Abstract
Influenza B virus (FLUBV) is an important pathogen that infects humans and causes seasonal influenza epidemics. To date, little is known about defective genomes of FLUBV and their roles in viral replication. In this study, by using a next-generation sequencing approach, we analyzed total mRNAs extracted from A549 cells infected with B/Brisbane/60/2008 virus (Victoria lineage), and identified four defective FLUBV genomes with two (PB1∆A and PB1∆B) from the polymerase basic subunit 1 (PB1) segment and the other two (M∆A and M∆B) from the matrix (M) protein-encoding segment. These defective genomes contained significant deletions in the central regions with each having the potential for encoding a novel polypeptide. Significantly, each of the discovered defective RNAs can potently inhibit the replication of B/Yamanashi/166/98 (Yamagata lineage). Furthermore, PB1∆A was able to interfere modestly with influenza A virus (FLUAV) replication. In summary, our study provides important initial insights into FLUBV defective-interfering genomes, which can be further explored to achieve better understanding of the replication, pathogenesis and evolution of FLUBV.
Keywords: defective RNA genomes, interfering, Influenza B virus
Introduction
Influenza viruses are classified as types A, B and C according to their distinct antigenic properties residing in two major structural proteins (matrix 1 and nucleocapsid) [1]. Recently, a new type of influenza virus with cattle as a primary reservoir, designated influenza type D, has been described [2, 3]. Influenza B virus (FLUBV) is a clinically important pathogen. It has been well-established that FLUBVs infect humans, and cause seasonal influenza epidemics along with some influenza A virus (FLUAV) strains that belong to H3N2 and H1N1 subtypes [4–7]. Similar to FLUAV, FLUBV infection can result in a spectrum of clinical diseases in humans, ranging from mild to severe respiratory illness requiring hospitalization and medical treatment [7, 8]. FLUBV-associated mortality has been frequently observed in seasonal influenza epidemics particularly in children and adolescents [9, 10]. The burden of respiratory diseases caused by FLUBV, coupled with the increasing strong links between FLUBV and fatal infections in humans [11, 12], calls for an urgent need for more basic and clinical investigations of FLUBV.
Humans are thought to be the primary host and reservoir of FLUBV, although sporadic infection episodes of FLUBVs have been described in seals and pigs [13, 14]. In contrast, FLUAV circulates in various mammals including humans, swine, horses and migratory or domestic waterfowl [1]. FLUBV evolves at an estimated rate of 2×10−3 nucleotide substitutions per site per year for both haemagglutinin (HA) and neuraminidase (NA) segments [15–17]. This evolutionary rate is approximately two–three times slower than the one observed in FLUAVs. Despite the relatively slower evolutionary rate, new antigenic variants of FLUBV emerge frequently, largely driven by genetic reassortments of co-circulating FLUBV strains [6, 18, 19]. FLUBV has evolved into two antigenically and genetically distinct lineages, Yamagata and Victoria [20, 21]. The antigenic diversity of FLUBVs has added barriers for successful influenza vaccination.
Similar to FLUAV, FLUBV contains eight negative-sense, single-stranded RNA segments. Despite similarities in the coding strategy and viral protein expression, there are some notable differences between these two types. The most striking difference lies in the NA segment [1]. In FLUAV this segment is monocistronic, only encoding the NA protein, while the NA segment of FLUBV is bicistronic, expressing two integral membrane proteins, NA and NB. The ORF of NB protein starts four nucleotides before the initiating AUG codon that directs the NA protein synthesis. Another difference is in the polymerase basic 1 (PB1) segment. In FLUBV, the PB1 segment only encodes the PB1 protein, whereas in some strains of FLUAV, it codes for PB1 and a small accessory protein PB1-F2 [22]. Recent studies have also provided evidence that FLUAV’s PB1 has the capacity to synthesize PB1-N40 by using alternative translation initiation strategy [23]. Another major difference between FLUAV and FLUBV occurs in the matrix (M) segment with regard to the expression of M1 and M2 proteins [1]. The M2 protein of FLUAV is translated from a spliced mRNA originating from the primary M segment-derived RNA transcript [24], while the expression of FLUBV M2 protein is produced from the primary RNA transcript of M segment through a coupled termination and reinitiation mechanism, also called translational stop-start, around the sequence-encoding M1 stop codon via a UAAUG pentanucleotide motif [25]. Moreover, in marked contrast to the nonspliced FLUBV M segment, the FLUAV M segment can undergo three different splicing events [24] and more than 90 % of M segment-derived mRNAs are spliced transcripts [26]. These spliced transcripts in the M and NS segments of FLUAV were recently speculated to have important roles in viral replication, host range and pathogenesis [27].
In addition to spliced transcripts, the replication and transcription of FLUAV segments give rise to more than 50 different defective interfering (DI) RNAs [28–32]. A DI RNA is a smaller (ranging from 200 to 700 nucleotides in size) viral RNA (vRNA) with similar or identical terminal sequences to its parental segment and a large internal deletion. The competition of DI RNAs with the parental full-length segments in replication, transcription and genome packaging results in a segment-specific inhibition of FLUAV replication [33]. The underlying mechanism for DI RNA formation has not been fully understood yet. However, there is evidence indicating that a vRNA polymerase slippage-based faulty replication process is a likely mechanism [34]. Despite the observations that DI genomes can originate from any segment, the general consensus is that the three polymerase segments of FLUAV are the primary donors of DI RNAs [34, 35]. DI vRNAs can be packaged into budding virions from the surface of infected cells in a way to out compete their parental segments, leading to the formation of DI particles. DI particles containing incomplete genomes are unable to replicate, unless the missing viral protein(s) is supplied by co-infection with replication-competent viruses. In addition to directly interfering with FLUAV replication, some DI genomes are potent inducers of the innate immune response [36–39]. Recent emerging data consistently indicate that the FLUAV-derived DI genomes can protect Madin-Darby canine kidney (MDCK) cells or mice from infections of FLUAV, FLUBV and other respiratory viral pathogens such as respiratory syncytial virus [35, 40–42]. Furthermore, FLUAV DI particles were identified in the respiratory tract of infected chickens and humans [43–45], suggesting a potential role in viral transmission and pathogenesis.
Despite significant progress on the characterization of the DI RNAs in FLUAV and harnessing them for broad-spectrum antivirals, and early descriptions of DI phenomena in FLUBV [46, 47], little is known about the molecular nature of FLUBV DI RNAs and their roles in viral replication. The investigation of FLUBV DI particles will likely improve our understanding of FLUBV pathogenesis and host immune response. A high-resolution analysis of dynamic changes in viral gene transcription following FLUBV infection has not been reported. In this study, we investigated FLUBV gene replication dynamics in FLUBV-infected human lung epithelial A549 cells using a next-generation sequencing (NGS) approach. Specifically, we identified four defective RNAs. Among them, two defective RNAs (PB1∆A and PB1∆B) were derived from the PB1 segment. The other two (M∆A and M∆B) were derived from the M segment. Interestingly, the majority of the defective transcripts were from the M segment with M∆A being the most abundant, which is substantially different from FLUAVs in which most DI RNAs are generated from the three polymerase segments. Furthermore, we showed that transcription of these four defective vRNAs strongly inhibited FLUBV replication. To our surprise, only PB1∆A was able to interfere with FLUAV replication in a moderate manner. The findings provide new insights into influenza DI genomes and further characterization of these vRNAs may help us to better understand the biology and evolution of FLUBV.
Results
Analysis of viral gene transcription by NGS
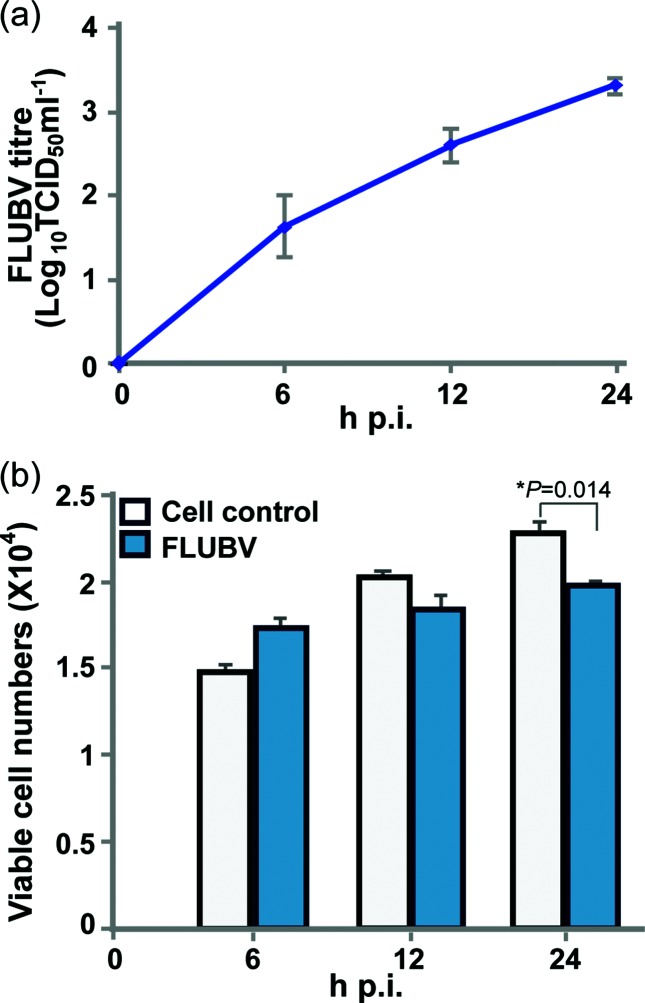
To investigate FLUBV transcription profile, we infected human lung epithelial A549 cells in duplicate with B/Brisbane/60/2008 (Victoria lineage) at a m.o.i. of 1.0. FLUBV growth kinetics in A549 cells showed that the viral replication was detected at 6 h post infection (h p.i.) at an appreciable level (101.5 TCID50 ml−1) and increased in a time-dependent manner over a 24 h period (Fig. 1a). At 24 h p.i., approximately 98 % of cells expressed FLUBV-specific antigens as determined by the FACS-based assay (data not shown), suggesting nearly complete infection was achieved. FLUBV-infected cells and uninfected controls exhibited similar cell proliferation profiles at 6 and 12 h p.i., but a statistically significant difference was observed at 24 h p.i. (Fig. 1b), indicating virus replication exerted a time-dependent effect on cell proliferation.
Fig. 1.
Viral replication kinetics and proliferation dynamics of FLUBV-infected A549 cells. (a) Viral titre in A549 cells infected with B/Brisbane/60/2008 virus. Supernatant samples of A549 cells infected by 1.0 m.o.i. of FLUBV were collected at 0, 6, 12 and 24 h p.i. The TCID50 of each sample was evaluated in MDCK cells. Mean TCID50 (+sd) from three independent experients in duplicate are shown. (b) A549 cell growth in the first 24 h of infection. MTS assay in 96-well plate format was performed at 0, 6, 12 and 24 h p.i. The bars represent mean cell numbers (+sd) from three independent experiments in triplicate. '*' indicates a significant difference using Student’s t-test (P<0.05).
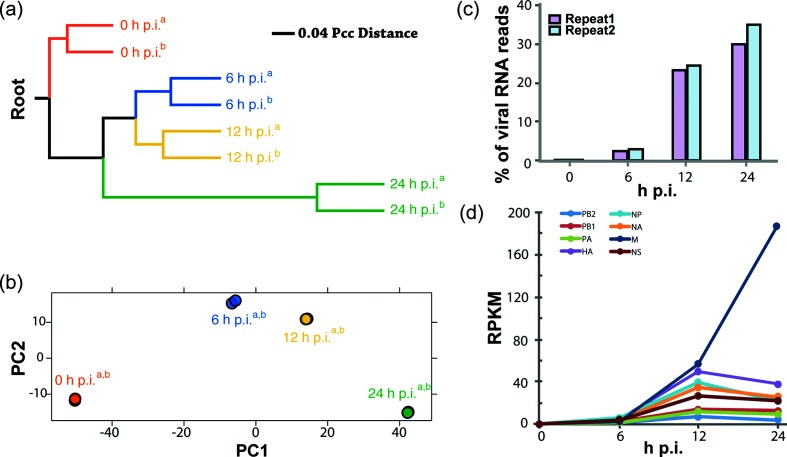
Total mRNAs were isolated from infected cells at 0, 6, 12 and 24 h p.i. and the corresponding cDNA libraries were sequenced using Illumina-based NGS technology. The NGS reads were then aligned to the eight-segment FLUBV genome as well as the human genome. To measure similarities of gene expression profiles at all time points, we calculated Pearson correlation coefficient (Pcc) distance for all pairs of samples and constructed an unweighted pair group method with arithmetic mean (UPGMA) dendrogram (Fig. 2a). The dendrogram showed that the replicate samples of all time points from two independent experiments clustered together first, suggesting the variations of expression profiles between repeats are lower than those displayed between time points. The Pcc distance of gene expression profiles between mock (0 h sample) and infected samples increased over time, suggesting cellular response to FLUBV infection was significantly changed. In addition, principal component (PC) analysis was used to further calculate the variations between gene expression profiles (Fig. 2b). The analysis showed that there was little variation between repeats of the same time point, indicating a high reproducibility of the experiments. The gene expression profiles of 6, 12 and 24 h p.i. showed gradual divergence from that of 0 h cell samples along principal component 1 (PC1). The 6 and 12 h p.i. profiles were also different from 0 h p.i. along PC2 while the profiles of 24 h p.i. had PC2 scores similar to 0 h p.i., implying the expression of some genes was mostly altered at 6 and 12 h p.i. and then gradually returned to basal levels at 24 h p.i.
Fig. 2.
Analysis of viral gene transcription by NGS. (a) Hierarchical clustering of expression profiles. The UPGMA tree was rerooted using the RNA-seq read from the two control samples as the root. The divergence of expression profiles at 0, 6, 12 and 24 h p.i. is shown in the UPGMA dendrogram. Two independent experiments (indicated with superscripts 'a' and 'b') at each time point are shown separately. (b) PC analysis of host gene expression profile divergence. FLUBV-induced expression differences are shown using the first two components (PC1 and PC2) of PC analysis. The two independent experiments at each time point are partially (6 h p.i.) or almost completely (0, 12 and 24 h p.i.) overlapping with each other for both PC1 and PC2. Two repeats (indicated with superscripts 'a' and 'b') are shown separately. (c) Percentage of viral reads in the NGS samples. Genome-wide total gene expression was quantified using RNA-seq. Two total RNA samples at each time point were extracted from A549 cells infected with 1.0 m.o.i. of FLUBV. Percentage of viral genome reads was shown, which represents the ratio of the FLUBV genome reads to the total reads of the genomes of Homo sapiens and FLUBV. (d) Expression level changes of the eight FLUBV segments over time. The expression level was displayed using reads per kilobases per million reads(RPKM).
For each sample, we obtained on average over 6 million 100-nucleotide (nt) reads mapping to either the FLUBV or human genome. At 6 h p.i., ~2.5 % of the total reads mapped to the viral genome, while at 12 h p.i., this proportion increased to ~24 %. At 24 h p.i., ~30 % of the total mapped reads were derived from FLUBV (Fig. 2c). The observed increase in viral reads over time correlated well with a step-wise increase in viral titres as shown in the viral replication kinetics experiment (Fig. 1a). During infection, M segment-derived viral transcripts were predominant as measured by reads per kilobase of transcript per million mapped reads (RPKM) at 12 and 24 h p.i. (Fig. 2d), followed by those of HA, NP, NA and NS segments. The viral transcripts of the polymerase segments (PA, PB1 and PB2) were detected with the lowest abundance. Intriguingly, the M segment transcripts continued to increase to 24 h p.i., which was a marked contrast to viral transcripts of all the other seven segments. For the latter, the numbers of transcripts either reached a plateau at 12 h p.i. for PA, PB1, PB2 and NS segments; or slightly decreased for HA, NP and NA segments at 24 h p.i.
Identification of defective RNAs from M and PB1 segments
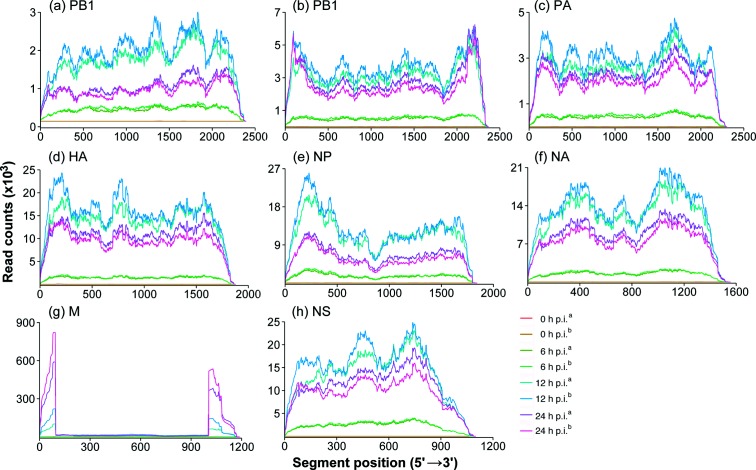
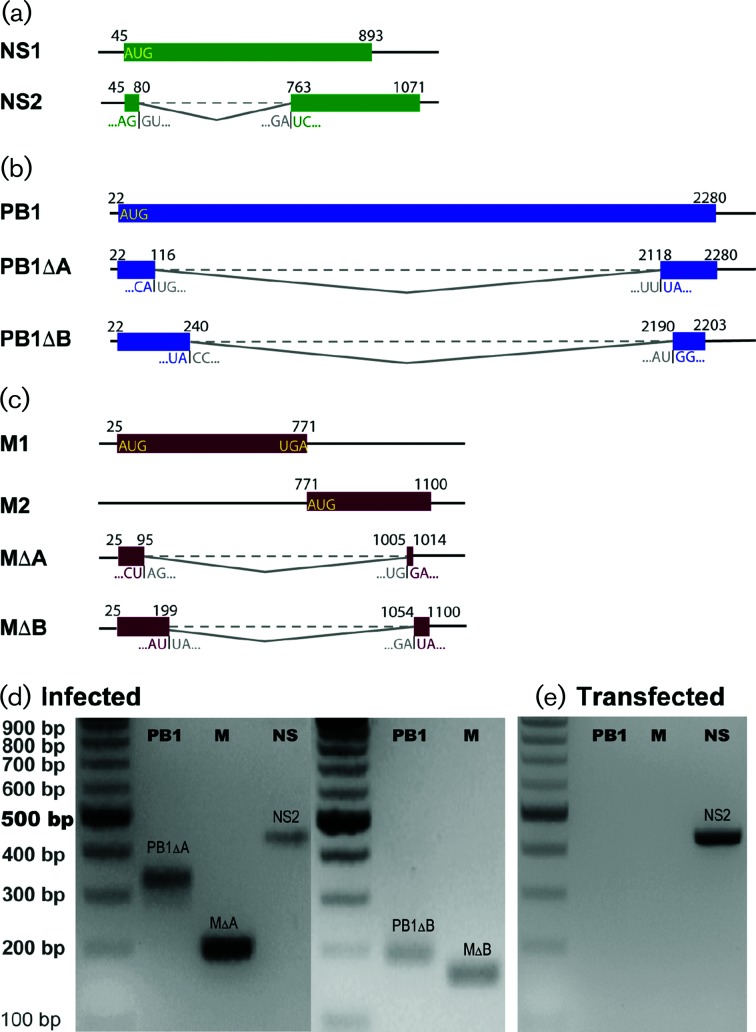
Closer examination of the position-dependent read coverage across the FLUBV genome showed that the M segment and, to a lesser extent, the PB1 segment had a large proportion of reads mapping to the 5′ and 3′ ends of segments, with a significant reduction in the number of reads mapping to the central regions (Fig. 3). The overrepresentation of sequencing reads in the 5′ and 3′ ends of the segments suggested the presence of internal deletions in defective transcripts of these genes. This observation prompted us to reveal the junction sites and to count the junction reads of the defective transcripts over the course of infection toward better understanding of the origin of them. To remove sequencing noises, we only focused on junction sites that were supported by more than 200 reads. Analysis of NS junction reads was included in this study as a control because the NS segment is known to utilize a splicing mechanism to produce NS2 mRNA. With sequencing coverage of more than 1000 reads, we observed a junction site between genomic positions 80 and 763 (nts 45–80 joined to 763–1071), matching the previously reported splicing donor and acceptor sites for the NS2 transcript [48] (Fig. 4a). This splicing event utilized a consensus dinucleotide motif GT/AG: GT present at the extreme 5′ (donor) and AG at the extreme 3′ (acceptor) ends of the intron. In addition, we identified two junction sites in the PB1 segment that joined genomic positions 116 with 2118, and 240 with 2190, respectively, which resulted in the production of two previously uncharacterized defective transcripts, PB1∆A and PB1∆B (Fig. 4b). Moreover, two junction sites in the M segment that joined genomic positions 95 with 1005, and 199 with 1054, respectively, were observed (Fig. 4c). Further analysis indicated that none of these junction sites conformed to the canonical splicing donor and acceptor dinucleotide motif GT/AG that are present in the splicing sites of human and influenza viral genes [49]. Follow-up reverse transcription (RT)-PCR) and Sanger sequencing experiments confirmed the sequences of NS2, PB1∆A, PB1∆B, M∆A and M∆B mRNAs in virus-infected A549 cells (Fig. 4d). All the primers used for the RT-PCRs are shown in detail in Table S1 (available in the online version of this article). Specifically, we amplified PB1∆A and M∆A (Fig. 4b, c). RT-PCRs specific to PB1∆B and M∆B successfully amplified PB1∆B and M∆B (Fig. 4d). The RT-PCR products were then sequenced and compared to the defective RNA sequences from NGS analysis. We have only noted one discordance in the junction site positions. In RNA-Seq, the junction site position for PB1∆B was 240/2190, while in RT-PCR and Sanger sequencing, the junction site position was 240/2189. The latter was chosen for GenBank sequence submission and the subsequent cloning of PB1∆B plasmids.
Fig. 3.
Positional sequencing depth of FLUBV gene segments over time. (a–h)- Positional sequencing depth for segments PB2, PB1, PA, HA, NP, NA, M and NS, respectively. The sequencing depth for each segment is shown as RNA-seq read counts at each position (5′→3′). Two replicates (indicated with superscript ‘a’ and ‘b’) from each time point (0, 6, 12 and 24 h p.i.) are shown separately.
Fig. 4.
Alternative splicing or junction sites and defective RNA genomes. (a) NS segment splicing. Diagram showing the known splicing site in the FLUBV NS segment that generates NS2 mRNA. (b) PB1 segment-derived defective RNAs. Schematic diagram shows the two newly identified defective RNAs from the PB1 segment termed PB1∆A and PB1∆B. (c) M segment-derived defective RNAs. Schematic diagram displays the two newly identified defective RNAs in the M segment termed M∆A and M∆B. The initiation, junction and termination positions (5′→3′) of an mRNA’s ORF are labelled with position numbers above. An mRNA’s two junction sequences are shown under ORFs. (a) Detection of PB1∆A, M∆A, PB1∆B, M∆B and NS2 RNAs in FLUBV-infected A549 cells. Segment-specific primers were used to amplify full-length PB1∆A, M∆A and NS2 by RT-PCR. Junction-specific primers (forward) and segmental primers (reverse) were used to amplify partial PB1∆B and M∆B transcripts. The cDNA was generated from total mRNA extracted from FLUBV-infected A549 cells. (e) Absence of PB1∆A and M∆A RNAs in HEK293T cells transfected with plasmid DNA encoding FLUBV PB1 and M segments. Segment-specific primers were used to amplify PB1∆A and M∆A by RT-PCR. NS2-specific RT-PCR was performed as a splicing control. The cDNA was generated from total mRNA extracted from transfected HEK293T cells with FLUBV PB1, M or NS segments.
Based on the fact that none of the detected junction sites for the four defective transcripts is consistent with the GT/AG splicing motif, we speculated that these small transcripts were not derived from mRNA splicing, instead, they are likely to be derived from defective vRNAs that are vRNA polymerase-dependent products. To test the hypothesis, we transfected each of the three segments (PB1, M and NS) driven by CMV promoter into HEK293T cells and extracted the total RNA. We then used RT-PCR to detect PB1∆A and M∆A mRNAs. NS2 was detected in both infected A549 cells and transfected HEK293T cells (Fig. 4d, e). In marked contrast, both PB1∆A and M∆A were detected only in infected A549 cells, but not in transfected HEK293T cells (Fig. 4d, e), indicating that the defective transcripts presented in the infected A549 cells were derived from defective vRNAs.
Abundances of defective RNAs
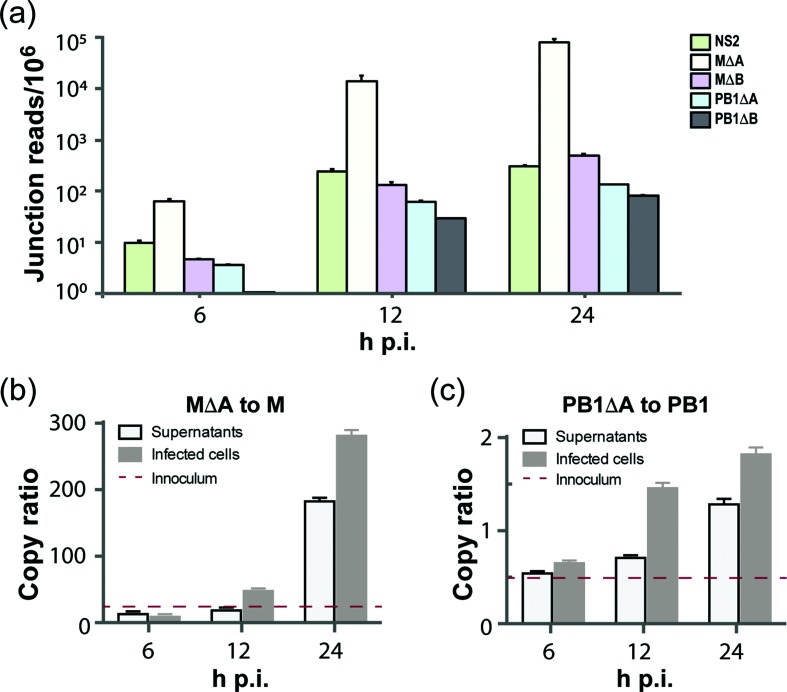
We then used the RNA-Seq data to determine the transcription dynamics of defective mRNAs in infected A549 cells. We estimated the relative abundance of defective mRNAs by calculating the number of junction reads per million mapped reads. The analysis showed that the levels of PB1∆A and PB1∆B were very low at 6 h p.i., and reached peak levels at 24 h p.i. Similar to PB1∆A and PB1∆B, M∆A and M∆B levels reached the highest at 24 h p.i. (Fig. 5a). Interestingly, the transcription level of M∆A was over 100-fold higher than M∆B. The positional read coverage at the M∆A junction sites was ~70-fold higher than M1 and M2 transcripts. This suggested that the transcription level of M∆A was significantly higher than these of M1 and M2.
Fig. 5.
Transcription profiles of defective RNAs and their relative abundances. (a) Transcription profiles of defective mRNAs. RNA-seq junction reads for the four defective RNAs (M∆A, M∆B, PB1∆A and PB1∆B) and NS2 are shown as junction reads per million total reads at indicated time points following FLUBV infection of A549 cells. (b) Ratio of M∆A vRNAs relative to full-length M vRNAs. (c) Ratio of PB1∆A vRNAs relative to full-length PB1 vRNAs. Ratios of M∆A to the full-length M segment and of PB1∆A to the full-length PB1 segment were measured respectively by droplet digital PCR (ddPCR). Both clarified supernatants and cell lysates of A549 cells infected with 1.0 m.o.i. of FLUBV were collected at 6, 12 and 24 h p.i. Total RNAs were then extracted and 200 ng RNA was used for reverse transcription with vRNA-specific primers. ddPCRs were conducted with primers and probes specific to amplify defective RNAs (the junction regions) and full-length segments. No-template controls were included in every run. The ratios of defective genomes to their corresponding full-length segments were normalized and shown as mean+sem from three separate experiments with each assaying samples in triplicate. Note that M∆A and PB1∆A were detected in the viral inoculum and copy ratios relative to their parental segments were indicated by red dashed lines in (b) and (c). Viral inoculum was generated from clarified and filtered supernatants from MDCK cells infected with 0.1 m.o.i. at 72 h p.i.
We next developed vRNA-sense reverse transcription [50] coupled with a two-colour probe droplet digital PCR (ddPCR) assay that enabled us to measure the relative abundance of the observed defective vRNAs compared to their parental segments in both intracellular and extracellular environments. Such an assay allowed us to target on vRNA-sense, not positive-sense RNA forms. We focused our analysis on M∆A and PB1∆A species here and during the rest of the study. Primers and probes used in the quantitative ddPCR experiment are described in Table S2.
By using the vRNA-sense RT-PCR coupled with the Sanger sequencing, we found that viral inoculum contained PB1∆A and M∆A (data not shown). Quantitative analysis with a ddPCR assay showed that MΔA was present in the viral inoculum with about a 20-fold higher level than its parent M segment, while PB1ΔA was present at approximately 50 % of the parental PB1 level (Fig. 5b, c). Additional analysis showed that M∆A (Fig. 5b) tended to be much more abundant relative to its parental segment than PB1∆A (Fig. 5c) in both infected cells and clarified supernatants at different time points following viral infection, which is in good agreement with our analysis of the viral inoculum (Fig. 5b, c). At 24 h p.i., M∆A was nearly 180-fold and 280-fold more abundant than the full-length M segment in infected cells and clarified supernatants, respectively (Fig. 5b). In marked contrast, PB1∆A only exhibited about 1.25- to 1.75-fold increase in copy numbers over the full-length PB1 in both infected cells and clarified supernatants at this time point (Fig. 5c). Significantly, the vRNA analysis correlated well with the mRNA profile (Fig. 5a). The results of these experiments provided evidence for the existence and the abundance of the negative-sense defective RNAs, which correlated with the level of virus replication.
Inhibition of FLUBV and FLUAV replication by defective vRNAs
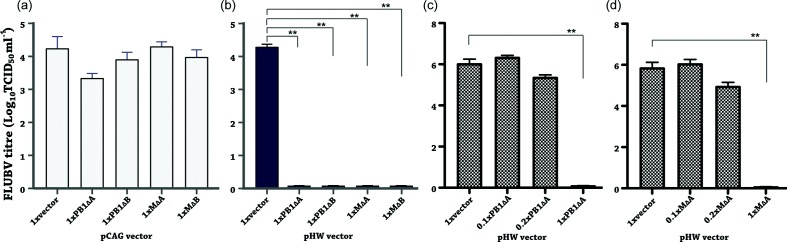
It is known that vRNA-sense DI genomes of FLUAV are capable of inhibiting virus replication [33, 51]. To determine whether the defective genomes were able to interfere with the replication of FLUBV, we placed each individual defective sequence into the pHW2000 (pHW) vector [52] with bidirectional promoters that allow both Pol I transcription of vRNAs and Pol II-driven transcription of mRNAs. In addition, we generated a set of control plasmids in the pCAGEN (pCAG) vector [53] that contains a Pol II promoter and no Pol I promoter. To test the effects of vRNA-sense defective genome transcription on virus replication, we employed a reverse genetics system (RGS) to generate B/Yamanashi/166/98 virus [54]. In the presence or absence of the defective plasmids (1×, 1 : 1 ratio of defective plasmids and the corresponding parental segment plasmids), viral infectivity was then determined. As shown in Fig. 6(b), FLUBV replication was completely inhibited in the presence of each defective genome in pHW vector. In contrast to the blockage of replications by the pHW series, the replication levels were not affected by the pCAG series except for a ~1 log reduction in the pCAG-PB1∆A experiment (Fig. 6a). This moderate inhibition by PB1∆A mRNA suggested that the potential ORF residing in this short form RNA may play a role in interference with viral replication, which should be investigated in future study. Overall the data suggested that the presence of each of the four defective transcripts in vRNA form, but not in mRNA form, was efficient to inhibit FLUBV viral replication.
Fig. 6.
Inhibition of FLUBV replication by defective RNAs. Co-culture of HEK293T and MDCK cells were transfected with each of the indicated defective genome plasmids (0.25 µg each) in the context of (a) pCAG vector (positive-sense mRNA production) or (b) pHW vector (both positive- and negative-sense RNA production), together with an eight-plasmid FLUBV RGS system (0.25 µg each plasmid). Culture supernatants collected at 24 h p.i. were titrated in MDCK cells to determine end-point titres (TCID50 ml−1). The dose-dependent effects of PB1∆A and M∆A genomes in interference with FLUBV replication (c, d) were investigated under similar experimental conditions by decreasing the amount of the defective genome plasmid used for transfection while maintaining the same concentration of the corresponding parental segment. Three relative ratios of the defective genome and parental segment plasmids: 1×, 1 : 1 (0.25 to 0.25 µg); 0.2×, 1 : 5 (0.05 to 0.25 µg); and 0.1×, 1 : 10 (0.025 to 0.25 µg), were used together with seven other FLUBV RGS plasmids (0.25 µg each plasmid) in the transfection/infection experiment. Data are shown as mean+sem from three separate experiments each in triplicate. One-way ANOVA was used to calculate significance with two stars indicating P<0.01.
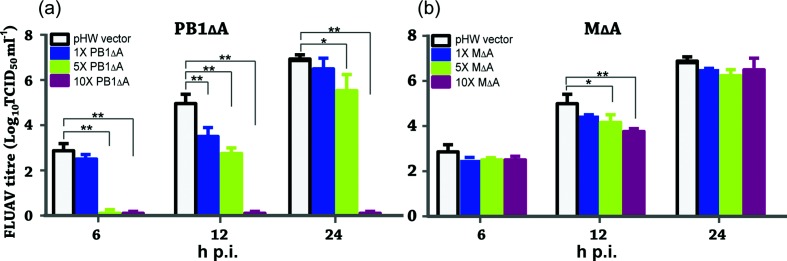
To further investigate the dose-dependent interfering effects of the defective vRNAs, we decreased the amount of the pHW-vectored defective plasmids used for transfection while maintaining the same amount of the parental plasmids and other RGS segment plasmids. Three ratios of the defective plasmids to the parental segment plasmids (1×, 1 : 1; 0.2×, 1 : 5; and 0.1×, 1 : 10) were used together with the other seven RGS plasmids that each had the same amount of the parental segment plasmids. Dose-dependent suppression of FLUBV replication by PB1∆A or M∆A was clearly demonstrated in Fig. 6(c, d). Specifically, 1× of both PB1∆A or M∆A were able to completely inhibit FLUBV replication. Reducing the amount of DI plasmid by 80 % (0.2×) resulted in a moderate ~1 log reduction of viral infectivity. Furthermore, there was no detectable reduction in viral infectivity observed in the 0.1× group where the PB1∆A or M∆A was further reduced by 90 %.
Next, we investigated whether expression of PB1∆A and M∆A defective vRNAs could interfere with FLUAV replication. An A/WSN/33 RGS [52] was employed to generate FLUAV using a similar approach as described above. For determining viral infectivity, we measured viral TCID50 ml−1 at 6, 12 and 24 h p.i., respectively. Similarly, three different dose groups (based on the ratios of the defective plasmids and parental segment plasmids) were used in this experiment, which were 1×, 1 : 1; 5×, 5 : 1; and 10×, 10 : 1. As demonstrated in Fig. 7, FLUAV replication was not substantially inhibited by either M∆A or PB1∆A when used in the same amount of plasmid DNA as the corresponding full-length M or PB1 segment (1× group). Interestingly, significant inhibitions of FLUAV replication were achieved when the PB1∆A plasmid was used five (5× group) or ten times (10× group) higher than the PB1 segment (Fig. 7a). For example, in the 10× group, no detectable viruses were found in the presence of PB1∆A at 6, 12 and 24 h p.i. In the 5× group, introduction of PB1∆A resulted in a complete inhibition of FLUAV replication at 6 h p.i. followed by ~3 log and ~1 log reductions relative to mock vector control at 12 and 24 h p.i., respectively (Fig. 7a). In marked contrast, neither 5× nor 10× M∆A groups showed significant inhibitory activity against FLUAV over the course of the experiment (Fig. 7b).
Fig. 7.
Inhibition of FLUAV replication by defective RNAs. Co-culture of HEF293T and MDCK cells was transfected with an eight-plasmid A/WSN/33 RGS in the absence or presence of different amounts of PB1∆A (a) and M∆A (b). Note 1×: 0.25 µg defective RNA plasmid and 0.25 µg of each of the RGS plasmids, 5×: 1.25 µg defective RNA plasmid and 0.25 µg of each of the RGS plasmids, and 10x: 2.5 µg defective RNA plasmid and 0.25 µg of each of the RGS plasmids. DNA amount in each transfection reaction was made equivalent by adding pHW empty vector. Culture supernatants collected at 6, 12 and 24 h p.i. were titrated for determining viral infectivity (TCID50 ml−1) in MDCK cells. Data are shown as mean+sem from three separate experiments each in triplicate. One-way ANOVA is used to calculate significance with one star indicating P<0.05 and two stars indicating P<0.01, respectively.
In summary, the above experimental data demonstrated that each of the four defective vRNAs derived from B/Brisbane/60/2008 virus (Victoria lineage) was able to potently inhibit replication of B/Yamanashi/166/98 (Yamagata lineage). Further investigation of PB1∆A and M∆A showed that PB1∆A, but not M∆A, was able to interfere with A/WSN/33 replication to a less pronounced degree when compared to that observed for FLUBV.
Discussion
DI genomes have been well-described in natural FLUAV infections [28–31, 34, 35, 40, 45]. The transmission of DI genomes has occurred between human patients infected with influenza A(H1N1)pdm09 virus [45]. Because the position of the internal deletion is highly variable in segments, a heterogeneous population of segment-specific DI RNAs has been frequently observed in FLUAV-infected cells and animals [40, 45]. To date, more than 50 different species of DI RNAs have been described in FLUAV-infected cells [32, 35]. Although for FLUBV the DI phenomenon has been reported in 1954 [46], and the presence of a substantial amount of putative FLUBV DIs in addition to FLUAV DIs in live attenuated influenza vaccine has been described recently [55], the molecular nature of these FLUBV-associated defective genomes and their roles in moderating viral replication are not clear.
Here we demonstrated that FLUBV, like FLUAV, is capable of producing defective genomes during viral replication. Specifically, by NGS coupled with RT-PCR and traditional Sanger sequencing, we have confirmed the presence of four defective genomes in A549 human lung epithelial cells that had been infected with B/Brisbane/60/2008. The stringent threshold (i.e. above 200 junction reads) employed in our sequence analysis may result in the detection of a relatively low number of defective genomes in FLUBV-infected A549 cells. An alternative explanation is our experimental conditions where no continuous virus passage or high infectious dose was employed (only 1 m.o.i. were employed in this study). Regardless of a precise cause, our data indicated that both PB1 and M segments likely serve as a major source for the production of defective RNAs upon FLUBV infection.
Each of the four identified defective RNAs, when transcribed into vRNA-sense, was able to potently inhibit the replication of FLUBV. The interfering activities of the defective genomes we had seen are similar to those of the well-characterized FLUAV DI genomes. Therefore, we propose that the four defective genomes should be categorized as DI genomes. Despite their unknown mechanism of action, by analogy with FLUAV, one can envision that the defective vRNAs inhibit the growth of FLUBV by competing with their corresponding full-length segment for the engagement of the viral polymerase complex during transcription of vRNA into cRNA as well as for the final packaging into budding virus particles. Interestingly, only PB1∆A had the ability to exert a moderate and dose-dependent interference effect on FLUAV replication, which was much less pronounced than that seen in the interference of cognate FLUBV replication (Figs 6 and 7). Our sequence analysis (data not shown) revealed that nucleotide sequence homology between the influenza A and B PB1 segment is approximately 60 %. In contrast, only approximately 28 % sequence homology in the M segment between two viruses is detected. High-level PB1 homology supports our observation in that the FLUBV PB1-derived defective genome inhibited the replication of FLUAV, while the FLUBV M-derived defective genome exerted no measurable inhibition. Furthermore, we speculate that the PB1 segment shares more similar features such as the panhandle structure (vRNA promoter for vRNA polymerase complex formed by the 5′ and 3′ termini of each segment) and the packaging signal sequence (containing the 5′ and 3′ non-coding regions and the terminal coding sequences of each segment) than the M segment between FLUAV and FLUBV [56]. Previous studies have shown that the panhandle structure and the packaging sequence play a decisive role in DI-mediated interference in viral replication [36–39]. The sequence and structural differences between FLUBV PB1 and M segments, probably subtle, warrant further investigation toward addressing the mechanism by which DI RNA from FLUBV PB1, not the M segment, interfere with FLUAV replication.
The presence of the highly abundant M∆A (i.e. 200-fold and 300-fold higher than the parental M segment, Fig. 5b) in both the virus-infected cell and clarified supernatants raised several interesting questions. First, the observed high abundance of M∆A indicates that the M gene is probably a major source, from which DI RNAs of FLUBV are derived. It is intriguing to note that Tobita and colleagues have previously described a novel type of interfering influenza B virus defective in the function of the M gene [47]. This defective FLUBV, designed clone 301, is capable of interfering with the replication of wild-type FLUBV replication as reported in our study. Interestingly, this clone grows normally in MDCK cells infected with a low m.o.i. but fails to produce infectious particles when MDCK cells were infected at a high multiplicity. The M protein synthesis in clone 301-infected cells with a high m.o.i. is significantly reduced but not completely abolished, suggesting that mutations rather than large deletions likely occurred in the M gene of clone 301. In contrast, two M gene-derived DI genomes (MΔA and MΔB) reported in this study have a large internal deletion in the M gene, which prevents them from the synthesis of both M1 and M2 proteins. Second, the presence of a predominant M segment-derived defective genome during active FLUBV replication may further discriminate FLUBV from FLUAV in defective genome production and M segment expression strategy. Numerous studies of FLUAV have shown that the three polymerase segments, not the M segment, are the major substrates driving the generation of DI genomes [28, 32, 45, 57]. Moreover, the M2 protein of FLUAV is translated from M2 mRNA, which is generated via alternative splicing [24]. However, FLUBV’s M segment depends on a translational stop-start strategy to synthesize BM2 protein [25]. To our knowledge, no splicing event has been previously observed in the FLUBV M segment. Intriguingly, the M segment in FLUAV is favoured for splicing because three independent splicing events have been identified, while its counterpart in FLUBV is largely selected for producing defective genomes. Further dissection of this notable difference may help us better appreciate the differences between these two closely related influenza viruses in biology and evolution. Third, why does FLUBV replication give rise to such abundant M∆A species? It can be assumed that M∆A is a perfect vRNA template that can be readily recognized and replicated by the vRNA polymerase complex. This situation, if true, may consume a substantial amount of cellular energy-providing compounds which the viral replication machinery could otherwise use for manufacturing more infectious viral particles for subsequent infection. Alternatively, it can be considered that M∆A abundance is intentionally required for efficient FLUBV replication and transmission. For example, it may behave as an accessory factor to facilitate viral replication or help FLUBV-infected cells recover from apoptosis or damage. Paradoxically, this model should reconcile the prevailing dogma that DI is a negative regulator of viral replication by competing out its full-length segment. An emerging role for DI such as antiviral innate immune response inducer should also be taken into consideration to address the potential opposing effects of DI in modulation of FLUBV replication in vitro and in vivo [36–39].
Despite decades of research, the molecular mechanism underlying DI RNA formation has not been fully elucidated. Current studies suggest that a vRNA polymerase slippage-based faulty replication process is a likely mechanism [34]. Heterologous populations of DI present in FLUAV and various species of segment-derived DIs in terms of the junction sites support this model. Our observation of over-abundant M∆A in FLUBV, however, may indicate that some DI-RNAs such as M∆A may be produced under certain experimental conditions through a specific mechanism, which warrants further investigation. In summary, we identified four DI genomes specific to FLUBV replication, with M segment-derived M∆A being the most abundant. The negative-sense defective vRNAs potently inhibit the replication of B/Yamanashi/166/98. PB1∆A was able to modestly interfere with FLUAV replication. Further characterization of these DI genomes should advance our understanding of the biology and evolution of FLUBV.
Methods
Cells and virus
Human lung alveolar carcinoma epithelial cell line A549 (ATCC CCL-185) cells, MDCK cells (ATCC CCL-34), and human Calu-3 (ATCC HTB-55) were maintained in Dulbecco's minimum essential medium (DMEM) supplemented with 10 % (v/v) fetal bovine serum (FBS, PAA Laboratories, Dartmouth, MA, USA) and 100 U ml−1 penicillin-streptomycin (Life Technologies, Carlsbad, CA, USA). B/Brisbane/60/2008 virus was provided by Drs Ruben Donis and Xiyan Xu (Centers for Disease Control and Prevention, USA). The viruses were originally replicated in 9-day-old specific-pathogen-free chicken embryonated eggs. In our laboratory, these viruses were propagated using MDCK cells at an m.o.i. of 0.1. The cells in a T75 tissue culture flask were allowed to reach only 60 to 70% confluence at the time of infection. Three additional passages in MDCK cells were made toward the generation of virus stock used in this study. The virus inoculum was suspended in 2 ml DMEM and incubated at 37 °C in 5 % CO2 for 1 h. Following infection, fresh DMEM with 0.5 µg ml−1 tolylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (Sigma, Saint Louis, MO, USA) was added for further incubation at 37 °C in 5 % CO2 for 3 days. After 3 days, the supernatant was harvested and spun at 500 g for 10 min at 4 °C to remove the cellular debris followed by TCID50 determination in MDCK cells. DMEM supplemented with 200 U ml−1 penicillin-streptomycin (Life Technologies, Carlsbad, CA, USA) and 1 µg ml−1 TPCK-treated trypsin (Sigma, Saint Louis, MO, USA) were used as the virus growth medium.
Viral growth kinetics in A549 cells
A549 cells were inoculated with B/Brisbane/60/2008 virus at an m.o.i. of 1.0. After 1 h-incubation at 37 °C in 5 % CO2, the viral inoculum was removed from the cells. Cells were washed three times with PBS and then fresh virus growth medium were added. At 0, 6, 12 and 24 h p.i. respectively, supernatant samples were collected and used for the determination of viral TCID50 in MDCK cells. Four independent experiments were performed with each conducted in duplicate.
Cell proliferation assay
A549 cells (~15000 cells well−1) were seeded onto 96-well plates overnight. Cells were then infected with B/Brisbane/60/2008 at an m.o.i. of 1.0. After 1 h incubation, the cells were washed with PBS three times, and then replaced with fresh virus growth medium. At 6, 12 and 24 h p.i., respectively, CellTiter 96 Aqueous One Solution (Promega, Madison, WI, USA) containing tetrazolium compound [3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] was added to a final concentration of 317 µg ml−1. After 1 h incubation with MTS, the absorbance was detected at 490 nm using a Synergy 2 microplate reader (Biotek, Winooski, VT, USA). Three independent experiments were performed with each run in triplicate. To estimate the cell numbers according to absorbance values measured for A549 cells, the corrected absorbance values at 490 nm versus cell number (from 5000 to 35 000 with 5000 increments) were used to plot a regression equation with an R squared of 0.992. Cell numbers at different time points (6, 12 and 24 h p.i.) for control cells and virus-infected cells were calculated according to this equation.
Next-generation sequencing
A549 cells were infected with B/Brisbane/60/2008 at an m.o.i. of 1.0. Total cellular RNA was isolated from mock-infected cells (0 h p.i. sample) and infected cells at, 6, 12 and 24 h p.i., respectively, with TRIZOL followed by RNA purification with ethanol. RNA quality was tested using a Bioanalyzer 2100 (Agilent, Palo Alto, CA) and then processed for cDNA library construction by using a cDNA library prep kit (Illumina, San Diego, CA) according to the manufacturer’s instruction. cDNA library construction and all other procedures were conducted in Genomics Core Research Facility at the University of Nebraska-Lincoln. Briefly, mRNAs were purified from the total RNA using oligo(dT) magnetic beads followed by fragmentation. The resultant mRNAs were reverse transcribed to cDNAs that were subjected to an end repair process followed by ligation to the adapters. After separation in agarose gel through electrophoresis, cDNA fragments with a size of about 200 bp were excised, extracted and amplified by PCR using two primers that match the ends of adaptors. PCR-enriched samples were then sequenced by an Illumina Genome Analyzer IIx sequencer in the GCRF at UNL. Please note that two independent experiments (virus infection and total RNA extraction) were performed and cDNA library preparation and RNA-Seq experiment were processed in parallel. The eight samples (two samples from each of four time-points 0, 6, 12 and 24 h p.i. from two experiments) were barcoded and sequenced in one lane. Each of the eight samples had approximately 5–7 million 100-nt single-end reads. All sequencing results passed quality control. The raw reads of the eight samples were submitted to NCBI SRA database under Biosample ID: SRS1328599.
Transcriptome read processing
The raw read quality was checked using FastQC (www.bioinformatics.babraham.ac.uk/projects/fastqc/). The five nucleotides at the 3′ end of each read, whose sequencing qualities were low, were removed using fastx_trimmer (http://hannonlab.cshl.edu/fastx_toolkit/index.html). GSNAP was then used to map raw reads to human (version: GRCh37.p13) and influenza B virus genomes [58]. Samtools was used to index bam files and remove PCR duplicates [59]. HTSeq was used to count the number of reads mapped to human genes defined by the Ensemble genome database and viral genes [60]. The percentage of vRNA was calculated as the ratio of [viral mRNA reads/total mRNA reads].
RT-PCR amplification and sequencing of defective RNA genomes
A549 cells were infected with B/Brisbane/60/2008 virus at an m.o.i. of 1.0. Total cellular RNAs were isolated from the 0 h sample and infected samples collected at 24 h p.i., respectively, with TRIZOL followed by subsequent RNA purification with ethanol. The extracted RNAs were subjected to segment-specific RT-PCRs for the amplification of defective RNA molecules. Following electrophoresis in 1 % agarose gel, amplified RT-PCR products were excised and sequenced by a commercial operator (www.genscript.com/) and contigs were then assembled using FLUBV PB1, M and NS segment sequences. The GenBank accession numbers of these defective RNA sequences are KX092351 for PB1∆A, KX092352 for PB1∆B, KX092353 for M∆A, and KX092354 for M∆B. Inclusion of NS segment in RT-PCR amplification served as a positive control because of a well-known splicing event originating from the NS segment. The validation primers are shown in Table S1. Briefly, cDNA samples specific to PB1 or M segments were reverse transcribed from total RNA with forward primers BPB1-1For and BM-For that targeted only negative-sense vRNAs. The method to reverse transcribe negative-sense vRNAs was as described by Kawakami E [50]. Each RT-PCR product was sequenced with the same set of primers for its PCR.
To determine whether or not the observed defective genomes could be produced from the segment-derived mRNA in the absence of active viral replication, HEK293T cells were transfected with PB1, M or NS segments of B/Yamanashi/166/98 RGS [54]. At 48 h p.i., total RNAs were extracted and then treated with DNase to remove residual DNA. Following the inactivation of DNase by phenol/chloroform extraction and ethanol precipitation, the treated RNAs were used in subsequent RT-PCR reactions with the above primers and experimental conditions. The amplified products were separated through electrophoresis in 1 % agarose gel.
Quantitative measurement of relative abundances of defective genomes to their parental full-length segments
To measure the ratios of M∆A to the parental M segment and of PB1∆A to the parental PB1 segment, A549 cells were infected with 1.0 m.o.i. of FLUBV and both clarified supernatants and cell lysates were collected at 6, 12 and 24 h p.i. vRNAs from supernatant and total cellular RNAs from cell lysates were then extracted using Trizol Reagent (Invitrogen, CA, USA) according to the manufacturer’s protocol. RNA concentration and purity were determined by NanoDrop 1000 spectrometer (Thermo Scientific) and 200 ng RNA was used for reverse transcription with vRNA-specific primers using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). Droplets were made and analysed on the Bio-Rad QX200 ddPCR system according to the manufacturor's guide. The ddPCR reaction consisted of 10 ul 2× Supermix for Probes (Bio-Rad), 900 nM primers, 25 nM probes and 8 ul cDNA into a final volume of 20 ul. The primers and probes (Table S2) were specifically designed to query both the truncated (via targeting junction regions) and full-length genomes. No-template controls were included in every run. Three separate experiments were performed with each assaying sample in triplicate.
Inhibition of viral replication by defective RNAs
293T and MDCK cells were co-cultured at approximately 70 % confluence in 12-well plates and transfected with each of the indicated defective RNA plasmids (0.25 µg each) in the context of pCAGEN vector (pCAG) or pHW2000 vector (pHW), together with an eight-plasmid B/Yamanashi/166/98 RGS system (0.25 µg of each plasmid). pHW2000 and the two RGS systems used in the research project were kindly provided by Erich Hoffmann and Jonathan McCullers [52, 54]. pCAGEN was a gift from Connie Cepko (Addgene plasmid 11160). Transfection system was prepared in Trans-LT1 (Mirus) and Opti-MEM I medium according to the manufacturer’s instructions. Culture supernatants were collected at 72 h p.i. and titrated in MDCK cells to determine end-point titres (TCID50 ml−1). The dose-dependent effects of PB1∆A and M∆A in interference with FLUBV replication were investigated under similar experimental conditions by decreasing the amount of defective plasmids used for transfection while maintaining the same concentration of the corresponding parental plasmids. Three relative ratios of the defective plasmids and its parental plasmids: 1×, 1 : 1 (0.25 to 0.25 µg); 0.2×, 1 : 5 (0.05 to 0.25 µg); and 0.1×, 1 : 10 (0.025 to 0.25 µg), were used together with seven other FLUBV RGS plasmids (0.25 µg of each plasmid) in the transfection/infection experiment. Three separate experiments were performed with each conducted in triplicate.
A similar approach was used to investigate whether expression of FLUBV-derived PB1∆A and M∆A defective genomes could interfere with FLUAV replication. Co-culture of HEF293T and MDCK cells was transfected with an eight-plasmid A/WSN/33 RGS (0.25 µg of each plasmid) in the absence or presence of different amounts of PB1∆A and M∆A. The amount of defective RNA plasmids used was 0.25, 1.25 and 2.5 µg, which resulted in three different dose groups (based on the relative ratios of the defective RNAs and its parental segment plasmids): 1×, 1 : 1 (0.25 to 0.25 µg); 5×, 5 : 1 (1.25 to 0.25 µg); and 10×, 10 : 1 (2.5 to 0.25 µg). Culture supernatants were collected at 24 h p.i. and viral TCID50 ml−1 were measured at 6, 12 and 24 h p.i., respectively. DNA amount in each transfection reaction here and above was made equivalent by adding pHW empty vector. Three separate experiments were performed with each conducted in triplicate.
Funding information
The study was funded by South Dakota Agricultural Experiment Station (3AH-477 to F. L.), the National Science Foundation/EPSCoR Cooperative Agreement #IIA-1355423, the South Dakota Research and Innovation Center, and BioSNTR, and NIH AI121906 (sub-award to D. W.).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Data
Footnotes
Abbreviations: ddPCR, droplet digital PCR; DI, defective interfering; DMEM, Dulbecco's minimum essential medium; FLUAV, influenza A virus; FLUBV, influenza B virus; h p.i., hours post infection; MDCK, Madin-Darby canine kidney; MTS, 3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)- 2H-tetrazolium; NGS, next-generation sequencing; PC, principal component; Pcc, Pearson correlation coefficients; RGS, reverse genetics system; TPCK, tolylsulfonyl phenylalanyl chloromethyl ketone; UPGMA, unweighted pair group method with arithmetic mean; vRNA, viral RNA.
Two supplementary tables are available with the online version of this article.
Reference
- 1.Palese P, Shaw M. Orthomyxoviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. pp. 1647–1689. (editors) [Google Scholar]
- 2.Hause BM, Collin EA, Liu R, Huang B, Sheng Z, et al. Characterization of a novel influenza virus in cattle and Swine: proposal for a new genus in the Orthomyxoviridae family. mBio. 2014;5:e00031-14. doi: 10.1128/mBio.00031-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hause BM, Ducatez M, Collin EA, Ran Z, Liu R, et al. Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog. 2013;9:e1003176. doi: 10.1371/journal.ppat.1003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Annu Rev Med. 2000;51:407–421. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- 5.McCullers JA, Facchini S, Chesney PJ, Webster RG. Influenza B virus encephalitis. Clin Infect Dis. 1999;28:898–900. doi: 10.1086/515214. [DOI] [PubMed] [Google Scholar]
- 6.McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B virus circulated between 1979 and 2003. J Virol. 2004;78:12817–12828. doi: 10.1128/JVI.78.23.12817-12828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monto AS. Epidemiology of influenza. Vaccine. 2008;26:D45–D48. doi: 10.1016/j.vaccine.2008.07.066. [DOI] [PubMed] [Google Scholar]
- 8.Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health. 2013;103:e43. doi: 10.2105/AJPH.2012.301137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutiérrez-Pizarraya A, Pérez-Romero P, Alvarez R, Aydillo TA, Osorio-Gómez G, et al. Unexpected severity of cases of influenza B infection in patients that required hospitalization during the first postpandemic wave. J Infect. 2012;65:423–430. doi: 10.1016/j.jinf.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Wu P, Goldstein E, Ho LM, Yang L, Nishiura H, et al. Excess mortality associated with influenza A and B virus in Hong Kong, 1998-2009. J Infect Dis. 2012;206:1862–1871. doi: 10.1093/infdis/jis628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullers JA, Hayden FG. Fatal influenza B infections: time to reexamine influenza research priorities. J Infect Dis. 2012;205:870–872. doi: 10.1093/infdis/jir865. [DOI] [PubMed] [Google Scholar]
- 12.Paddock CD, Liu L, Denison AM, Bartlett JH, Holman RC, et al. Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J Infect Dis. 2012;205:895–905. doi: 10.1093/infdis/jir861. [DOI] [PubMed] [Google Scholar]
- 13.Osterhaus AD, Rimmelzwaan GF, Martina BE, Bestebroer TM, Fouchier RA. Influenza B virus in seals. Science. 2000;288:1051–1053. doi: 10.1126/science.288.5468.1051. [DOI] [PubMed] [Google Scholar]
- 14.Ran Z, Shen H, Lang Y, Kolb EA, Turan N, et al. Domestic pigs are susceptible to infection with influenza B viruses. J Virol. 2015;89:4818–4826. doi: 10.1128/JVI.00059-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa N, Higashi N, Nakagawa T. Cocirculation of antigenic variants and the vaccine-type virus during the 2004-2005 influenza B virus epidemics in Japan. J Clin Microbiol. 2009;47:352–357. doi: 10.1128/JCM.01357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nerome R, Hiromoto Y, Sugita S, Tanabe N, Ishida M, et al. Evolutionary characteristics of influenza B virus since its first isolation in 1940: dynamic circulation of deletion and insertion mechanism. Arch Virol. 1998;143:1569–1583. doi: 10.1007/s007050050399. [DOI] [PubMed] [Google Scholar]
- 17.Shen J, Kirk BD, Ma J, Wang Q. Diversifying selective pressure on influenza B virus hemagglutinin. J Med Virol. 2009;81:114–124. doi: 10.1002/jmv.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin JH, Chiu SC, Shaw MW, Lin YC, Lee CH, et al. Characterization of the epidemic influenza B viruses isolated during 2004-2005 season in Taiwan. Virus Res. 2007;124:204–211. doi: 10.1016/j.virusres.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 19.McCullers JA, Wang GC, He S, Webster RG. Reassortment and insertion-deletion are strategies for the evolution of influenza B viruses in nature. J Virol. 1999;73:7343–7348. doi: 10.1128/jvi.73.9.7343-7348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glezen WP. Editorial commentary: Changing epidemiology of influenza B virus. Clin Infect Dis. 2014;59:1525–1526. doi: 10.1093/cid/ciu668. [DOI] [PubMed] [Google Scholar]
- 21.Vijaykrishna D, Holmes EC, Joseph U, Fourment M, Su YC, et al. The contrasting phylodynamics of human influenza B viruses. elife. 2015;4:e05055. doi: 10.7554/eLife.05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hai R, Schmolke M, Varga ZT, Manicassamy B, Wang TT, et al. PB1-F2 expression by the 2009 pandemic H1N1 influenza virus has minimal impact on virulence in animal models. J Virol. 2010;84:4442–4450. doi: 10.1128/JVI.02717-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise HM, Foeglein A, Sun J, Dalton RM, Patel S, et al. A complicated message: Identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA. J Virol. 2009;83:8021–8031. doi: 10.1128/JVI.00826-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamb RA, Lai CJ, Choppin PW. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc Natl Acad Sci USA. 1981;78:4170–4174. doi: 10.1073/pnas.78.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horvath CM, Williams MA, Lamb RA. Eukaryotic coupled translation of tandem cistrons: identification of the influenza B virus BM2 polypeptide. EMBO J. 1990;9:2639–2647. doi: 10.1002/j.1460-2075.1990.tb07446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robb NC, Fodor E. The accumulation of influenza A virus segment 7 spliced mRNAs is regulated by the NS1 protein. J Gen Virol. 2012;93:113–118. doi: 10.1099/vir.0.035485-0. [DOI] [PubMed] [Google Scholar]
- 27.Dubois J, Terrier O, Rosa-Calatrava M. Influenza viruses and mRNA splicing: doing more with less. mBio. 2014;5:e00070-14. doi: 10.1128/mBio.00070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis AR, Hiti AL, Nayak DP. Influenza defective interfering viral RNA is formed by internal deletion of genomic RNA. Proc Natl Acad Sci USA. 1980;77:215–219. doi: 10.1073/pnas.77.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis AR, Nayak DP. Sequence relationships among defective interfering influenza viral RNAs. Proc Natl Acad Sci USA. 1979;76:3092–3096. doi: 10.1073/pnas.76.7.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janda JM, Davis AR, Nayak DP, De BK. Diversity and generation of defective interfering influenza virus particles. Virology. 1979;95:48–58. doi: 10.1016/0042-6822(79)90400-8. [DOI] [PubMed] [Google Scholar]
- 31.Nayak DP, Sivasubramanian N, Davis AR, Cortini R, Sung J. Complete sequence analyses show that two defective interfering influenza viral RNAs contain a single internal deletion of a polymerase gene. Proc Natl Acad Sci USA. 1982;79:2216–2220. doi: 10.1073/pnas.79.7.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noble S, Dimmock NJ. Characterization of putative defective interfering (DI) A/WSN RNAs isolated from the lungs of mice protected from an otherwise lethal respiratory infection with influenza virus A/WSN (H1N1): a subset of the inoculum DI RNAs. Virology. 1995;210:9–19. doi: 10.1006/viro.1995.1312. [DOI] [PubMed] [Google Scholar]
- 33.Odagiri T, Tashiro M. Segment-specific noncoding sequences of the influenza virus genome RNA are involved in the specific competition between defective interfering RNA and its progenitor RNA segment at the virion assembly step. J Virol. 1997;71:2138–2145. doi: 10.1128/jvi.71.3.2138-2145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimmock NJ, Easton AJ. Defective interfering influenza virus RNAs: time to reevaluate their clinical potential as broad-spectrum antivirals? J Virol. 2014;88:5217–5227. doi: 10.1128/JVI.03193-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimmock NJ, Easton AJ. Cloned defective interfering influenza RNA and a possible pan-specific treatment of respiratory virus diseases. Viruses. 2015;7:3768–3788. doi: 10.3390/v7072796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baum A, Sachidanandam R, García-Sastre A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc Natl Acad Sci USA. 2010;107:16303–16308. doi: 10.1073/pnas.1005077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frensing T, Pflugmacher A, Bachmann M, Peschel B, Reichl U. Impact of defective interfering particles on virus replication and antiviral host response in cell culture-based influenza vaccine production. Appl Microbiol Biotechnol. 2014;98:8999–9008. doi: 10.1007/s00253-014-5933-y. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Cidoncha M, Killip MJ, Oliveros JC, Asensio VJ, Fernández Y, et al. An unbiased genetic screen reveals the polygenic nature of the influenza virus anti-interferon response. J Virol. 2014;88:4632–4646. doi: 10.1128/JVI.00014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ngunjiri JM, Buchek GM, Mohni KN, Sekellick MJ, Marcus PI. Influenza virus subpopulations: exchange of lethal H5N1 virus NS for H1N1 virus NS triggers de novo generation of defective-interfering particles and enhances interferon-inducing particle efficiency. J Interferon Cytokine Res. 2013;33:99–107. doi: 10.1089/jir.2012.0070. [DOI] [PubMed] [Google Scholar]
- 40.Dimmock NJ, Rainsford EW, Scott PD, Marriott AC. Influenza virus protecting RNA: an effective prophylactic and therapeutic antiviral. J Virol. 2008;82:8570–8578. doi: 10.1128/JVI.00743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Easton AJ, Scott PD, Edworthy NL, Meng B, Marriott AC, et al. A novel broad-spectrum treatment for respiratory virus infections: influenza-based defective interfering virus provides protection against pneumovirus infection in vivo. Vaccine. 2011;29:2777–2784. doi: 10.1016/j.vaccine.2011.01.102. [DOI] [PubMed] [Google Scholar]
- 42.Scott PD, Meng B, Marriott AC, Easton AJ, Dimmock NJ. Defective interfering influenza A virus protects in vivo against disease caused by a heterologous influenza B virus. J Gen Virol. 2011;92:2122–2132. doi: 10.1099/vir.0.034132-0. [DOI] [PubMed] [Google Scholar]
- 43.Bean WJ, Kawaoka Y, Wood JM, Pearson JE, Webster RG. Characterization of virulent and avirulent A/chicken/Pennsylvania/83 influenza A viruses: potential role of defective interfering RNAs in nature. J Virol. 1985;54:151–160. doi: 10.1128/jvi.54.1.151-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers TM, Webster RG. Defective interfering virus associated with A/Chicken/Pennsylvania/83 influenza virus. J Virol. 1987;61:1517–1523. doi: 10.1128/jvi.61.5.1517-1523.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saira K, Lin X, DePasse JV, Halpin R, Twaddle A, et al. Sequence analysis of in vivo defective interfering-like RNA of influenza A H1N1 pandemic virus. J Virol. 2013;87:8064–8074. doi: 10.1128/JVI.00240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Magnus P. Incomplete forms of influenza virus. Adv Virus Res. 1954;2:59–79. doi: 10.1016/s0065-3527(08)60529-1. [DOI] [PubMed] [Google Scholar]
- 47.Tobita K, Odagiri T, Tanaka T. Isolation of a novel type of interfering influenza B virus defective in the function of M gene. Arch Virol. 1986;90:223–236. doi: 10.1007/BF01317372. [DOI] [PubMed] [Google Scholar]
- 48.Briedis DJ, Lamb RA. Influenza B virus genome: sequences and structural organization of RNA segment 8 and the mRNAs coding for the NS1 and NS2 proteins. J Virol. 1982;42:186–193. doi: 10.1128/jvi.42.1.186-193.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheth N, Roca X, Hastings ML, Roeder T, Krainer AR, et al. Comprehensive splice-site analysis using comparative genomics. Nucleic Acids Res. 2006;34:3955–3967. doi: 10.1093/nar/gkl556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawakami E, Watanabe T, Fujii K, Goto H, Watanabe S, et al. Strand-specific real-time RT-PCR for distinguishing influenza vRNA, cRNA, and mRNA. J Virol Methods. 2011;173:1–6. doi: 10.1016/j.jviromet.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boergeling Y, Rozhdestvensky TS, Schmolke M, Resa-Infante P, Robeck T, et al. Evidence for a novel mechanism of influenza virus-induced type I interferon expression by a defective RNA-encoded protein. PLoS Pathog. 2015;11:e1004924. doi: 10.1371/journal.ppat.1004924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci USA. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffmann E, Mahmood K, Yang CF, Webster RG, Greenberg HB, et al. Rescue of influenza B virus from eight plasmids. Proc Natl Acad Sci USA. 2002;99:11411–11416. doi: 10.1073/pnas.172393399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gould PS, Easton AJ, Dimmock NJ. Live attenuated influenza vaccine contains substantial and unexpected amounts of defective viral genomic RNA. Viruses. 2017;9:269. doi: 10.3390/v9100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu MT, Parvin JD, Gupta S, Krystal M, Palese P. Genomic RNAs of influenza viruses are held in a circular conformation in virions and in infected cells by a terminal panhandle. Proc Natl Acad Sci USA. 1987;84:8140–8144. doi: 10.1073/pnas.84.22.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jennings PA, Finch JT, Winter G, Robertson JS. Does the higher order structure of the influenza virus ribonucleoprotein guide sequence rearrangements in influenza viral RNA? Cell. 1983;34:619–627. doi: 10.1016/0092-8674(83)90394-X. [DOI] [PubMed] [Google Scholar]
- 58.Wu TD, Nacu S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics. 2010;26:873–881. doi: 10.1093/bioinformatics/btq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.