Abstract
Perturbations to the amygdala have been observed in neurological disorders characterized by abnormalities in social behavior, such as autism and schizophrenia. Here we quantitatively examined the amygdala in the postmortem human brains of male and female individuals diagnosed with Williams Syndrome (WS), a neurodevelopmental disorder caused by a well-defined deletion of ~26 genes, and accompanied by a consistent behavioral profile that includes profound hypersociability. Using unbiased stereological sampling, we estimated nucleus volume, number of neurons, neuron density, and neuron soma area in four major amygdaloid nuclei- the lateral nucleus, basal nucleus, accessory basal nucleus, and central nucleus- in a sample of five adult and two infant WS brains and seven age-, sex- and hemisphere matched typically developing control (TD) brains. Boundaries of the four nuclei examined were drawn on Nissl-stained coronal sections as four separate regions of interest for data collection. We found that the lateral nucleus contains significantly more neurons in WS compared to TD. WS and TD do not demonstrate significant differences in neuron number in the basal, accessory basal, or central nuclei, and there are no significant differences between WS and TD in nuclei volume, neuron density, and neuron soma area in any of the four nuclei. A similarly designed study reported a decrease in lateral nucleus neuron number in autism, mirroring the opposing extremes of the two disorders in the social domain. These results suggest that the number of neurons in the lateral nucleus may contribute to pathological disturbances in amygdala function and sociobehavioral phenotype.
Keywords: Williams syndrome, neuropathology, neuroanatomy, neuron number, amygdala
INTRODUCTION
Williams Syndrome (WS) is a rare neurodevelopmental disorder (1 in 7,500 to 1 in 12,000 births; Stromme et al. 2002) caused by a hemizygous microdeletion of ~26 consecutive genes in chromosome band 7q11.23. WS is characterized by a consistent behavioral phenotype broadly described as a hyperaffiliative social drive, marked by an atypically strong desire for social engagement, exaggerated gregariousness, a lack of inhibition in approaching and interacting with unfamiliar conspecifics, and impaired social perceptual ability (Jarvinen-Pasley et al. 2010). While WS is rare, it has garnered broad interest in behavioral neurobiology- not only does the syndrome present a unique opportunity to study the relationship between genotype, neural structure, and social behavior, but WS also demonstrates strong links to the much more prevalent autism spectrum disorders (ASD). WS and ASD display behavioral phenotypes that include hypersociability and social avoidance, respectively, representing opposing extremes in the social domain (Jarvenin, Korenberg, and Bellugi 2012). Intriguingly, some ASD cases include a duplication of the WS deletion (Merla et al. 2010), and some WS cases with atypical deletions demonstrate behavioral phenotypes more fitting to an ASD diagnosis (Edelmann et al. 2007; Sakuri et al. 2011).
The amygdala has been critically implicated in social behavior and cognition (Adolphs et al., 1994, 1999; Adolphs 2001; Meunier et al. 1999; Emery et al. 2001; Bauman et al. 2006). Neuroimaging and postmortem histological studies have found significant alterations to the amygdala in ASD, including an increase in amygdala volume in childhood that is no longer detectable in adolescence, and reduced neuron numbers in the lateral nucleus of the amygdala across age groups (Schumann et al. 2004; Schumann and Amaral 2006; Mosconi et al. 2009). Neuroimaging studies in WS have demonstrated enlargement of the amygdala in adolescence and adulthood (Reiss et al. 2004; Martens et al. 2009; Haas et al. 2014; but see Meyer-Lindenberg et al. 2004, Meda et al. 2012, which found no change), and reduced activation of the amygdala in response to negative social stimuli (Haas et al. 2009, 2010). However, little is known about WS at the histological level. A single case report on a postmortem adult WS brain noted that the lateral nucleus of the amygdala appeared smaller than expected relative to the other nuclei (Galaburda and Bellugi 2000). A stereological analysis of the cortex in six adult WS postmortem brains and six matched controls found a significant decrease in neuron density in the infragranular layers of the orbitofrontal cortex (OFC) in WS (Lew et al. 2017), and another study examining dendritic branching in the same cortical areas in two WS brains found that dendritic branching may be reduced in the WS OFC (Hrvoj-Mihic et al., 2017). The OFC demonstrates significant connections to subcortical structures including the amygdala (Barbas 1995), so such findings could indicate atypical downstream input.
Utilizing a sample of seven postmortem WS brains, comprising two early postnatal infants and five adults, and seven typically developing matched controls (TD), we measured volume, neuron number, neuron density, and neuron soma area of four major subdivisions of the amygdala: the lateral nucleus, basal nucleus, and accessory basal nucleus, most often implicated in the cognitive/sociobehavioral functions of the amygdala (Saddoris et al. 2005; Murray 2007; Seymour and Dolan 2008; Janak and Tye 2015; but see Goossens et al. 2009 for fMRI data demonstrating evidence of superficial amygdaloid nuclei involvement in human social behavior), and the central nucleus, involved in autonomic functions of the amygdala (Gallagher and Holland 1994). This is the first study to quantitatively examine the WS amygdala utilizing unbiased stereological methods, and to our knowledge, the first postmortem stereological study to examine the human amygdala in early postnatal infants. Given findings from previous studies in ASD and WS, we expected to observe differences in neuron number in the lateral nucleus of the amygdala in WS, as well as differences in volume throughout the WS amygdala. While amygdala volume would be smaller in the infants than the adults, we expected the infant subjects to demonstrate neuron numbers similar to their respective adult diagnostic groups (WS or TD), given that most amygdala neurogenesis and migration is complete before birth (Chareyron et al. 2012; Kordower et al. 1992; but see Bernier et al. 2002 for evidence of some adult neurogenesis in the amygdala in nonhuman primates).
MATERIALS AND METHODS
The materials of this study include amygdala tissue from seven WS subjects and seven TD subjects (Table 1). Only subjects free of seizures or other neurological disorders were used in this study. Fluorescence in-situ hybridization (FISH) probes for elastin, a gene consistently deleted in the WS hemideletion, were used to determine genetic diagnosis in the WS cases, and all WS subjects used in this study demonstrate the typical WS genetic deletion. TD and WS subjects were matched for age, sex, and hemisphere (right), with the exception of one pair, that was matched for age and sex only. Due to the rare occurrence of the disorder, our TD sample was matched to meet the availability of WS tissue, and comprises two early postnatal infant pairs and five adult pairs. While developmental differences between infant and adult cohorts will be present in the volume of the amygdala, neuron number should be similar in infants and adults, as neurogenesis of the amygdala is complete well before birth (Chareyron et al. 2012; Kordower et al. 1992). This study includes one hemisphere per subject (in all but a single case, right hemisphere), as both postmortem and neuroimaging studies have found an absence of asymmetry in the human amygdala (Barger et al. 2007; Brierley et al. 2002).
Table 1.
Subject background
| Subject ID | Age at Death | Diagnosis | Gender | Hemisphere | Cause of Death | Postmortem Interval (hours) |
|---|---|---|---|---|---|---|
| WS 11 | 26 days | Williams syndrome | Male | Right | Unknown | 34.5 |
| TD 4353 | 34 days | Typically developing | Male | Right | SIDS | 5 |
| WS 7 | 114 days | Williams syndrome | Male | Right | Multiple organ failure | 30 |
| TD 5183 | 107 days | Typically developing | Male | Right | SIDS | 13 |
| WS 10 | 17 years | Williams syndrome | Male | Right | Cardiac complications | 24 |
| TD 4916 | 19 years | Typically developing | Male | Right | Drowning | 5 |
| WS 14 | 42 years | Williams syndrome | Female | Right | Cardiac complications | 18 |
| TD 5445 | 42 years | Typically developing | Female | Right | Pulmonary thromboembolism | 10 |
| WS 9 | 43 years | Williams syndrome | Female | Right | Cardiac complications | 12 |
| TD 5758 | 43 years | Typically developing | Female | Right | Sepsis | 22 |
| WS 4 | 46 years | Williams syndrome | Female | Right | Breast cancer | 28 |
| TD 4640 | 47 years | Typically developing | Female | Right | Pneumonia | 5 |
| WS 8 | 48 years | Williams syndrome | Male | Left | Respiratory illness | 30 |
| TD 4598 | 45 years | Typically developing | Male | Right | Unknown non-neurological | 6 |
All WS tissue was obtained from the Ursula Bellugi Williams Syndrome Brain Collection, an ongoing donation-based program run by the Laboratory for Human Comparative Neuroanatomy at UCSD (La Jolla). TD brain tissue was obtained through the NIH NeuroBioBank.
Tissue Preparation
All brains were immersed in 10% buffered formalin after autopsy (see Table 1 for PMI), and remained in formalin until processed for experimentation, with fixation time ranging from several months to over 20 years. For each subject, a 4cm block containing the entire rostro-caudal extent of the amygdala was removed from a single hemisphere. This large block was then bisected into 2-3 smaller blocks along the coronal plane in order to fit on the microtome stage (Schumann and Amaral 2005). These smaller blocks were cryoprotected until saturated in a gradient of sucrose and 0.1M phosphate buffer solutions (10%, 20%, 30%) in preparation for cutting. Tissue was frozen with dry ice and cut along the coronal plane on a Leica SM sliding microtome. Infant tissue was cut in five series of 80 micrometer (m) sections, and adult tissue was cut in three series of 80 m sections and four series of 40 m sections, so that each series represented the entire rostro-caudal extent of the amygdala, and the distance between consecutive sections in each series was 400 m. The number of sections per series ranged from 23-26 sections in adult subjects and 20-21 sections in infant subjects. One 80 m series per individual was mounted on gelatin-coated glass slides and stained for Nissl substance with 0.25% thionin for analysis in this study, and additional series were stored in the freezer for future experiments.
Region of interest
We examined four amygdaloid nuclei: the lateral nucleus, basal nucleus, and accessory basal nucleus, which together make up the basolateral amygdala, and the central nucleus. We chose these nuclei for three primary reasons: 1) tracer studies in nonhuman primates have found that the basolateral nuclei are the primary site of amygdalar connectivity with higher-order cortical areas associated with social and emotional cognition (Aggleton et al. 1980; Leichnetz and Astruc 1976, 1977; Leichnetz et al. 1976; Carmichael and Price 1995; Stefanacci and Amaral 2000, 2002); 2) these nuclei demonstrate distinct anatomical boundaries in Nissl-stained tissue, and can therefore be identified with high accuracy, while boundaries of the individual superficial amygdaloid nuclei are less clear and thus less suitable for the purpose of the present study; 3) similarly designed postmortem human studies in typically developing brains and brains from individuals with ASD have examined the same nuclei (Schumann and Amaral 2005, 2006). Anatomical boundaries within the amygdala were defined cytoarchitectonically (Figure 1) and guidelines, briefly summarized below, were adapted from previous studies (Sorvari et al. 1995; Schumann and Amaral 2005 and Barger et al. 2012):
Figure 1.
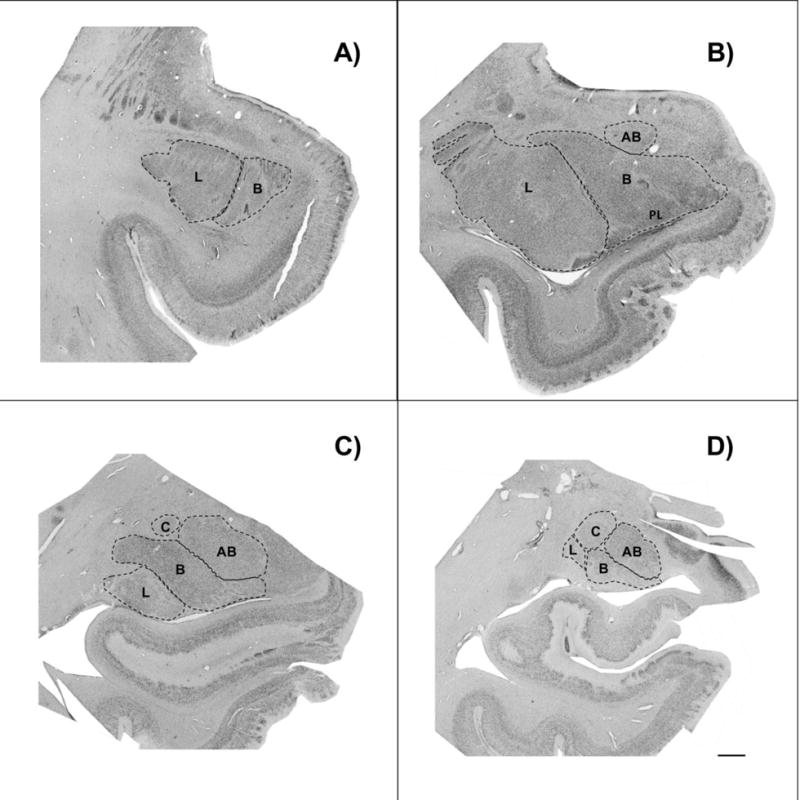
Brightfield photomicrograph of Nissl-stained coronal sections through rostral A), midrostro-caudal B) and C), and caudal D) levels of the amygdala from a typically developing subject, demonstrating nuclei boundaries used in data collection. L= lateral nucleus of the amygdala; B= basal nucleus of the amygdala; AB= accessory basal nucleus of the amygdala; C= central nucleus of the amygdala. Scale bar= 2 mm.
Lateral nucleus: The lateral boundary of the lateral nucleus is the border of the external capsule, while white matter forms the dorsal and ventral borders. The lateral medullary lamina marks the medial border of the lateral nucleus (and lateral border of the basal nucleus). In addition, the presence of smaller, more compact cells distinguish the lateral nucleus from the adjacent basal nucleus. In caudal sections, the larger cells in the dorsal region of the lateral nucleus distinguish it from the putamen.
Basal nucleus: The medial border is defined by the intermediate medullary lamina, which separates the basal nucleus from the accessory basal nucleus. The dorsomedial region of the basal nucleus can be distinguished from the accessory basal nucleus by the presence of large cells, which are absent in the accessory basal nucleus. The paralaminar nucleus, a narrow, densely packed band of small, darkly stained cells, forms the ventral border of the basal nucleus throughout most of the amygdala. The paralaminar nucleus is difficult to distinguish from the basal nucleus, and is therefore included here as part of the basal nucleus.
Accessory basal nucleus: In addition to being defined laterally by the intermediate medullary lamina, the accessory basal nucleus can be further identified by the absence of the large cells that are present in the adjacent basal nucleus. The medial medullary lamina forms the medial border, and separates the accessory basal nucleus from the superficial cortical nuclei. The ventral region of the accessory basal nucleus can be distinguished from the basal nucleus by smaller cell size.
Central nucleus: the central nucleus is only visible in more caudal sections, and lies dorsal to the basolateral nuclei. The central nucleus is distinguished from the rest of the amygdala by fiber tracts. A fiber tract also divides the central nucleus into two subdivisions: a lateral region, which has small, densely packed neurons that stain lightly, and a medial region that is stained more darkly and has more loosely packed neurons. The basal nucleus is ventrolateral to the central nucleus, and the accessory basal nucleus is ventromedial to the central nucleus. The dorsolateral border of the central nucleus forms part of the outer border of the amygdala.
Unbiased, design-based stereology
Data was collected by a single investigator (CHL) after establishing inter-rater reliability in neuron count estimations in the amygdala of a TD sample previously reported in the literature (Schumann and Amaral 2005) to 95% concordance. Sections were coded prior to data collection to blind the investigator. Data collection was conducted using the Cavalieri, Optical Fractionator, and Nucleator probes in Stereoinvestigator software (MBF BioScience, Williston, VT) on a Dell workstation receiving live video feed from a Lumenera color video camera (Ottawa, Ontario) attached to an Eclipse 80i microscope equipped with a Ludl MAC5000 stage (Hawthorn, NY) and a Heiden z-axis encoder (Plymouth, MN). The entire rostro-caudal extent of the basolateral nuclei and central nucleus was defined on every 80 m-thick section in which the nuclei were present in the series (see Table 2 for average number of sections per nucleus in infant and adult subjects) and the distance between each section was 0.4 mm. Cytoarchitectonic boundaries were drawn at 1× magnification. The Cavalieri method was used to determine the post-processing volume of each nucleus. The Cavalieri method (Garcia-Finata et al. 2003) operates by randomly overlaying a lattice of points (0.15 mm distance between each point) over each region of interest on each section, and counting the number of points within each region to calculate area. Volume is then calculated by multiplying the area by the average section thickness (measured at each counting site). Due to absolute volume differences of the nuclei (e.g. central nucleus is smallest, lateral nucleus is largest), different grid sizes were used for each nucleus (Schumann and Amaral 2006), as well as for the infant and adult subjects (Table 2).
Table 2.
Stereology parameters
| Amygdaloid nucleus | Mean no. of sections | Dissector grid area (mm2) |
|---|---|---|
| Infant | ||
| Lateral | 20 | 4.00 |
| Basal | 21 | 2.25 |
| Accessory Basal | 20 | 1.00 |
| Central | 12 | 0.50 |
|
| ||
| Adult | ||
| Lateral | 23 | 6.25 |
| Basal | 26 | 4.00 |
| Accessory Basal | 23 | 2.25 |
| Central | 15 | 1.00 |
Utilizing the same boundaries drawn for volumetric measurements, neuron number in each amygdaloid nucleus was estimated using the Optical Fractionator probe in combination with fractionator sampling. This quantitative method counts neurons using optical disectors (a three-dimensional probe) in a reference space, and is independent of volume measurements so that estimation of numbers of neurons is unaffected by tissue shrinkage (Gunderson and Jensen 1987; West and Gunderson 1990). Tissue was viewed at high magnification (100×, 1.4 numerical aperture, oil lens). Similar to the set-up of the Cavalieri probe, different grid sizes were used for each nucleus and age cohort to account for differences in absolute volume. A disector height of 10 m and a counting frame of 60 m × 60 m was used, and mounted section thickness was measured at each counting site. Neurons were counted by placing a marker on each neuron within the counting frame and not touching the red line of exclusion, for which the nucleolus was visible (Figure 2). We excluded cells with a diameter smaller than 5 m, given the difficulty in distinguishing glia from some very small neurons in the amygdala. Neuron density was calculated by dividing neuron number by nucleus volume in each amygdaloid nucleus of each subject.
Figure 2.
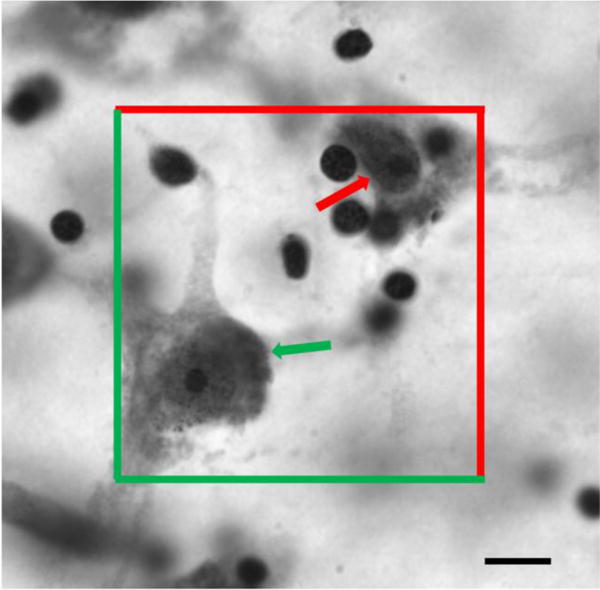
Photomicrograph of a Nissl-stained section illustrating the optical fractionator probe in the lateral nucleus of the amygdala at 100× from an individual with Williams syndrome. Neurons within the counting frame and not touching the red line of exclusion are counted (green arrow), while neurons outside the counting frame or touching the line of exclusion (red arrow) are not counted. Scale bar= 10 m.
Neuron soma area for each amygdaloid nucleus was determined by using the Nucleator probe during neuron counts. For every tenth neuron counted with optical fractionator, a grid of four radially extending lines, centered on the nucleolus, overlaid the cell body, and the point of intersection with the edge of the neuron body was marked on each line. Neuron soma area is defined as the cross-sectional area of the cell body. We opted to report soma area rather than volume given that three-dimensional measures of soma volume and cell shape are not optimal in the stereological literature (Schumann and Amaral 2006; Gittins and Harrison 2011) due to the fixed orientation of the tissue sections.
Statistical analyses
Statistical analyses were performed using Graphpad Prism statistical software (v7b; La Jolla, CA). Two-tailed student’s t-tests (P<0.05) were used to determine differences in volume, neuron number, neuron density, and neuron soma area in WS and TD. Correlation tests were run to identify any effects of age or sex. Given the age range of our sample, in the case of a result reaching or approaching significance, an analysis of covariance was performed to determine whether the finding was still significant with age as a covariate. Additionally, t-tests were calculated with the infant subjects excluded from the sample to determine that including or excluding the infants did not affect the significance of findings. All data was run through a Grubbs test (P<0.05) to identify any outliers.
RESULTS
Findings from comparisons between WS and TD groups in each nucleus (Figure 4) are discussed below. The Grubbs test revealed no significant outliers in our sample. Mean age at death was similar between WS and TD (Table 3). TD neuron number values found here were consistent with other TD neuron number values reported in the literature (Schumann and Amaral 2005, 2006). No effects of sex were observed in any of the parameters examined. Similar statistical significance findings were observed in all nuclei on all measures examined when omitting the infants from the sample, with the exception of neuron density in the lateral nucleus, which was approaching significance when calculated in the adult sample only (Table 4). There was no correlation between subject age at death and number of neurons in the lateral nucleus, accessory basal nucleus, or central nucleus in either WS or TD.
Figure 4.
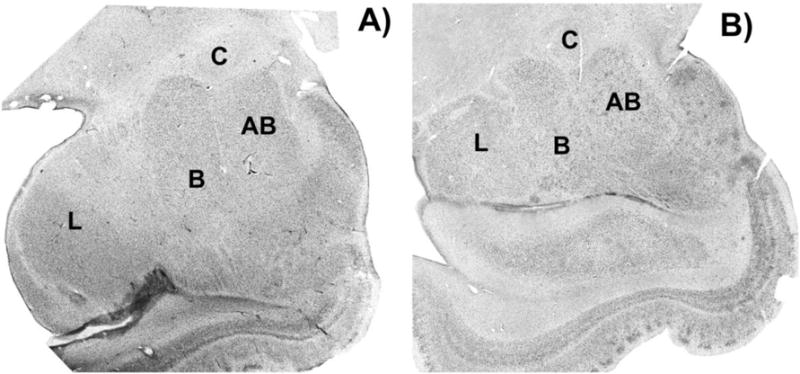
Brightfield photomicrograph of Nissl-stained coronal sections of the amygdala from a Williams Syndrome subject A), and a typically developing subject B) with all four amygdaloid nuclei present. L= lateral nucleus of the amygdala; B= basal nucleus of the amygdala; AB= accessory basal nucleus of the amygdala; C= central nucleus of the amygdala.
Table 3.
Mean stereological results in WS and TD
| WS, n= 7 | TD, n= 7 | |
|---|---|---|
| Age (years) | 28.13 ± 21.61 | 28.06 ± 21.21 |
| Nucleus Volume (mm3) | ||
| Lateral nucleus | 375.13 ± 128.30 | 362.00 ± 102.90 |
| Basal nucleus | 241.90 ± 93.71 | 266.30 ± 90.08 |
| Accessory basal nucleus | 102.00 ± 32.20 | 109.50 ± 30.96 |
| Central nucleus | 24.83 ± 8.32 | 25.12 ± 7.37 |
| Neuron Number (× 106) | ||
| Lateral nucleus** | 5.16 ± 0.31 | 4.39 ± 0.37 |
| Basal nucleus | 3.79 ± 0.43 | 3.56 ± 0.42 |
| Accessory basal nucleus | 1.44 ± 0.26 | 1.45 ± 0.23 |
| Central nucleus | 0.36 ± 0.03 | 0.35 ± 0.04 |
| Neuron density (neurons/cm3) | ||
| Lateral nucleus | 15.53 ± 6.38 | 13.144 ± 4.23 |
| Basal nucleus | 17.63 ± 6.15 | 14.88 ± 5.33 |
| Accessory basal nucleus | 15.24 ± 4.60 | 14.20 ± 4.26 |
| Central nucleus | 16.23 ± 7.08 | 15.42 ± 6.06 |
| Neuron Soma Area (m2) | ||
| Lateral nucleus | 214.00 ± 48.50 | 205.00 ± 61.40 |
| Basal nucleus | 223.30 ± 36.55 | 211.20 ± 34.86 |
| Accessory basal nucleus | 223.40 ± 52.58 | 226.70 ± 61.35 |
| Central nucleus | 183.10 ± 41.40 | 158.40 ± 47.50 |
indicates significant difference between WS and TD
Table 4.
Statistical results of student’s T-test comparing WS and TD:The first value listed represents the P-value of WS versus TD, all subjects included; the italicized value listed represents the P-value of WS versus TD adult subjects only, infant subjects excluded.
| Lateral nucleus | Basal nucleus | Accessory basal nucleus | Central nucleus | |
|---|---|---|---|---|
| Nucleus Volume | 0.8367 (0.4911) | 0.6280 (0.4026) | 0.6653 (0.4833) | 0.9461 (0.9385) |
| Neuron number | 0.0012**(0.0012**) | 0.3372 (0.2018) | 0.8248 (0.6707) | 0.9475 (0.8102) |
| Neuron density | 0.4254 (0.0952) | 0.3875 (0.2066) | 0.6707 (0.5636) | 0.8102 (0.9814) |
| Neuron soma area | 0.7831 (0.9994) | 0.5715 (0.6022) | 0.9223 (0.7716) | 0.3600 (0.4851) |
indicates significant difference between WS and TD.
Lateral Nucleus
There was a significant difference in neuron number in the lateral nucleus, with WS demonstrating significantly more neurons compared to TD (P-val= 0.0012; Table 4; Figure 3). This difference remained significant even when age was included as a covariate (P-val= 0.0017). Lateral nucleus volume and neuron density were slightly greater in WS compared to TD (Table 3), although this difference was not significant. Neuron soma area in the lateral nucleus was similar between the two groups (Table 3, 4).
Figure 3.
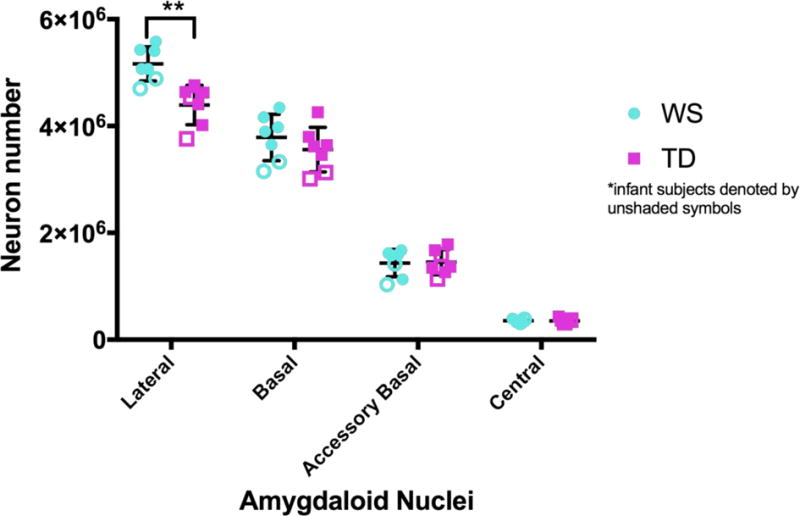
Number of neurons in the lateral, basal, accessory basal, and central nuclei of the amygdala in Williams syndrome (blue circle) and typically developing control (purple square) brains. Infant subjects are denoted by unshaded symbols. ** indicate significant difference (p-val= 0.0012) between Williams syndrome and TD controls in neuron number of the lateral nucleus.
Basal Nucleus
All four measures in the basal nucleus- volume, neuron number, neuron density, and neuron soma area -were slightly greater in WS compared to TD (Table 3; Figure 3), although this difference was not significant (Table 4). In both WS and TD, there was a slight correlation between subject age and number of neurons in the basal nucleus (r= 0.6276; p= 0.0163).
Accessory Basal Nucleus
Accessory basal nucleus volume was slightly smaller in WS compared to TD, while neuron number and neuron density were slightly higher in WS compared to TD (Table 3; Figure 3). None of these differences reached significance (Table 4). Neuron soma area was similar between the two groups (Table 3, 4).
Central Nucleus
Central nucleus volume, neuron number, and neuron density were similar in WS and TD (Table 3, 4; Figure 3). Neuron soma area was slightly larger in WS compared to TD, although this difference was not significant (Table 3, 4).
DISCUSSION
Our main finding was that the number of neurons is significantly increased in the lateral nucleus of the amygdala in WS compared to TD. No other measure demonstrated significant differences between the two groups. While the infant subjects were at the lower end of the range of values in some nuclei, the infant pairs followed adult trends of WS/TD differences, and excluding infants from the sample did not change the significance of the results. Furthermore, the increase in neuron number in the lateral nucleus in WS was still significant when age was included as a covariate. These findings demonstrate that the infant subjects are similar to the adult counterparts of their respective diagnostic group, and that processes leading to atypical development of the amygdala in WS likely occur during fetal development. Interestingly, we found a slight correlation between age and neuron number in the basal nucleus that was present in both WS and TD, with infant subjects containing fewer neurons. Our boundaries of the basal nucleus include the paralaminar region, which contains a large pool of neurons that are immature at birth (Chareyron et al. 2012). Cells with a diameter smaller than 5 m were not counted during data collection (see Methods), so it is likely that small, immature neurons were present but excluded from analysis in the infant basal nucleus.
While not significant, some trends differentiated the two groups. Comparing group means, the WS lateral nucleus was larger and had a greater density of neurons compared to TD. The WS basal nucleus was smaller, with greater neuron number and density of neurons compared to TD. The WS accessory basal nucleus was smaller and had a greater neuron density compared to TD, although neuron number was similar between the two groups. In contrast, the WS and TD group means for measures in the central nucleus were highly similar, suggesting that the central nucleus is relatively unaffected in WS.
Postmortem human studies, and particularly those examining rare neurological disorders, are limited by small sample sizes and this study is no exception. The group differences in the basal and accessory basal nuclei could potentially reach significance with a larger sample size. Furthermore, developmental differences may become apparent with a more complete age spectrum- one neuroimaging study employing multiple age cohorts in ASD found evidence of atypical brain growth in two different developmental phases in infancy (Courchesne et al. 2003), and another found evidence of atypical amygdala growth in childhood that was not detectable in adolescence or adulthood in ASD (Schumann et al 2004). Future postmortem studies are needed to examine neuronal and glial subtype populations, as well as neuronal dendritic branching, in order to further identify specific microstructural characteristics disrupted in individuals with an altered social phenotype.
A similarly designed study examined the amygdala in nine postmortem ASD and ten TD brains in a developmental sample (ages 10 years to 44 years) and found that neuron number and density are significantly decreased in the lateral nucleus in ASD, while all other measures examined are similar between the two groups (Schumann and Amaral 2006). Postmortem studies of the amygdala in schizophrenia found that the lateral nucleus demonstrates significant microstructural changes in that disorder as well, including a reduction in volume and a decrease in neuron number (Kreczmanski et al. 2007; Beretta et al. 2007). Neuroimaging studies have long demonstrated that alterations of the amygdala are a common occurrence in neurodevelopmental and neuropsychiatric disorders (Schumann, Bauman, and Amaral 2011), and it is possible that microstructural changes to the lateral nucleus underlie these findings.
The lateral nucleus is the primary recipient of multimodal sensory cortical input in the amygdala (Stefanacci and Amaral 2000, 2002). It is thought to be involved in the categorization of emotional saliency of sensory stimuli, as well as integration of this information with information about social context sent from orbitofrontal cortical projections in the basal and accessory basal nuclei (LeDoux et al. 1990; Barbas 1995; Stefanacci and Amaral 2002). Reduced activation of the amygdala in response to social stimuli has been observed in WS (Munoz et al. 2009), and increased activation of the amygdala in response to social stimuli has been observed in ASD (Aschwin et al. 2007). Behavioral studies suggest that both WS and ASD individuals struggle with determining saliency of conspecific stimuli in social interactions (Vivanti et al. 2017). Atypical function of the lateral nucleus as a result of altered microstructural development may contribute to the associated cognitive and behavioral deficits observed in these disorders.
Our results yield two major questions that deserve examination in future studies: 1) What mechanisms underlie the increase in neuron number in the lateral nucleus of the amygdala in WS, and 2) What events in fetal development result in the individual amygdaloid nuclei to be differentially affected in WS? Regarding the first question, there are two possibilities that would result in an increase in neuron number in the lateral nucleus of the WS amygdala- either an atypical initial over-proliferation of neurons, or a later disruption of the programmed cell death critical to typical neural development, resulting in a greater number of neurons. Future postmortem studies examining neuronal markers for cell proliferation and apoptosis are critical, as no data currently exists that would enable us to support or reject either possibility. A study examining WS neural progenitor cells found higher rates of apoptosis in WS cortex compared to TD (Chailangkarn et al. 2016), which would suggest that an over-proliferation of neurons in the lateral nucleus is a more likely scenario.
The developmental processes in WS underlying the increase of neurons in the lateral nucleus, yet relative preservation of typical neuron number in the central nucleus, are also unclear. Our findings show that the early postnatal infant WS amygdala already demonstrates the same pathological changes to the lateral nucleus seen in the mature WS adult amygdala, indicating a prenatal origin. Distinct amygdaloid nuclei are first present in the human fetus at gestational week six (Muller and O’Rhahilly 2006), and are under differential timing in fetal development (Kordower, Piecinski, and Rakic 1992; Nikolic and Kostovic 1986; Muller and O’Rhahilly 2006). However, neurogenesis does not appear to respect neuroanatomical subdivisions in the amygdala, but rather occurs in a smooth medial to lateral gradient, such that neurogenesis in the central nucleus begins earlier and is completed first, and neurogenesis in the lateral nucleus begins later and is completed last (Kordower, Piecinski, and Rakic 1992). Interestingly, the differences in mean neuron number in WS compared to TD reported here reflect this lateral to medial gradient: differences are greatest in the lateral nucleus, followed by the basal nucleus, then the accessory basal nucleus, and finally by the central nucleus, where values are most similar between the two groups. Therefore, it is possible that these differences are related to a dysfunction of developmental events along this gradient. A few possible genetic candidates in the WS deletion have been identified that may underlie our findings, such as transcription factor WBSCR14, which regulates tissue-specific gene expression controlling neurogenesis (de Luis, Valero, and Jurardo 2000; Meng et al. 1998), Gtf2i, involved in the regulation of several genes that are critical to embryonic neural development (Sakurai et al. 2011), FZD9, involved in timing of cell division and apoptosis (Chailangkarn et al. 2016), and PSD-95, demonstrated to play a role in differential cellular morphology in the basolateral nuclei, but not the central nucleus of the amygdala in PSD-95 mouse knockouts (Feyder et al. 2010).
This is the first postmortem study to provide quantitative evidence that the cellular microstructure of the amygdala is altered in WS. The current study follows our previous histological investigation of the cortex in WS, which found a decrease in neuron density in the infragranular layers of the OFC in WS (Lew et al. 2017), a region that shares significant connectivity with the basolateral amygdala (Barbas and Pandya 1989). Both studies found that the neuroanatomical regions affected in WS are associated with functional specificity in the socio-behavioral domain, while adjacent regions not implicated in social function remain relatively preserved, supporting the hypothesis of an impaired social brain network in WS. The present finding that the lateral nucleus of the amygdala is specifically affected in WS is a feature shared with other neurological disorders that include alterations in social behavior, such as ASD and schizophrenia (Schumann, Bauman, and Amaral 2011; Beretta et al. 2007; Rubinow et al. 2016). It is of interest that the basolateral amygdala has undergone significant reorganization in human evolution, with changes in size and neuron number that indicate an increasing emphasis on the lateral nucleus in humans compared to nonhuman apes (Barger et al. 2007, 2012). Together, these findings may indicate a possible link between recent evolutionary change and susceptibility to dysfunction, and warrants further investigation.
This study is the first step towards defining the neural architecture of the amygdala in light of a disorder characterized by a distinct genetic and behavioral profile. Such studies can contribute to future research examining the cellular composition of the brain and its relationship to the genotype and behavior, an essential step in identifying targets of future therapeutics in disorders of social dysfunction.
Acknowledgments
This research was supported by the National Institutes of Health P01 NICHD033113, 5R03MH103697and R56MH109587. We wish to thank the tissue donors and their families whose gift to science made this study possible, and especially Terry Monkaba and the Williams Syndrome Association. WS human tissue was obtained under the Ursula Bellugi WS Brain Collection, curated by KS at UC San Diego. Typically developing human tissue was obtained from the University of Maryland Brain and Tissue Bank, which is a Brain and Tissue Repository of NIH NeuroBioBank. We thank Chelsea Brown, Valerie Judd, Hailee Orfant and Deion Cuevas for tissue processing assistance, and Kari Hanson, Branka Hrvoj, and Linnea Wilder for feedback.
Footnotes
ETHICAL STATEMENT: The authors declare they have no conflict of interest. This article does not contain any studies with human participants or authors performed by any of the authors. For this type of study formal consent is not required.
References
- Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, Anderson A, Lee GP, Damasio AR. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O’Riordan M, Bullmore ET. Differential activation of the amygdala and the “social brain” during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barger N, Stefanacci L, Semendeferi K. A Comparative Volumetric Analysis of the Amygdaloid Complex and Basolateral Division in the Human and Ape Brain. Am J Phys Anthropol. 2007;403:392–403. doi: 10.1002/ajpa.20684. [DOI] [PubMed] [Google Scholar]
- Barger N, Stefanacci L, Schumann CM, Sherwood CC, Annese J, Allman JM, Buckwalter JA, Hof PR, Semendeferi K. Neuronal populations in the basolateral nuclei of the amygdala are differentially increased in humans compared with apes: a stereological study. J Comp Neurol. 2012;520:3035–3054. doi: 10.1002/cne.23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason Wa, Lavenex P, Amaral DG. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Lange N. Neuron Numbers and Volume of the Amygdala in Subjects Diagnosed with Bipolar Disorder or Schizophrenia. Biol Psychiatry. 2007;62:884–893. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chailangkarn T, et al. A human neurodevelopmental model for Williams syndrome. Nature. 2016;536(7616):338–343. doi: 10.1038/nature19067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Amaral DG, Lavenex P. Postnatal development of the amygdala: A stereological study in macaque monkeys. J Comp Neurol. 2012;520:1965–1984. doi: 10.1002/cne.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- de Luis O, Valero MC, Pérez Jurado LA. WBSCR14, a putative transcription factor gene deleted in Williams-Beuren syndrome: complete characterisation of the human gene and the mouse ortholog. Eur J Hum Genet. 2000;8:215–222. doi: 10.1038/sj.ejhg.5200435. [DOI] [PubMed] [Google Scholar]
- Edelmann L, Prosnitz A, Pardo S, Bhatt J, Cohen N, Lauriat T, Ouchanov L, Jimenez Gonzalez P, Manghi ER, Bondy P, Esquivel M, Monge S, Fallas M, Splendore A, Francke U, Burton BK, McInnes LA. An atypical deletion of the Williams syndrome intervalimplicates genes associated with defective visuospatial processing and autism. J Med Genet. 2007;44:136–143. doi: 10.1136/jmg.2006.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason Wa, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:515–544. [PubMed] [Google Scholar]
- Feyder M, et al. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams’ syndrome. Am J Psychiatry. 2010;167:1508–1517. doi: 10.1176/appi.ajp.2010.10040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, Bellugi U. V. Multi-level analysis of cortical neuroanatomy in Williams syndrome. J Cogn Neurosci. 2000;12:74–88. doi: 10.1162/089892900561995. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Holland PC. The amygdala complex: multiple roles in associative learning and attention. Proc Natl Acad Sci U S A. 1994;91:11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Fiñana M, Cruz-Orive LM, Mackay CE, Pakkenberg B, Roberts N. Comparison of MR imaging against physical sectioning to estimate the volume of human cerebral compartments. Neuroimage. 2003;18:505–516. doi: 10.1016/s1053-8119(02)00021-6. [DOI] [PubMed] [Google Scholar]
- Gittins RA, Harrison PJ. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J Affect Disord. 2011;133:328–332. doi: 10.1016/j.jad.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Goossens L, Kukolja J, Onur OA, Fink GR, Maier W, Griez E, Schruers K, Hurlemann R. Selective processing of social stimuli in the superficial amygdala. Hum Brain Mapp. 2009;30:3332–3338. doi: 10.1002/hbm.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson HJG, Jensen EB. The efficiency of systematic of systematic smapling in stereology and its prediction. Microscopy. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Haas BW, Hoeft F, Searcy YM, Mills D, Bellugi U, Reiss A. Individual differences in social behavior predict amygdala response to fearful facial expressions in Williams syndrome. Neuropsychologia. 2009;48:1283–1288. doi: 10.1016/j.neuropsychologia.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Hoeft F, Searcy YM, Mills D, Bellugi U, Reiss A. Individual differences in social behavior predict amygdala response to fearful facial expressions in Williams syndrome. Neuropsychologia. 2010;48:1283–1288. doi: 10.1016/j.neuropsychologia.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Sheau K, Kelley RG, Thompson PM, Reiss AL. Regionally specific increased volume of the amygdala in Williams syndrome: evidence from surface-based modeling. Hum Brain Mapp. 2014;35:866–874. doi: 10.1002/hbm.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrvoj-Mihic B, Hanson KL, Lew CH, Stefanacci L, Jacobs B, Bellugi U, Semendeferi K. Basal dendritic morphology of cortical pyramidal neurons in Williams syndrome: prefrontal cortex and beyond. Frontiers in Neuroscience. 2017;11:1–13. doi: 10.3389/fnins.2017.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen A, Korenberg JR, Bellugi U. The social phenotype of Williams syndrome. Curr Opin Neurobiol. 2013;23:414–422. doi: 10.1016/j.conb.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Adolphs R, Yam A, Hill KJ, Grichanik M, Reilly J, Mills D, Reiss AL, Korenberg JR, Bellugi U. Affiliative behavior in Williams syndrome: social perception and real-life social behavior. Neuropsychologia. 2010;48:2110–2119. doi: 10.1016/j.neuropsychologia.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak S, Brimble E, Zhang W, Zaslavsky K, Strong E, Ross PJ, Hendry J, Mital S, Salter MW, Osborne LR, Ellis J. Human induced pluripotent stem cell derived neurons as a model for Williams-Beuren syndrome. Mol Brain. 2015;8:77–81. doi: 10.1186/s13041-015-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Piecinski P, Rakic P. Neurogenesis of the amygdaloid nuclear complex in the Rhesus monkey. Dev Brain Res. 1992;68:9–15. doi: 10.1016/0165-3806(92)90242-o. [DOI] [PubMed] [Google Scholar]
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, Korr H, Steinbusch HWM, Hof PR, Schmitz C. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain. 2007;130:678–692. doi: 10.1093/brain/awl386. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichnetz GR, Astruc J. The efferent projections of the medial prefrontal cortex in the squirrel monkey (Saimiri sciureus) Brain Res. 1976;109:455–472. doi: 10.1016/0006-8993(76)90027-5. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Astruc J. The course of some prefrontal corticofugals to the pallidum, substantia innominata, and amygdaloid complex in monkeys. Exp Neurol. 1977;54:104–109. doi: 10.1016/0014-4886(77)90238-2. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Povlishock JT, Astruc J. A prefronto-amygdaloid projection in the monkey: Light and electron microscopic evidence. Neurosci Lett. 1976;2:261–265. doi: 10.1016/0304-3940(76)90157-9. [DOI] [PubMed] [Google Scholar]
- Lew CH, Brown C, Bellugi U, Semendeferi K. Neuron density is decreased in the prefrontal cortex in Williams syndrome. Autism Res. 2017;10:99–112. doi: 10.1002/aur.1677. [DOI] [PubMed] [Google Scholar]
- Martens Ma, Wilson SJ, Dudgeon P, Reutens DC. Approachability and the amygdala: Insights from Williams syndrome. Neuropsychologia. 2009;47:2446–2453. doi: 10.1016/j.neuropsychologia.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Meda SA, Pryweller JR, Thornton-Wells TA. Regional brain differences in cortical thickness, surface area and subcortical volume in individuals with williams syndrome. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Lu X, Li Z, Green ED, Massa H, Trask BJ, Morris CA, Keating MT. Complete physical map of the common deletion region in Williams syndrome and identification and characterization of three novel genes. Hum Genet. 1998;103:590–599. doi: 10.1007/s004390050874. [DOI] [PubMed] [Google Scholar]
- Merla G, Brunetti-Pierri N, Micale L, Fusco C. Copy number variants at Williams-Beuren syndrome 7q11.23 region. Hum Genet. 2010;128:3–26. doi: 10.1007/s00439-010-0827-2. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray Ea, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris Ca, Berman KF. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Mosconi M. Longitudinal study of amygdala volume and joint attention in 2-to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. The amygdaloid complex and the medial and lateral ventricular eminences in staged human embryos. J Anat. 2006;208:547–564. doi: 10.1111/j.1469-7580.2006.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz KE, Meyer-Lindenberg A, Hariri AR, Mervis CB, Mattay VS, Morris Ca, Berman KF. Abnormalities in neural processing of emotional stimuli in Williams syndrome vary according to social vs. non-social content. Neuroimage. 2010;50:340–346. doi: 10.1016/j.neuroimage.2009.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Nikolić I, Kostović I. Development of the lateral amygdaloid nucleus in the human fetus: transient presence of discrete cytoarchitectonic units. Anat Embryol (Berl) 1986;174:355–360. doi: 10.1007/BF00698785. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Mahajan G, May W, Overholser JC, Jurjus GJ, Dieter L, Herbst N, Steffens DC, Miguel-Hidalgo JJ, Rajkowska G, Stockmeier CA. Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain Structure and Function. 2016;221(1):171–184. doi: 10.1007/s00429-014-0900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Eckert Ma, Rose FE, Karchemskiy A, Kesler S, Chang M, Reynolds MF, Kwon H, Galaburda A. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci. 2004;24:5009–5015. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Dorr NP, Takahashi N, McInnes LA, Elder GA, Buxbaum JD. Haploinsufficiency of Gtf2i, a gene deleted in Williams Syndrome, leads to increases in social interactions. Autism Res. 2011;4:28–39. doi: 10.1002/aur.169. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological estimation of the number of neurons in the human amygdaloid complex. J Comp Neurol. 2005;491:320–329. doi: 10.1002/cne.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, Decision Making, and the Amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Paljarvi L, Karkola K, Pitkanen A. Distribution of parvalbumin-immunoreactive cells and fibers in the human amygdaloid complex. J Comp Neurol. 1995;360:185–212. doi: 10.1002/cne.903600202. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: A retrograde tracing study. J Comp Neurol. 2000;421:52–79. doi: 10.1002/(sici)1096-9861(20000522)421:1<52::aid-cne4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: An anterograde tracing study. J Comp Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Strømme P, Bjørnstad PG, Ramstad K. Prevalence Estimation of Williams Syndrome. J Child Neurol. 2002;17:269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- Vivanti G, Hocking DR, Fanning P, Dissanayake C. The social nature of overimitation: Insights from Autism and Williams syndrome. Cognition. 2017;161:10–18. doi: 10.1016/j.cognition.2017.01.008. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]


