Abstract
Metabolic syndrome (MetS), a cluster of cardiovascular disease risk factors, is increasingly common in people living with HIV; however, data on prevalence and the role of antiretroviral therapy (ART) as a risk factor for MetS in sub-Saharan Africa are lacking. We conducted a cross-sectional study to assess the prevalence and risk factors for MetS among ART-naive and ART-experienced HIV-infected adults without preexisting cardiometabolic disorders in Western Kenya using validated questionnaires and laboratory tests after overnight fasting. We used logistic regression to identify associations between traditional risk factors, HIV disease characteristics, ART, and MetS. Study participants included 164 ART-experienced patients, majority (56%) on tenofovir/lamivudine/nevirapine regimen, and 136 ART-naive patients. The median age was 40 (interquartile range, 33–46) years and 64% were women. Median HIV infection and ART use were 4.6 (1.7–7.9) and 4.8 (2.7–7.8) years, respectively. Prevalence of MetS did not differ between ART-experienced (16.9%) and -naive (15.2%) groups. ART-experienced patients had higher rates of elevated fasting blood sugars and lower rates of low high-density lipoprotein-cholesterol. The prevalence of abnormal waist circumference, elevated blood pressure, and hypertriglyceridemia were comparable between the two groups. Older age, female sex, and high body mass index were independently associated with diagnosis of MetS. Traditional risk factors rather than ART-related effects were more important predictors of MetS in this cohort and may have been influenced by ART type and exclusion of preexisting hypertension and diabetes. HIV-infected patients without preexisting cardiometabolic disorders should be monitored for metabolic abnormalities regardless of ART.
Keywords: : HIV, cardiovascular diseases, antiretroviral therapy, sub-Saharan Africa, metabolic syndrome, Kenya
Introduction
The global scale-up of antiretroviral therapy (ART) has resulted in the reduction of AIDS-related mortality in low-resource settings, including Kenya.1–4 As HIV-infected patients live longer, they are exposed to traditional environmental and behavioral risk factors known to increase risk of metabolic and cardiovascular diseases (CVD) prevalent in their communities.5 Studies from developed countries have shown that in addition to the traditional risk factors, some ART regimens and HIV infection itself may increase risk of cardiometabolic diseases and CVD.6–10 In these studies, patients with HIV were twice as likely to develop stroke or myocardial infarction compared with their HIV-uninfected counterparts.9,10 Although greater contact with the healthcare system has been associated with better control of hypertension and diabetes among HIV-infected women compared with HIV-uninfected women, CVD risk remains from nonlipid causes and suboptimal control CVD risk factors.11 Despite sub-Saharan Africa (SSA) being a region highly affected by HIV, few studies have assessed aging-related HIV comorbidities, particularly CVD, metabolic syndrome (MetS), and their associated risk factors.
MetS is a complex cluster of factors, including hyperglycemia, elevated blood pressure, dyslipidemia, and abdominal obesity that are shown to predict risk of CVD and type 2 diabetes.12,13 Prevalence of MetS is increasing worldwide and is closely linked to the presence of obesity and dyslipidemia in the adult population in high-income countries.13,14 Among HIV-infected individuals, the prevalence of MetS worldwide ranges from 7% to 52% depending on the defining criteria, study population, study design, and sample size.15–17 Recently published meta-analysis data show a similar prevalence of MetS in the general population as in those with HIV.15 Other studies, however, have reported an increase in the incidence of MetS in HIV-infected patients on ART compared with ART-naive and HIV-uninfected counterparts, indicating that antiretroviral drugs may be associated with increased risk of MetS.18,19 Most of these studies were conducted in higher income countries and a greater number of patients were obese, consuming tobacco and on protease inhibitors (PIs) compared with populations in low and middle income settings.20 The prevalence of MetS in SSA ranges from 11.1% to 47% depending on the study setting, ART type and ART experience.16 There has been limited information from SSA particularly on the role of ART in the development of MetS. Moreover, most existing SSA studies have focused primarily on HIV-infected adults on ART, making it difficult to determine the independent effect of ART drugs on MetS. The present work examines the prevalence and associated risk factors for the development of MetS in HIV-infected adults without previous clinical diagnosis of CVD who are seeking care at one of the largest HIV clinics in Western Kenya.
Methods
Study population and design
This was part of a larger study to assess CVD risk factors, knowledge, perception, and attitudes toward CVD among HIV-infected patients in Western Kenya who had no previous diagnosis of cardiovascular-related disease.21 We conducted a cross-sectional survey between July and September 2014 in a sample of HIV-infected adults attending an HIV clinic at the Academic Model Providing Access to Healthcare (AMPATH) program within the Moi Teaching and Referral Hospital (MTRH) in Western Kenya. We enrolled 300 consecutive HIV-infected men and women (>18 years of age) who presented for care at the MTRH HIV outpatient clinic in Eldoret, Kenya. Pregnant women, patients who reported or had recorded history of cardiometabolic disorders, including hypertension, those with diabetes, or history of CVD, were ineligible to participate. AMPATH program provides care to over 150,000 HIV-infected people with a broad mix of urban middle class, urban poor, and rural populations.22 The AMPATH program is a collaboration between MTRH, Moi University School of Medicine, and a consortium of North American universities that focuses on improving the health of the people of Western Kenya as previously described.23 Moi University is the hub of clinical research in cardiopulmonary diseases in Western Kenya.24
Data collection
Our data collection methods have been previously described in detail.21 In brief, data were collected by structured questionnaires, physical measurements, and venous blood sample analysis. A trained research assistant administered the questionnaire in English, Swahili, or a local language. Each interview was followed by physical measurements, including height, weight, and blood pressure. Participants were asked to return to clinic the following day after fasting for 8 h for blood sample collection. All participants were tested for fasting blood lipids and glucose level. The research participants were expected to complete all the components of the research examination on the second visit. HIV-related characteristics and information, including the use of ART, the duration and type of ART regime, previous WHO clinical stage, pre-ART nadir, lowest and highest CD4 T cell count, and HIV RNA viral load were obtained from the participant and/or the medical record. Any findings that warranted immediate medical attention were reported to the participant and their physician.
We used the harmonized consensus criteria for MetS to define high BP as systolic BP ≥130 mmHg, diastolic BP ≥85 mmHg, or currently on antihypertensive drug treatment, central obesity as waist circumference of ≥80 cm (women) and ≥94 cm (men), and elevated fasting blood glucose (FBG) as FBG ≥7 mmol/L (126 mg/dL).25 Dyslipidemia was defined as total cholesterol ≥5.2 mmol/L (200 mg/dL) or high-density lipoprotein (HDL) cholesterol <1.03 mmol/L (40 mg/dL), low-density lipoprotein (LDL) cholesterol ≥3.4 mmol/L (130 mg/dL), and triglycerides >4.0 mmol/L (350 mg/dL) according to the American Heart Association and American College of Cardiology Foundation.26 Overweight referred to body mass index (BMI) greater than or equal to 25–29.9 kg/m2 and obesity was BMI greater than or equal to 30 kg/m2.
HIV-infected patients fulfilling Kenyan national criteria for ART are started on treatment and seen monthly at the AMPATH clinic. Criteria for starting ART at the time of the study included all HIV-infected adults with CD4 T cell count <350 cells/mm3 irrespective of WHO stage and stage III/IV disease regardless of CD4 T cell count. The first-line ART regimen consisted of either tenofovir/lamivudine or zidovudine/lamivudine + nevirapine or efavirenz. PIs were only given as second-line ART in accordance with Kenya national guidelines for antiretroviral drug therapy.27
Primary outcome
Our primary outcome, MetS was defined according to International Diabetes Federation (IDF); central obesity; waist circumference ≥80 cm in women and ≥94 cm in men, plus two of the following: triglycerides ≥150 mg/dL (1.7 mmol/L), HDL cholesterol <50 mg/dL (1.29 mmol/L) for women or ≤40 mg/dL (1.03 mmol/L) for men, FBG ≥100 mg/dL (5.6 mmol/L), systolic blood pressure ≥130 mmHg, or diastolic blood pressure ≥85 mmHg.25
Statistical analysis
Baseline characteristics of study participants were compared between ART-experienced and ART-naive participants. The primary outcome of the study was diagnosis of MetS, which was compared between the HIV-infected ART-experienced group and the HIV-infected ART-naive control group. Continuous variables were summarized using mean [standard deviation (SD)] or medians (interquartile range) and compared using two-sample t-tests if normality assumptions were met. We estimated the prevalence of MetS and its components by ART status. Categorical variables were summarized using counts and proportions and compared using Pearson's chi-square tests or Fisher's exact tests, as appropriate. Using bivariate and multivariate logistic regression, we also obtained unadjusted and adjusted odds ratios, respectively, for the association between known risk factors and diagnoses of MetS. Data analysis was done using STATA® version 13 (San Antonio, TX). p Values less than 0.05 were considered significant.
Ethical statement
The Institutional Research and Ethics Committee of Moi University School of Medicine approved the study. All participants provided a written informed consent.
Results
A total of 300 HIV-infected patients (36% male) were enrolled in the study. Table 1 shows characteristics of HIV-infected adult participants. Fifty-five percent were receiving combination ART and 45% were ART naive. Mean (SD) age of ART-experienced and ART-naive participants were 43 (9) and 38 (9) years, respectively. Forty percent of the participants were between 35 and 44 years of age, 42.7% for the ART-experienced and 41.9% for the ART-naive groups. ART-experienced participants were more likely to be older and male, and less likely to have received any formal education (Table 1). In addition, ART-experienced participants had significantly higher mean waist circumference and BMI, and were more likely to have dyslipidemia and higher fasting plasma glucose; they had similar rates of alcohol intake and tobacco use as ART-naive adults (Table 1).
Table 1.
Characteristics of HIV-Infected Participants By Antiretroviral Therapy Status
| ART experienced (N = 164) | ART naive (N = 136) | p | |
|---|---|---|---|
| Demographics | |||
| Female | 94 (57.3) | 98 (72.1) | 0.008 |
| Age, years | 43.1 (9.4) | 37.9 (8.9) | <0.001 |
| Age groups, years | <0.001 | ||
| 18–34 | 29 (17.7) | 48 (35.3) | |
| 35–44 | 70 (42.7) | 57 (41.9) | |
| 45–54 | 37 (22.6) | 23 (16.9) | |
| >55 | 28 (17.1) | 8 (5.9) | |
| Education level | |||
| High school or more | 55 (33.5) | 15 (11) | 0.03 |
| Cardiovascular risk factors | |||
| Current tobacco use | 5 (3) | 5 (3.7) | 0.95 |
| Current alcohol drinking | 37 (22.6) | 24 (17.7) | 0.29 |
| Diabetes | 2 (1.5) | 5 (3.1) | 0.35 |
| Overweight/obese | 71 (43.3) | 50 (36.8) | 0.25 |
| Physical activity, work related | 122 (74.4) | 89 (65.4) | 0.09 |
| HDL, mmol/L | 1.5 ± 0.6 | 1.1 ± 0.5 | <0.001 |
| LDL, mmol/L | 2.8 ± 0.9 | 2.5 ± 0.1 | 0.001 |
| Triglycerides, mmol/L | 1.6 ± 1.1 | 1.5 ± 0.9 | 0.21 |
| Total cholesterol, mmol/L | 4.8 ± 1.1 | 4.1 ± 1.0 | <0.001 |
| BMI, kg/m2 | 25.1 ± 5.9 | 23.8 ± 5.2 | 0.05 |
| Waist circumference, cm | 87.8 ± 12.7 | 84.6 ± 9.4 | 0.01 |
| SBP, mmHg | 116.3 ± 5.5 | 115.7 ± 5.4 | 0.40 |
| DBP, mmHg | 65.5 ± 5.6 | 65.9 ± 6.1 | 0.57 |
| FBS, mmol/L | 5.0 ± 1.0 | 5.1 ± 1.0 | 0.34 |
| HIV-related factors | |||
| Nadir CD4a, cells/mm3 | 191.4 ± 127.8 | 417.9 ± 206.2 | <0.001 |
| Peak viral load,b copies/mL | 120,646.1 (538–112.9) | 189,710 (199–678.9) | 0.59 |
| WHO clinical stage 4 | 15 (9.2) | 4 (2.9) | <0.001 |
| HIV duration, years | 6.6 ± 4.7 | 3.7 ± 4.4 | <0.001 |
| ART duration, years | 5.4 ± 3.3 | ||
| Type of ART | |||
| PI-based | |||
| Lopinovir/ritonavir | 11 (6.7) | ||
| NRTI-based | |||
| Stavudine | 4 (2.4) | ||
| Zidovudine | 57 (34.8) | ||
| Lamuvidine | 152 (92.7) | ||
| Tenofovir | 109 (66.5) | ||
| NNRTI-based | |||
| Nevirapine | 99 (60.4) | ||
| Efavirenz | 51 (31.1) | ||
| Others | 9 (5.5 | ||
Values are n (%), median (interquartile range) or mean ± SD.
N = 188.
N = 38.
ART, antiretroviral therapy; SBP, systolic BP; DBP, diastolic BP; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; BMI, body mass index; FBS, fasting blood sugar; PI, protease inhibitor; SD, standard deviation.
Patients on ART had worse history of immune function, with lower nadir CD4 cell count, more WHO stage 4 disease, and longer mean duration of HIV infection compared with the ART-naive adults (6.6 vs. 3.7 years), respectively (Table 1). Among the ART experienced, mean duration on ART treatment was 5.4 years, with a large number on first-line ART (82.3%). Current ART use included 92%, non-nucleoside reverse transcriptase inhibitors (NNRTI) and 6.7% PI. Nearly all (99%) were on nucleoside reverse transcriptase inhibitors (NRTI). A large majority (56%) of these patients were on nevirapine-based (tenofovir/lamivudine/nevirapine) regimen and one third (36%) were on efavirenz-based (tenofovir/lamivudine/efavirenz) regimen.
MetS prevalence and risk factors
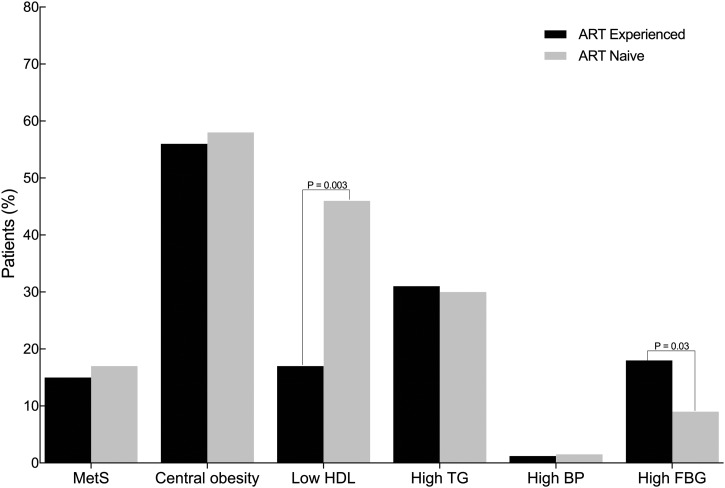
MetS frequencies by ART status are presented in Fig. 1. MetS prevalence among ART experience and ART naive were 16.9% and 15.2%, respectively. Although Mets prevalence was slightly higher in patients receiving ART, it was not statistically different (p > 0.05). The distribution of individual components of MetS by ART status is shown in Fig. 1. Over half of our participants met the criteria for abnormal waist circumference with the pattern not varying by ART status. ART experienced were more likely to have elevated blood glucose, whereas ART naive were more likely to have low HDL levels (Fig. 1) Frequencies of hypertriglyceridemia and elevated blood pressure were similar between the two groups. Significantly more ART experienced (24%) met the criteria for abnormal waist circumference and one additional component of MetS compared with the naive group (14%), p = 0.03.
FIG. 1.
Prevalence of metabolic syndrome by ART status. All (n = 300) participants were included. Central obesity was defined as waist circumference of ≥80 cm (women) and ≥90 cm (men). High TG was defined as triglycerides ≥150 mg/dL (1.7mmol/L), low HDL was HDL cholesterol levels of <50 mg/dL (1.29 mmol/L) for women and ≤40 mg/dL (1.03 mmol/L) for men, high FBG was fasting blood glucose of ≥100 mg/dL (5.6 mmol/L), high BP was a systolic blood pressure of ≥130 mmHg, or diastolic blood pressure of ≥85 mmHg. ART, antiretroviral therapy; FBG, fasting blood glucose; HDL, high-density lipoprotein; TG, triglycerides.
In unadjusted analyses, HIV-infected patients with MetS were more likely to be female, have a higher level of LDL cholesterol, BMI of at least 25 kg/m2, and age above 55 years (Table 2) compared with those without MetS. After adjustment for demographic characteristics and HIV-related characteristics in a multivariate logistic regression analysis, female sex, older age ≥55 years, and higher BMI remained independently associated with the presence of MetS (Table 3). We did not observe any association among the use of ART, specific ART drugs, HIV infection duration, and MetS. The ART regimen commonly used and the exclusion of those with hypertension in the study may have influenced the ART-related effects on MetS.
Table 2.
Characteristics of HIV Infected Participants By Metabolic Syndrome Status
| With MetS (N = 48) | Without MetS (N = 252) | p | |
|---|---|---|---|
| Gender | |||
| Female | 40 (83.3) | 152 (60.3) | 0.002 |
| Age, years mean (SD) | 43.2 ± 9.83 | 40.2 ± 9.42 | 0.05 |
| Age groups, years | |||
| 18–34 | 8 (16.7) | 69 (27.4) | 0.12 |
| 34–55 | 30 (62.5) | 157 (62.3) | 0.98 |
| >55 | 10 (20.8) | 26 (10.3) | 0.04 |
| Current tobacco users | 0 (0.0) | 10 (3.8) | 0.17 |
| Current alcohol drinking | 8 (16.7) | 53 (21.0) | 0.49 |
| Dyslipidemia | 43 (89.6) | 133 (52.8) | <0.001 |
| BMI ≥25 kg/m2 | 32 (66.7) | 93 (36.9) | <0.001 |
| Nadir CD4,a cells/mm3 | 251.9 (196.0) | 261.7 (185.9) | 0.79 |
| Peak viral load,b copies/mL | 195472.3 (185216.3) | 126071.9 (86873.08) | 0.82 |
| Length of HIV infection, years | 5.42 (4.9) | 5.3 (4.8) | 0.85 |
| ART duration, years | 4.7 (3.0) | 5.5 (3.3) | 0.2 |
| ART use | 25 (52.1) | 139 (55.2) | 0.70 |
| Type of ART | |||
| Lopinovir/ritonavir | 2 (8.0) | 9 (6.5) | 0.78 |
| Zidovudine | 11 (44.0) | 46 (33.1) | 0.29 |
| Lamuvidine | 24 (96.0) | 128 (92.1) | 0.49 |
| Tenofovir | 13 (52.0) | 96 (69.1) | 0.10 |
| Nevirapine | 13 (52.0) | 86 (61.9) | 0.35 |
| Efavirenz | 11 (44.0) | 40 (28.8) | 0.13 |
| Others | 2 (8.0) | 7 (5.0) | 0.55 |
Values are n (%) or mean ± SD.
N = 188.
N = 38.
ART, antiretroviral therapy; MetS, metabolic syndrome; BMI, body mass index; SD, standard deviation.
Table 3.
Factors Associated with Metabolic Syndrome Among HIV-Infected Patients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | Adjusted OR | 95% CI | p | |
| Male sex | 0.30 | 0.14–0.68 | 0.004 | 0.22 | 0.09–0.56 | 0.001 |
| Age groups,a years | ||||||
| ≥55 | 2.54 | 1.08–5.95 | 0.03 | 3.35 | 1.20–9.33 | 0.02 |
| ART use | 0.88 | 0.48–1.64 | 0.70 | — | ||
| HIV duration | 1.01 | 0.95–1.07 | 0.84 | — | ||
| BMI ≥25 kg/mb | 3.42 | 1.78–6.5 | <0.001 | 2.83 | 1.38–5.82 | 0.005 |
| LDL | 1.02 | 1.01–1.05 | 0.01 | — | ||
| HIV viral load >100,000 copies/mL | 2.88 | 0.66–12.49 | 0.16 | — | ||
| HIV stage 4c | 0.96 | 0.70–1.31 | 0.79 | — | ||
| CD4 nadird <200 cells/mm3 | 1.26 | 0.64–2.46 | 0.51 | — | ||
Versus age groups 18–54 years.
Reference BMI <25 kg/m2.
Reference HIV stages 1–3.
Reference Nadir CD4 ≥ 200 cells/mm3.
CI, confidence interval; ART, antiretroviral therapy; LDL, low-density lipoprotein; BMI, body mass index; OR, odds ratio.
Discussion
With introduction to ART, HIV infection has now evolved into a chronic disease with increased morbidity and mortality due to non-AIDs-defining conditions, including CVD.28–30 The consequences of HIV-related comorbidities have not been well documented in SSA, but are likely to be of significant magnitude considering the existing poor health systems, lack of qualified health professionals, and the sheer number of HIV-infected patients on ART in this region. Since MetS is a known risk factor for CVD and diabetes,31,32 in this study we document the burden of MetS among HIV patients with no previous screening or diagnosis of cardiovascular-related diseases. These data identify factors associated with MetS and draw attention to the substantial prevalence of unrecognized and untreated risk factors for CVD in this population.
In this study of relatively young HIV-infected adults, MetS was common and similar among HIV-infected adults who were on ART and who were ART naive. The most common MetS criteria were increased waist circumference, low HDL, and high triglyceride levels for both ART naive and ART experienced. The specific component of MetS most influenced by ART exposure was fasting glucose and HDL cholesterol. In addition to older age, high BMI and female sex were independently associated with MetS and no association was found between ART exposure or specific antiretroviral drugs and MetS. Our results suggest that traditional risk factors may be stronger predictors of MetS than ART use. Given the projected increase of the aging HIV-infected population, our findings highlight the need to incorporate CVD risk factor screening within the HIV care packages across Kenya to identify those in need of CVD risk reduction interventions irrespective of ART status.
Estimates of MetS prevalence among HIV-infected individuals, ART naive/ART treated, in SSA region are limited and the use of different diagnostic criteria for MetS has made the comparison difficult. The 16% prevalence of MetS in our study is similar to what has been reported in other studies conducted in African countries with comparable diagnostic criteria, including in Cameroon (16%),33 Burkina Faso (18%),34 and Nigeria (17%).35 However, it is lower than what was reported in other studies.11,20,36 A larger study of 850 HIV-infected adults in the United States reported MetS prevalence of 26%.20 In another study among 250 HIV-infected adults on non-nucleoside-based ART regimens in Uganda, MetS prevalence was reported at 58%; similar to our study, factors associated with MetS included older age, higher BMI, and female sex.36The discrepancy in the results is likely due to the MetS diagnosis criteria used in the Uganda study, which used two rather than three components in defining MetS, and the limited number of patients on PI class of drugs in our study (7%) versus United States (31%). The Mets prevalence observed among our participants was substantially lower than prior estimates among Kenyan adults from the urban areas, where majority of the participants qualified for MetS due to hypertension and abdominal obesity.37 The difference in patient's characteristics (urban vs. rural) and exclusion of patients with preexisting CVD-related diseases, including the known hypertensives and diabetics, may therefore have led to the underestimation of MetS prevalence in our study.
ART has been frequently associated with numerous metabolic alterations. Our finding of similar MetS frequencies between ART-experienced and -naive HIV-infected adults was therefore unexpected and contradicts those of Jantarapakde et al. who reported a higher prevalence of MetS among ART-experienced patients compared with ART naive in Thailand.17 In that study and like in many similar studies, MetS risk was strongly associated with PI and stavudine use likely due to their effects on triglycerides and blood glucose.38,39 Of note, only 15 out of 164 patients were receiving PI or/and stavudine, hence we may not have had adequate power to detect any significant effect of these drugs on MetS or its component. Nevirapine drug was associated with increased levels of HDL, an observation that has been well described by others and could partially explain the lower-than-reported prevalence of MetS among the ART-exposed group consistent with a randomized control trial in Spain, which reported an increase of HDL levels by 44% in ART-naive patients after 12 months of nevirapine therapy.41,42 Similarly, ACTG Longitudinal Linked Randomized Trials found no association among NNRTI use, NRTI use, and MetS.39 Therefore, increased use of NNRTIs, a known metabolic friendly drug in our setting, may partially explain the lack of difference in MetS rates between the two groups. Our study further supports the protective nature of ART toward CVDs as reported in the SMART study, where patients randomized in the interrupted course of ART had increased risk CVD and cardiovascular-related mortality.42 Nevertheless, a higher proportion of ART experienced than ART naive had 1 or 2 components of MetS, suggesting a potential effect of ART on specific rather than all components of MetS. Longitudinal studies are warranted to confirm these results.
Previous studies have demonstrated that longer duration of HIV infection, high viral load, and longer duration of ART confers an excess risk of MetS. In our analysis, we did not find any significant association between the duration of HIV infection and duration of ART use with MetS. A possible explanation for the results is that the duration of HIV was self-reported hence the accuracy of these data could not be confirmed. We also did not find any association between self-reported alcohol intake and tobacco use with MetS, consistent with previous reports from other studies in the region.20,35,36,43
The finding of a twofold risk of MetS in those with high BMI suggests that both overweight/obesity was the main driver for the MetS in our study. This is consistent with a previous finding from the region that showed increased risk of MetS among overweight and obese patients both in general and HIV-infected populations.35,43 A high proportion of women in our study were overweight/obese compared with men, which may partially explain increased prevalence of MetS among females. Kenya, like many other African countries, is undergoing epidemiological transition, and epidemic rates of obesity and other metabolic abnormalities have been noted during recent years.44 A recent population-wide survey in Kenya reported overweight/obesity prevalence of 28%, which disproportionally affected women.5 This prevalence was lower than that found in our study (41%) raising a concern about the possible influence of HIV infection and other unique HIV-related characteristics in addition to lifestyle behaviors (i.e., poor diet and sedentary lifestyle) on obesity and consequently MetS.
Abrahams et al. reported an increase in weight and body fat distribution changes in black South African HIV-infected adults after initiation of ART.45 They demonstrated that percentage of trunk fat and the waist circumference in women, but not men, increased significantly months after ART initiation. Our finding that mean waist circumference was significantly increased in treated women, but compared with untreated women is in keeping with the findings of this study. By contrast, the study found an overall increase in weight after ART initiation. We showed that mean BMI was higher in ART-treated patients, but the difference was not statistically significant likely due to the exclusion of patients with cardiometabolic disease.
Our study had several strengths. It is the first study of prevalence of MetS among HIV-infected adults stratified by ART use in Kenya. Since it was conducted before universal treatment, it provides a rare opportunity to compare the effect of commonly used antiretroviral drugs on risk of MetS, a risk factor for CVD and diabetes. Also, it examined adults without known diagnosis of CVD-related diseases, thus showing the true risk of MetS before onset of CVD. We had complete data for all parameters required to make diagnosis of MetS. Thus, our study results can be generalizable to similar settings. Limitation of our study included the cross-sectional design, which prohibited a definitive determination of the temporal association between some independent variables and MetS. This current study examined the role of current self-reported behaviors (i.e., smoking, alcohol consumption etc.), which may not entirely reflect past behaviors that may have influenced the development of MetS. We were not able to assess the association of current CD4 cell count, viral load with MetS in our cohort because those data were not available. The exclusion of patients with preexisting CVD-related diseases, including the known hypertensives, a component for MetS, may have resulted in underestimation of the true prevalence of MetS in this population. Likewise, inclusion of HIV-negative adults would have made it possible to compare biochemical changes due to HIV infection in the absence of treatment.
MetS was prevalent among ART-naive and ART-experienced HIV-infected adults without preexisting cardiometabolic disorders, and traditional risk factors most strongly influenced the MetS diagnosis. Our findings emphasize the importance of addressing traditional risk factors for metabolic abnormalities as the HIV-infected populations' age. Future longitudinal studies comparing HIV-uninfected and infected adults are needed to confirm these results and to assess the predictive role of MetS toward CVD in HIV-infected populations in SSA.
Acknowledgments
This project was supported by NIH Research Training Grant R25TW009337 funded by the Fogarty International Center. The authors acknowledge Belinda Korir and the Cardiovascular and Pulmonary Disease Center of Excellence in Kenya (HHSN268200900031C), President's Emergency Plan for AIDS Relief (PEPFAR)/USAID (AID-623-A-12-0001). A.O. is supported by the Fogarty International Center and The Office of Research on Women's Health (ORWH) of the National Institutes of Health under Award K43TW010363. T.T. is supported by grant R21TW010459-02S1 from the Fogarty International Center of the National Institutes of Health (NIH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Trickey A, May MT, Vehreschild JJ, et al. . Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet HIV 2017;4:e349–e356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nsanzimana S, Remera E, Kanters S, et al. . Life expectancy among HIV-positive patients in Rwanda: A retrospective observational cohort study. Lancet Glob Health 2015;3:e169–e177 [DOI] [PubMed] [Google Scholar]
- 3.Mills EJ, Bakanda C, Birungi J, et al. . Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: A cohort analysis from Uganda. Ann Intern Med 2011;155:209–216 [DOI] [PubMed] [Google Scholar]
- 4.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: Comparisons with general population. Curr Opin HIV AIDS 2016;1:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health, Division of Non-Communicable Diseases. Kenya STEPwise survey for non-communicable diseases risk factors 2015 report. Available at: http://aphrc.org/wp-content/uploads/2016/04/Steps-Report-NCD-2015.pdf (Last accessed February2018)
- 6.Friis-Moller N, Sabin CA; Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group. Combination anti- retroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993–200314627784 [Google Scholar]
- 7.Holmberg SD, Tong TC; HIV Outpatient Study (HOPS) Investigators. Protease inhibitor drug use and adverse cardiovascular outcomes in ambulatory HIV-infected persons. Lancet 2002;360:1747–1748 [DOI] [PubMed] [Google Scholar]
- 8.Mary-Krause M, Cotte L, Simon A; Clinical Epidemiology Group from the French Hospital Database. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS 2003;17:2479–2486 [DOI] [PubMed] [Google Scholar]
- 9.Freiberg MS, Chang CC, Kuller LH, et al. . HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;173:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus JL, Leyden WA, Chao CR, et al. . HIV infection and incidence of ischemic stroke. AIDS 2014;28:1911–1919 [DOI] [PubMed] [Google Scholar]
- 11.Hanna DB, Jung M, Xue X, et al. . Trends in non-lipid cardiovascular disease risk factor management in the Women's Interagency HIV Study and association with adherence to antiretroviral therapy. AIDS Patient Care STDS 2016;30:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—A new worldwide definition. Lancet 2005;366:1059–1062 [DOI] [PubMed] [Google Scholar]
- 13.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all causes: Findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis 2004;17:309–314 [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among US adults. Diabetes Care 2004;27:2444–2449 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen KA, Peer N, Mills EJ, et al. . A meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS One 2016;11:e0150970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naidu S, Ponnampalvanar S, Kamaruzzaman SB, et al. . Prevalence of metabolic syndrome among people living with HIV in developing countries: A systematic review. AIDS Patient Care STDS 2017;31:1–3 [DOI] [PubMed] [Google Scholar]
- 17.Jantarapakde J, Phanuphak N, Chaturawit C, et al. . Prevalence of metabolic syndrome among antiretroviral-naive and antiretroviral-experienced HIV-1 infected Thai adults. AIDS Patient Care STDS 2014;28:331–340 [DOI] [PubMed] [Google Scholar]
- 18.Sobieszczyk ME, Werner L, Mlisana K, et al. . Metabolic syndrome after HIV acquisition in South African women. J Acquir Immune Defic Syndr 2016;73:438–445 [DOI] [PubMed] [Google Scholar]
- 19.Drelichowska J, Kwiatkowska W, Knysz B, et al. . Metabolic syndrome in HIV-positive patients. HIV AIDS Rev 2015;14:35–41 [Google Scholar]
- 20.Mondy K, Overton ET, Grubb J, et al. . Metabolic syndrome in HIV-infected patients from an urban, midwestern US outpatient population. Clin Infect Dis 2007;44:726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Temu TM, Kirui N, Wanjalla C, et al. . Cardiovascular health knowledge and preventive practices in people living with HIV in Kenya. BMC Infect Dis 2015;15:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inui TS, Nyandiko WM, Kimaiyo SN, et al. . AMPATH: Living proof that no one has to die from HIV. J Gen Intern Med 2007;22:1745–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Einterz RM, Kimaiyo S, Mengech HNK, et al. . Responding to the HIV pandemic: The power of an academic medical partnership. Acad Med 2007;82:812–818 [DOI] [PubMed] [Google Scholar]
- 24.Bloomfield GS, Kimaiyo S, Carter EJ, et al. . Chronic non-communicable cardiovascular and pulmonary disease in sub-Saharan Africa: An academic model for countering the epidemic. Am Heart J 2011;161:842–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberti KG, Eckel RH, Grundy SM, et al. . Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 26.Smith Jr SC, Benjamin EJ, Bonow RO, et al. . World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011;124:2458–2473 [DOI] [PubMed] [Google Scholar]
- 27.NASCOP Kenya guidelines for antiretroviral drug therapy, 4th edition 2011. Available at: http://healthservices.uonbi.ac.ke/sites/default/files/centraladmin/healthservices/Kenya%20Treatment%20Guidelines%202011.pdf (Last accessed February15, 2018)
- 28.Benjamin LA, Corbett EL, Connor MD, et al. . HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: A case–control study. Neurology 2016;86:324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friis-Møller N, Thiébaut R, Reiss P, et al. . Predicting the risk of cardiovascular disease in HIV-infected patients: The data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil 2010;17:491–501 [DOI] [PubMed] [Google Scholar]
- 30.Triant VA, Lee H, Hadigan C, et al. . Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gami AS, Witt BJ, Howard DE, et al. . Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 2007;49:403–414 [DOI] [PubMed] [Google Scholar]
- 32.Isomaa BO, Almgren P, Tuomi T, et al. . Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001;24:683–689 [DOI] [PubMed] [Google Scholar]
- 33.Bekolo CE, Nguena MB, Ewane L, et al. . The lipid profile of HIV-infected patients receiving antiretroviral therapy in a rural Cameroonian population. BMC Public Health 2014;14:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guira O, Tiéno H, Diendéré AE, et al. Features of metabolic syndrome and its associated factors during highly active antiretroviral therapy in Ouagadougou (Burkina Faso). J Int Assoc Provid AIDS Care 2016;15:159–163 [DOI] [PubMed] [Google Scholar]
- 35.Ayodele OE, Akinboro AO, Akinyemi SO, et al. . Prevalence and clinical correlates of metabolic syndrome in Nigerians living with human immunodeficiency virus/acquired immunodeficiency syndrome. Metab Syndr Relat Disord 2012;10:373–379 [DOI] [PubMed] [Google Scholar]
- 36.Muyanja D, Muzoora C, Muyingo A, et al. . High prevalence of metabolic syndrome and cardiovascular disease risk among people with HIV on stable ART in Southwestern Uganda. AIDS Patient Care STDS 2016;30:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omuse G, Maina D, Hoffman M, et al. . Metabolic syndrome and its predictors in an urban population in Kenya: A cross sectional study. BMC Endocr Disord 2017;17:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maloberti A, Giannattasio C, Dozio D, et al. . Metabolic syndrome in human immunodeficiency virus–positive subjects: Prevalence, phenotype, and related alterations in arterial structure and function. Metab Syndr Relat Disord 2013;11:403–411 [DOI] [PubMed] [Google Scholar]
- 39.Krishnan S, Schouten JT, Atkinson B, et al. . Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naive HIV-infected individuals. J Acquir Immune Defic Syndr 2012;61:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franssen R, Sankatsing RR, Hassink E, et al. . Nevirapine increases high-density lipoprotein cholesterol concentration by stimulation of apolipoprotein AI production. Arterioscler Thromb Vasc Biol 2009;29:1336–1341 [DOI] [PubMed] [Google Scholar]
- 41.Van der Valk M, Kastelein JJ, Murphy RL, et al. . Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS 2001;15:2407–2414 [DOI] [PubMed] [Google Scholar]
- 42.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count–guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–2296 [DOI] [PubMed] [Google Scholar]
- 43.Tchounga BK, Hønge BL; IeDEA West Africa collaboration. Effect of sex and age on outcomes among HIV-2-infected patients starting antiretroviral therapy in West Africa. AIDS 2016;30:2707–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bloomfield GS, Hogan JW, Keter A, et al. . Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS One 2011;6:e22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrahams Z, Levitt N, Lesosky M, et al. . Changes in body fat distribution on dual-energy x-ray absorptiometry in black South Africans starting first-line antiretroviral therapy. AIDS Patient Care STDS 2016;30:455–462 [DOI] [PubMed] [Google Scholar]