were lower and hydroxyproline concentrations were higher in low BMD menopausal women using capillary electrophoresis/mass spectrometry (CE-MS). You et al. [22] found higher glutamine concentrations and lower lactate and acetone concentrations associated with low BMD in Taiwanese women using 1H NMR Spectroscopy.
Amino acids modulate bone marrow stem cell (BMSC) function, signalling, proliferation, and differentiation in the bone marrow. Arginine is the precursor for the synthesis of many molecules including urea, nitric oxide, proline, and glutamate [57]. Disorders in arginine metabolism are suggested to cause decalcification, disturbance in calcium absorption, and osteomalcia. Growth hormone and insulin-like growth factor-I, both bone-forming growth factors, are stimulated by arginine and a disorder in these factors cause an increase in inflammatory cytokines and osteoporosis [58]. Proline and its metabolite hydroxyproline are the major amino acids components in collagen, and serum hydroxyproline can be used as bone collagen degradation marker [16,59]. Homocysteine is a metabolite in methionine metabolism and at high levels it interferes with collagen cross-linking, suggesting that increased homocysteine levels can lead to increased fracture risk in ageing [60]. Further, aminopropinitrile has been reported in in vitro studies as a collagen cross-linking inhibitor and, with homocysteine, caused decreased bone strength [61]. The mechanistic implications of altered metabolites and their association to bone remodeling and the link to BMD are not clear, but both BMSC differentiation and collagen formation are critical factors that influence BMD, suggesting that perturbations of amino acids under menopausal osteoporosis may partly contribute to bone loss in elderly women. Information on the association between polar metabolites and SC menopausal women is scarce and needs to be corroborated with further work. As we were unable to test for a possible relationship between polar metabolites and femoral neck BMD due to our small study groups and the high variability among participants, our findings need to be kept in perspective.
The limitations of our study include the small number of participants and higher variability among them such as genetic, age, environment, diet and lifestyle, and body composition. However, this was addressed when we analyzed two different datasets, and both the entire cohort and subset findings were consistent as the subset study showed similar compounds to those already detected in the entire cohort. Supporting these findings is the fact the same compounds were associated with BMD in both datasets’ analyses for the pathology of osteoporosis. Overall, future studies on larger populations are needed to confirm these findings and enable more reliable results for assessing the association between metabolome and femoral neck BMD for prognosis of osteoporosis in SC menopausal women.
5. Conclusions
This pilot study suggested that plasma lipids and polar metabolites differed between women of normal versus low BMD, and these are involved in several metabolic pathways such as sphingolipid metabolism, phospholipid metabolism, and fatty acid β-oxidation. Moreover, this study demonstrates that lipidomic and polar metabolic profiling are promising tools for finding novel biomarkers for bone loss in SC women. Further, prospective studies on larger populations are needed to corroborate these findings and elucidate their key roles in osteoporosis development and progression in SC menopausal women. Thus, understanding the cellular responses to molecular changes in bone metabolism and how these are associated with bone loss in menopausal women may offer the potential of discovering new biomarkers for prognosis of bone loss in elderly women.
Acknowledgments
The authors would like to thank to Nan Xin Wang and Huann Rong Koh who contributed to participant recruitment, blood collection, and BMD measurements. This work was funded by Agency for Science, Technology and Research (A*STAR), Singapore; Singapore-New Zealand Foods for Health Grant (BMRC grant 14/1/16/24/008), Ministry of Business, Innovation and Employment, New Zealand; Singapore-New Zealand Foods for Health Grant (MAUX1309), AgResearch Core funding (contract #A21072) and The Mexican National Council for Science and Technology (Scholarship No. 383233).
Abbreviations
The following abbreviations are used in this manuscript:
| ACN | Acetonitrile |
| BMD | Bone mineral density |
| BMI | Body mass index |
| BMUs | Basic multicellular units |
| CTx-1 | c-terminal telopeptide of type I collagen |
| DG | Diacylglycerol |
| DXA | Dual X-ray absorptiometry |
| EDTA | Ethylenediamine tetraacetic acid |
| HILIC LC-MS | Hydrophilic interaction chromatography liquid chromatography mass spectrometry |
| IL-1 | Interleukin-1 |
| IK-6 | Interleukin-6 |
| CerP | Ceramide-1-phosphate |
| S1P | Sphingosine-1-phosphate |
| M-CSF | Macrophage colony-stimulating factor |
| MSCs | Mesenchymal stem cells |
| OPLS | Orthogonal partial least squares |
| OVX | Ovariectomized |
| PA | Phosphatidic acid |
| PI | Phosphatidylinositol |
| Plasmenyl-PE | Plasmenylphosphatidylethanolamine |
| PE | Phosphatidylethanolamine |
| PPARy | Peroxisome proliferator-activated receptor |
| PS | Phosphatidylserine |
| PTH | parathyroid hormone |
| ROS | Reactive oxygen species |
| RP (LC-MS) | Reverse-phase liquid chromatography mass spectrometry |
| SC | Singaporean-Chinese |
| TNF-α | Tumor necrosis factor-α |
| QC | Quality control |
| WHO | World Health Organization |
Author Contributions
M.K., F.M.W., N.C.R., J.J.T., C.J.H., D.C.-S. and K.F.: conceived of study. C.J.H.: supervised patient recruitment. D.C., M.K. F.M.W., N.C.R., and K.F.: contributed to the study execution, analysis, and interpretation of data. D.C. and K.F.: performed data analysis and interpretation. D.C.: wrote the manuscript. M.K., F.M.W., N.C.R., J.J.T., C.J.H., D.C.-S. and K.F.: reviewed manuscript. D.C., M.K, F.M.W., N.C.R., J.J.T., C.J.H., D.C.-S. and K.F.: contributed to draft, edit and approve the final version of this paper for submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Aaseth J., Boivin G., Andersen O. Osteoporosis and trace elements—An overview. J. Trace Elem. Med. Biol. 2012;26:149–152. doi: 10.1016/j.jtemb.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C., Cole Z.A., Holroyd C.R., Earl S.C., Harvey N.C., Dennison E.M., Melton L.J., Cummings S.R., Kanis J.A. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos. Int. 2011;22:1277. doi: 10.1007/s00198-011-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weitzmann M.N., Pacifici R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolagas S.C. From Estrogen-Centric to Aging and Oxidative Stress: A Revised Perspective of the Pathogenesis of Osteoporosis. Endocr. Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redlich K., Smolen J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012;11:234. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 6.Maggio D., Barabani M., Pierandrei M., Polidori M.C., Catani M., Mecocci P., Senin U., Pacifici R., Cherubini A. Marked Decrease in Plasma Antioxidants in Aged Osteoporotic Women: Results of a Cross-Sectional Study. J. Clin. Endocrinol. Metab. 2003;88:1523–1527. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 7.Mody N., Parhami F., Sarafian T.A., Demer L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001;31:509–519. doi: 10.1016/S0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 8.Riggs B.L. The mechanisms of estrogen regulation of bone resorption. J. Clin. Investig. 2000;106:1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jilka R.L., Hangoc G., Girasole G., Passeri G., Williams D.C., Abrams J.S., Boyce B., Broxmeyer H., Manolagas S.C. Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science. 1992;257:88. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 10.Kanis J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 11.Patti G.J., Yanes O., Siuzdak G. Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012;13:263. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y.-R., Huang R.-Q., Xiao B.-K., Yang J.-Y., Dong J.-X. 1H NMR metabolic profiling analysis offers evaluation of Nilestriol treatment in ovariectomised rats. Mol. Cell. Endocrinol. 2014;387:19–34. doi: 10.1016/j.mce.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y.-R., Xiao B.-K., Yang J.-Y., Guo C.-H., Shen S.-J., Tang Z.-S., Dong J.-X., Huang R.-Q. 1H-NMR and HPLC–MS/MS-based global/targeted metabolomic evaluation of Hypericum perforatum L. intervention for menopause. J. Funct. Foods. 2015;17:722–741. doi: 10.1016/j.jff.2015.06.023. [DOI] [Google Scholar]
- 14.Iida M., Harada S., Kurihara A., Fukai K., Kuwabara K., Sugiyama D., Takeuchi A., Okamura T., Akiyama M., Nishiwaki Y., et al. Profiling of plasma metabolites in postmenopausal women with metabolic syndrome. Menopause. 2016;23:749–758. doi: 10.1097/GME.0000000000000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke C., Hou Y., Zhang H., Yang K., Wang J., Guo B., Zhang F., Li H., Zhou X., Li Y., et al. Plasma Metabolic Profiles in Women are Menopause Dependent. PLoS ONE. 2015;10:e0141743. doi: 10.1371/journal.pone.0141743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma B., Liu J., Zhang Q., Ying H., Jiye A., Sun J., Wu D., Wang Y., Li J., Liu Y. Metabolomic profiles delineate signature metabolic shifts during estrogen deficiency-induced bone loss in rat by GC-TOF/MS. PLoS ONE. 2013;8:e54965. doi: 10.1371/journal.pone.0054965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M.Y., Kim H.Y., Singh D., Yeo S.H., Baek S.Y., Park Y.K., Lee C.H. Metabolite profiling reveals the effect of dietary Rubus coreanus vinegar on ovariectomy-induced osteoporosis in a rat model. Molecules. 2016;21:149. doi: 10.3390/molecules21020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue L., Wang Y., Liu L., Zhao L., Han T., Zhang Q., Qin L. A 1HNMR-Based Metabonomics Study of Postmenopausal Osteoporosis and Intervention Effects of Er-Xian Decoction in Ovariectomized Rats. Int. J. Mol. Sci. 2011;12:7635–7651. doi: 10.3390/ijms12117635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long W.-F., Li L., Chen H.-Q., Tang Y., He X.-L., Jing R.-Z. 1H-NMR-based metabonomics analysis of plasma from osteoporotic rats induced by ovariectomy. J. Sichuan Univ. Med. Sci. Ed. 2009;40:843–847. [PubMed] [Google Scholar]
- 20.Miyamoto T., Hirayama A., Sato Y., Koboyashi T., Katsuyama E., Kanagawa H., Miyamoto H., Mori T., Yoshida S., Fujie A., et al. A serum metabolomics-based profile in low bone mineral density postmenopausal women. Bone. 2017;95:1–4. doi: 10.1016/j.bone.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Liu X., Zhang S., Lu X., Zheng S., Li F., Xiong Z. Metabonomic study on the anti-osteoporosis effect of Rhizoma Drynariae and its action mechanism using ultra-performance liquid chromatography–tandem mass spectrometry. J. Ethnopharmacol. 2012;139:311–317. doi: 10.1016/j.jep.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 22.You Y.-S., Lin C.-Y., Liang H.-J., Lee S.-H., Tsai K.-S., Chiou J.-M., Chen Y.-C., Tsao C.-K., Chen J.-H. Association Between the Metabolome and Low Bone Mineral Density in Taiwanese Women Determined by 1H NMR Spectroscopy. J. Bone Miner. Res. 2014;29:212–222. doi: 10.1002/jbmr.2018. [DOI] [PubMed] [Google Scholar]
- 23.Maunsell Z., Wright D.J., Rainbow S.J. Routine isotopedilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin. Chem. 2005;51:1683–1690. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 24.Organization W.H. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group [Meeting Held in Rome from 22 to 25 June 1992] WHO; Geneva, Switzerland: 1994. [PubMed] [Google Scholar]
- 25.Xu J., Begley P., Church S.J., Patassini S., Hollywood K.A., Jüllig M., Curtis M.A., Waldvogel H.J., Faull R.L.M., Unwin R.D., et al. Graded perturbations of metabolism in multiple regions of human brain in Alzheimer’s disease: Snapshot of a pervasive metabolic disorder. Biochim. Biophys. Acta. 2016;1862:1084–1092. doi: 10.1016/j.bbadis.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith C.A., Want E.J., O’Maille G., Abagyan R., Siuzdak G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 27.Zelena E., Dunn W.B., Broadhurst D., Francis-McIntyre S., Carroll K.M., Begley P., O’Hagan S., Knowles J.D., Halsall A., Wilson I.D., et al. Development of a Robust and Repeatable UPLC−MS Method for the Long-Term Metabolomic Study of Human Serum. Anal. Chem. 2009;81:1357–1364. doi: 10.1021/ac8019366. [DOI] [PubMed] [Google Scholar]
- 28.Wishart D.S., Jewison T., Guo A.C., Wilson M., Knox C., Liu Y., Djoumbou Y., Mandal R., Aziat F., Dong E., et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith C.A., Maille G.O., Want E.J., Qin C., Trauger S.A., Brandon T.R., Custodio D.E., Abagyan R., Siuzdak G. METLIN: A Metabolite Mass Spectral Database. Ther. Drug Monit. 2005;27:747–751. doi: 10.1097/01.ftd.0000179845.53213.39. [DOI] [PubMed] [Google Scholar]
- 30.Chong J., Soufan O., Li C., Caraus I., Li S., Bourque G., Wishart D.S., Xia J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018 doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liland K.H. Multivariate methods in metabolomics—From pre-processing to dimension reduction and statistical analysis. TrAC Trends Anal. Chem. 2011;30:827–841. doi: 10.1016/j.trac.2011.02.007. [DOI] [Google Scholar]
- 32.Manolagas S.C., Parfitt A.M. What old means to bone. Trends Endocrinol. Metab. 2010;21:369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng X., McDonald J.M. Disorders of Bone Remodeling. Ann. Rev. Pathol. Mech. Dis. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui L.-H., Shin M.-H., Chung E.-K., Lee Y.-H., Kweon S.-S., Park K.-S., Choi J.-S. Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporos. Int. 2005;16:1975–1981. doi: 10.1007/s00198-005-1977-2. [DOI] [PubMed] [Google Scholar]
- 35.Almeida M., O’Brien C.A. Basic Biology of Skeletal Aging: Role of Stress Response Pathways. J. Gerontol. Ser. A. 2013;68:1197–1208. doi: 10.1093/gerona/glt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muruganandan S., Roman A.A., Sinal C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J.-W., Gao Z.-L., Wang Y., Mei H., Li Y.-L. Differentiation of Human Mesenchymal Stem Cells: The Potential Mechanism for Estrogen-Induced Preferential Osteoblast Versus Adipocyte Differentiation. Am. J. Med. Sci. 2011;341:460–468. doi: 10.1097/MAJ.0b013e31820865d5. [DOI] [PubMed] [Google Scholar]
- 38.Almeida M., Ambrogini E., Han L., Manolagas S.C., Jilka R.L. Increased Lipid Oxidation Causes Oxidative Stress, Increased Peroxisome Proliferator-activated Receptor-γ Expression, and Diminished Pro-osteogenic Wnt Signaling in the Skeleton. J. Biol. Chem. 2009;284:27438–27448. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dieudonne M.N., Pecquery R., Leneveu M.C., Giudicelli Y. Opposite Effects of Androgens and Estrogens on Adipogenesis in Rat Preadipocytes: Evidence for Sex and Site-Related Specificities and Possible Involvement of Insulin-Like Growth Factor 1 Receptor and Peroxisome Proliferator-Activated Receptorγ 21. Endocrinology. 2000;141:649–656. doi: 10.1210/endo.141.2.7293. [DOI] [PubMed] [Google Scholar]
- 40.Takeshita S., Namba N., Zhao J.J., Jiang Y., Genant H.K., Silva M.J., Brodt M.D., Helgason C.D., Kalesnikoff J., Rauh M.J., et al. SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat. Med. 2002;8:943. doi: 10.1038/nm752. [DOI] [PubMed] [Google Scholar]
- 41.Wada T., Nakashima T., Hiroshi N., Penninger J.M. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Campos A.M., Maciel E., Moreira A.S.P., Sousa B., Melo T., Domingues P., Curado L., Antunes B., Domingues M.R.M., Santos F. Lipidomics of Mesenchymal Stromal Cells: Understanding the Adaptation of Phospholipid Profile in Response to Pro-Inflammatory Cytokines. J. Cell. Physiol. 2016;231:1024–1032. doi: 10.1002/jcp.25191. [DOI] [PubMed] [Google Scholar]
- 43.Kilpinen L., Tigistu-Sahle F., Oja S., Greco D., Parmar A., Saavalainen P., Nikkilä J., Korhonen M., Lehenkari P., Käkelä R., et al. Aging bone marrow mesenchymal stromal cells have altered membrane glycerophospholipid composition and functionality. J. Lipid Res. 2013;54:622–635. doi: 10.1194/jlr.M030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jové M., Maté I., Naudí A., Mota-Martorell N., Portero-Otín M., de la Fuente M., Pamplona R. Human Aging Is a Metabolome-related Matter of Gender. J. Gerontol. Ser. A. 2016;71:578–585. doi: 10.1093/gerona/glv074. [DOI] [PubMed] [Google Scholar]
- 45.Vinayavekhin N., Sueajai J., Chaihad N., Panrak R., Chokchaisiri R., Sangvanich P., Suksamrarn A., Piyachaturawat P. Serum lipidomics analysis of ovariectomized rats under Curcuma comosa treatment. J. Ethnopharmacol. 2016;192:273–282. doi: 10.1016/j.jep.2016.07.054. [DOI] [PubMed] [Google Scholar]
- 46.Brownbill R.A., Ilich J.Z. Lipid Profile and Bone Paradox: Higher Serum Lipids Are Associated with Higher Bone Mineral Density in Postmenopausal Women. J. Women’s Health. 2006;15:261–270. doi: 10.1089/jwh.2006.15.261. [DOI] [PubMed] [Google Scholar]
- 47.Makovey J., Chen J.S., Hayward C., Williams F.M.K., Sambrook P.N. Association between serum cholesterol and bone mineral density. Bone. 2009;44:208–213. doi: 10.1016/j.bone.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Pacifici R., Brown C., Puscheck E., Friedrich E., Slatopolsky E., Maggio D., McCracken R., Avioli L.V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc. Natl. Acad. Sci. USA. 1991;88:5134. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou S., Zilberman Y., Wassermann K., Bain S.D., Sadovsky Y., Gazit D. Estrogen modulates estrogen receptor α and β expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. J. Cell. Biochem. 2001;81:144–155. doi: 10.1002/jcb.1096. [DOI] [PubMed] [Google Scholar]
- 50.Zheng S.X., Vrindts Y., Lopez M., de Groote D., Zangerle P.F., Collette J., Franchimont N., Geenen V., Albert A., Reginster J.Y. Increase in cytokine production (IL-1β, IL-6, TNF-α but not IFN-γ, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas. 1997;26:63–71. doi: 10.1016/S0378-5122(96)01080-8. [DOI] [PubMed] [Google Scholar]
- 51.Arana L., Gangoiti P., Ouro A., Trueba M., Gómez-Muñoz A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010;9:15. doi: 10.1186/1476-511X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gómez-Muñoz A. Ceramide-1-phosphate: A novel regulator of cell activation. FEBS Lett. 2004;562:5–10. doi: 10.1016/S0014-5793(04)00211-X. [DOI] [PubMed] [Google Scholar]
- 53.Gangoiti P., Granado M.H., Wang S.W., Kong J.Y., Steinbrecher U.P., Gómez-Muñoz A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell. Signal. 2008;20:726–736. doi: 10.1016/j.cellsig.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Gómez-Muñoz A., Kong J.Y., Salh B., Steinbrecher U.P. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J. Lipid Res. 2004;45:99–105. doi: 10.1194/jlr.M300158-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Lee S.H., Lee S.-Y., Lee Y.-S., Kim B.-J., Lim K.-H., Cho E.-H., Kim S.-W., Koh J.-M., Kim G.S. Higher Circulating Sphingosine 1-Phosphate Levels Are Associated with Lower Bone Mineral Density and Higher Bone Resorption Marker in Humans. J. Clin. Endocrinol. Metab. 2012;97:E1421–E1428. doi: 10.1210/jc.2012-1044. [DOI] [PubMed] [Google Scholar]
- 56.Qi H., Bao J., An G., Ouyang G., Zhang P., Wang C., Ying H., Ouyang P., Ma B., Zhang Q. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC-MS. Mol. BioSyst. 2016;12:2265–2275. doi: 10.1039/C6MB00181E. [DOI] [PubMed] [Google Scholar]
- 57.Morris J.S.M. Arginine: Beyond protein1–4. Am. J. Clin. Nutr. 2006;83:508S–512S. doi: 10.1093/ajcn/83.2.508S. [DOI] [PubMed] [Google Scholar]
- 58.Chevalley T., Rizzoli R., Manen D., Caverzasio J., Bonjour J.P. Arginine increases insulin-like growth factor-I production and collagen synthesis in osteoblast-like cells. Bone. 1998;23:103–109. doi: 10.1016/S8756-3282(98)00081-7. [DOI] [PubMed] [Google Scholar]
- 59.Wood S.L., Westbrook J.A., Brown J.E. Omic-profiling in breast cancer metastasis to bone: Implications for mechanisms, biomarkers and treatment. Cancer Treat. Rev. 2014;40:139–152. doi: 10.1016/j.ctrv.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Van Meurs J.B.J., Dhonukshe-Rutten R.A.M., Pluijm S.M.F., van der Klift M., de Jonge R., Lindemans J., de Groot L.C.P.G.M., Hofman A., Witteman J.C.M., van Leeuwen J.P.T.M., et al. Homocysteine Levels and the Risk of Osteoporotic Fracture. N. Engl. J. Med. 2004;350:2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 61.Turecek C., Fratzl-Zelman N., Rumpler M., Buchinger B., Spitzer S., Zoehrer R., Durchschlag E., Klaushofer K., Paschalis E.P. Collagen Cross-Linking Influences Osteoblastic Differentiation. Calcif. Tissue Int. 2008;82:392–400. doi: 10.1007/s00223-008-9136-3. [DOI] [PubMed] [Google Scholar]