Abstract
Grain size is important for brewing-rice cultivars, but the genetic basis for this trait is still unclear. This paper aims to identify QTLs for grain size using novel chromosomal segment substitution lines (CSSLs) harboring chromosomal segments from Yamadanishiki, an excellent sake-brewing rice, in the genetic background of Koshihikari, a cooking cultivar. We developed a set of 49 CSSLs. Grain length (GL), grain width (GWh), grain thickness (GT), 100-grain weight (GWt) and days to heading (DTH) were evaluated, and a CSSL-QTL analysis was conducted. Eighteen QTLs for grain size and DTH were identified. Seven (qGL11, qGWh5, qGWh10, qGWt6-2, qGWt10-2, qDTH3, and qDTH6) that were detected in F2 and recombinant inbred lines (RILs) from Koshihikari/Yamadanishiki were validated, suggesting that they are important for large grain size and heading date in Yamadanishiki. Additionally, QTL reanalysis for GWt showed that qGWt10-2 was only detected in early-flowering RILs, while qGWt5 (in the same region as qGWh5) was only detected in late-flowering RILs, suggesting that these QTLs show different responses to the environment. Our study revealed that grain size in the Yamadanishiki cultivar is determined by a complex genetic mechanism. These findings could be useful for the breeding of both cooking and brewing rice.
Keywords: grain size, QTL, CSSLs, brewing-rice cultivar, QTL-by-environment interaction
Introduction
Rice (Oryza sativa L.) is one of the most important crops in the world. It is not only a staple food for the Japanese population, but also the raw material for the alcoholic beverage known as sake. Brewing-rice cultivars have characteristic traits adapted to sake brewing, such as large grain size and a high percentage of white-core grain. These traits are favorable for high-grade grain polishing (Aramaki et al. 1995), fast water absorption (Horigane et al. 2014, Nagato and Ebata 1959) and amylolysis efficiency in the process of sake brewing (Yanagiuchi et al. 1997). As such they are important targets for the breeding of a brewing cultivar. Yamadanishiki is of very high quality and is the highest-yielding brewing-rice cultivar in Japan; therefore, Okada et al. (2017) and Yoshida et al. (2002) used Yamadanishiki as a crossing parent to conduct QTL analysis of favorable traits for sake brewing. These papers detected common QTLs for grain length on chromosomes 4 and 11, and found the QTL for grain width and weight on chromosome 5. However, it is still necessary to verify these putative QTLs, so they can be used in the breeding of sake-brewing rice. Because grain size is associated with yield, the QTLs for large grain size in brewing-rice cultivars may also facilitate the breeding of high-yield cooking cultivars.
A set of chromosomal segment substitution lines (CSSLs) has a genetic background that is almost completely homogenous to the recipient parent, except with one chromosomal segment from the donor parent. A complete CSSL set represents the entire genome of the donor reproduced in the background of the recipient. Therefore, the CSSLs can be used to evaluate the genetic effects derived from the donor in detail, and to elucidate the complex genetic mechanisms behind agronomic traits (Ebitani et al. 2005). A large number of CSSL sets have been developed to identify the QTLs for the agronomic traits of rice, such as grain size and heading date (Ando et al. 2008, Bian et al. 2010, Ebitani et al. 2005, Furuta et al. 2014, Murata et al. 2014).
Yamasaki and Ideta (2013) classified the Japanese paddy rice population into six subgroups: Kirara397, Reimei, Nipponbare, Koshihikari, Asahi, and Kamenoo. The brewing-rice cultivars, Omachi, Yamadanishiki, and Gohyakumangoku belong to the Kamenoo subgroup. Since Yamadanishiki has been the most popular and highest-yielding brewing cultivar in recent years, it was selected for the genetic analysis (Okada et al. 2017) and breeding of novel brewing cultivars (Kaji et al. 2013). There is a large genetic difference between the Koshihikari and Yamadanishiki cultivars in the Japanese rice population (Yamasaki and Ideta 2013); therefore, the use of a Koshihikari/Yamadanishiki segregating population to conduct genetic analysis of their agronomic traits could contribute to the identification of new QTLs and to next-generation rice breeding.
There are three elements that determine rice grain size: grain length (GL), grain width (GWh), and grain thickness (GT). Many QTLs for grain size have recently been detected (Huang et al. 2013). Moreover, Nagata et al. (2015) identified a large number of QTLs for GL and GWh despite using mapping populations of a single-crossing combination derived from Koshihikari and IR64. They indicated that grain shape was controlled by many QTLs, which suggests a complex genetic mechanism. Reviews by Zuo and Li (2014) and Zheng et al. (2015) indicate that rice grain size is determined by: a proteasomal degradation pathway related to genes such as GW2 (Song et al. 2007) and GW5/qSW5 (Shomura et al. 2008, Weng et al. 2008), a G-protein signaling pathway related to genes such as GS3 (Fan et al. 2006, Takano-Kai et al. 2009), a phytohormone pathway related to genes such as TGW6 (Ishimaru et al. 2013) and OsBRI1 (Morinaka et al. 2006), and other pathways related to genes such as GS5 (Li et al. 2011) and GW8 (Wang et al. 2012a). Because the characteristics of these pathways are unclear, it is necessary to define the mechanisms controlling grain size.
In this study, we report the development of novel CSSLs, i.e., chromosomal segments from the most popular brewing-rice cultivar, Yamadanishiki, in the genetic background of Koshihikari, an elite Japanese cooking cultivar. By examining these CSSLs for grain size and heading date, we identified novel QTLs and validated several other QTLs that had previously been detected using F2 and recombinant inbred lines (RILs) derived from Koshihikari and Yamadanishiki crosses (Okada et al. 2017). In addition, one of the major QTLs for 100-grain weight (GWt) detected by the RILs was not identified in the CSSL-QTL analysis, suggesting that this QTL might be affected by the environment. To verify the effect of the environment on the QTLs for GWt, we reanalyzed the Koshihikari/Yamadanishiki RILs (Okada et al. 2017).
Materials and Methods
Development of CSSLs
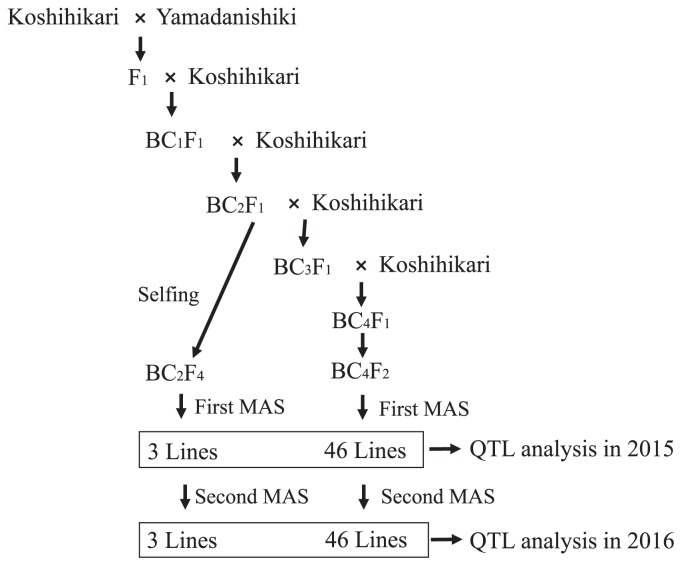
The CSSL development process is illustrated in Fig. 1. Koshihikari was crossed with Yamadanishiki, and the resultant F1 was backcrossed with Koshihikari. Two or four generations of backcrossing yielded BC2F1 and BC4F1. The BC2F1 population produced 156 plants in the BC2F4 generation after four generations of self-pollination. In the BC4F2 generation, 2,136 plants were generated from 89 plants from the BC4F1 generation. The leaves of the BC2F4 and BC4F2 populations were collected and DNA was extracted using the method described by Dellaporta et al. (1983) with minor modifications. One hundred and seventy-eight bulked BC4F1 DNA samples were produced by grouping the samples from BC4F2 plants in batches of 12, using an automated pipetting machine (epMotion 5070; Eppendorf, Hamburg, Germany). We performed a whole-genome survey (First MAS) of the BC2F4 and the bulked BC4F1 samples (Fig. 1), using 125 DNA markers including: 71 simple sequence repeat (SSR) markers, 36 cleaved amplified polymorphic sequence (CAPS) markers, 17 derived CAPS (dCAPS) markers, and one PCR-confronting two-pair primer (PCR-CTPP) marker (Supplemental Table 1). The average distance between adjacent markers was about 3.11 Mb. The CAPS and dCAPS markers were constructed from the linkage maps of Koshihikari/Yamadanishiki (Okada et al. 2017). After selecting heterozygous lines from the bulked BC4F1 samples, homozygous plants from BC4F2 were selected as candidate CSSLs (First MAS, Fig. 1). This resulted in 49 CSSL candidate plants derived from the BC2F4 and BC4F2 populations. Furthermore, the CSSLs cultivated in 2015 were selected to decrease foreground and background heterozygosity (Second MAS, BC2F5 and BC4F3 generations, Fig. 1). The CSSLs from both years were genotyped using an array of 768 SNPs selected from Nagasaki et al. (2010) and Yamamoto et al. (2010), using the BeadStation 500G system (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions (Supplemental Table 2).
Fig. 1.
Developmental scheme of the CSSLs for Yamadanishiki in the genetic background of Koshihikari. First MAS indicates the first round of marker-assisted selection (MAS), the whole-genome survey that was conducted using 125 DNA markers, while the second MAS was conducted to clear foreground and background heterozygosity.
Trait evaluation
For trait evaluation, the 49 CSSLs were sown on 7 May 2015 and 6 May 2016, and 24 plants per line were transplanted to an experimental field at Kobe University, Food Resources Education and Research Center (Kasai City, Hyogo Prefecture Japan; 34.88°N, 134.86°E) on 6 June 2015 and 4 June 2016. We evaluated the grain traits and days to heading (DTH) of six plants per CSSL in both 2015 and 2016.
The plants were harvested over 45 days after their flowering date, and the harvested grains were air-dried for three days. After the grains were dehulled, we selected 100 grains per plant, excluding broken and immature grains, and measured the following grain traits: GL, GWh, GT, and GWt. The first three were measured using a RGQI20A rice grain analyzer (SATAKE Corporation, Higashi-Hiroshima City, Hiroshima Prefecture, Japan), and GWt was measured using an electric balance to an accuracy of 0.01 g. DTH was defined as the number of days from the sowing date to the initial flowering date.
QTL identification by CSSL-QTL analysis
To identify QTLs, we first performed Dunnett’s multiple comparisons test to compare Koshihikari with each CSSL at the significance level of P < 0.05, using R (ver. 3.2.0, R Core Team 2015). Next, a regression analysis was conducted with 51 lines (the CSSLs and their parents), using the BayesC model in the R package “VIGoR” (Onogi and Iwata 2016). BayesC is a variable selection method that infers the probability of being included in the regression model (i.e., inclusion probability) for each marker (Habier et al. 2011). The SNP genotypes were coded additively: 0 indicates homozygous Koshihikari alleles, 1 indicates heterozygosity, and 2 indicates homozygous Yamadanishiki alleles. Prior to analysis, missing genotypes were imputed as follows: when both of the genotypes adjacent to the missing genotype were the same, the missing genotype was imputed as the same genotype; when the adjacent genotypes differed, the missing genotype was imputed as the average of the adjacent genotypes; and when the chromosome end was missing, the missing genotype was imputed as the genotype before the chromosome end. In total, 0.11% of genotypes were missing and imputed. To ensure robust QTL detection, we conducted a sub-sampling procedure using BayesC. We randomly selected 80% of the lines and inferred the inclusion probability and the marker effect by fitting with BayesC. We repeated this subsampling 1,000 times and calculated the average of the inclusion probability and the marker effect for each marker. The prior distributions from BayesC were determined using the function “hyperpara” in the VIGoR package, with the assumption that 3% of markers were included in the model and that the included markers explained all the phenotypic variance observed. Statistical significance was assessed using permutation tests. First, we permutated the phenotypes, conducted the subsampling procedure 1,000 times as described above, and calculated the average inclusion probabilities for each marker. We then repeated this permutation test 1,000 times and obtained the null distribution of the average inclusion probability. Significance levels were set to 1% and 5%. We identified robust QTLs using both methods in both years, and named these QTLs according to the nomenclature guidelines by McCouch et al. (1997).
QTL reanalysis of RILs
Okada et al. (2017) performed a QTL analysis for grain traits using RILs derived from Koshihikari/Yamadanishiki. Since the CSSLs in the present study revealed unexpected reactivity in a major QTL for GWt on chromosome 5 that was detected by Okada et al. (2017) and Yoshida et al. (2002), we focused on the distribution of DTH. The histograms of DTH for these RILs revealed two peaks (Supplemental Fig. 1). The population of 190 RILs was divided into 88 early-flowering lines (eRILs), 92 late-flowering lines (lRILs), and 10 residual lines (rRILs; Supplemental Fig. 1). QTL analysis for GWt in the eRILs and lRILs from both years of the study (2013 and 2014) was conducted. Windows QTL cartographer 2.5 (Wang et al. 2012b) was used for QTL analysis, and QTLs were detected using the composite interval mapping method (Zeng 1994) with a window size and walk speed of 5 cM and 1 cM, respectively. The empirical threshold values as determined by 1,000 permutation tests were significant at the 5% level (Churchill and Doerge 1994).
Results
Characteristics of the CSSLs
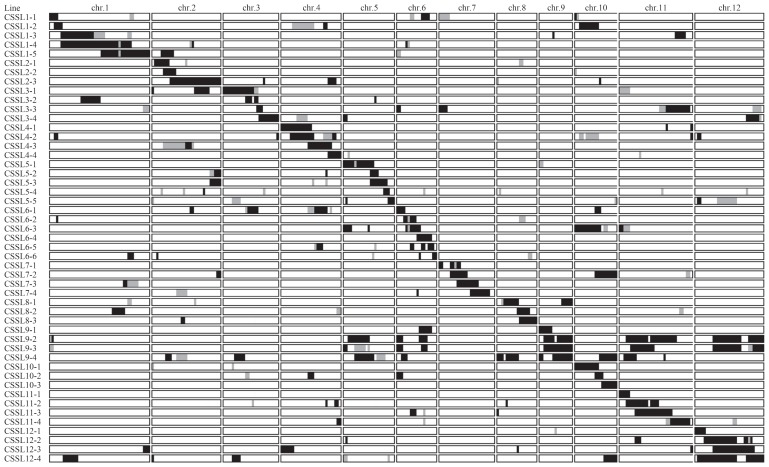
The present study reveals the development of 49 CSSLs harboring chromosomal segments from Yamadanishiki in the Koshihikari genetic background (Fig. 2, Supplemental Table 2). The CSSLs contained the target chromosomal region, as well as non-target regions, from Yamadanishiki. Given that recombination events occur at the midpoint between two adjacent markers, the non-target regions ranged from 0–74.7 Mb with a mean length of 13.7 Mb; however, on average, each CSSL contained 93% of the Koshihikari genome (Fig. 2, Supplemental Table 2). When combined, the CSSLs covered more than 98% of the Yamadanishiki genome, although there were gaps in the target regions on chromosomes 2 (1.0 Mb), 7 (2.1 Mb and 0.5 Mb), 8 (2.0 Mb), and 11 (1.3 Mb). These gaps were partially covered by non-target regions; e.g., the gap on chromosome 2 was partially covered by CSSL3-1 or CSSL12-4 (Fig. 2, Supplemental Table 2).
Fig. 2.
Graphical genotypes of the 49 Yamadanishiki CSSLs in the Koshihikari genetic background, from 2016. Black and white bars indicate fragments homozygous with Yamadanishiki and Koshihikari, respectively. Gray bars represent heterozygous segments, and striped bars indicate missing data.
Trait evaluation and QTL identification using the CSSLs
Nine and four of the CSSLs had significantly longer and shorter average GLs than Koshihikari, respectively (Table 1). To consider the genetic effects of both target and non-target chromosomal regions, we identified robust QTLs using two methods: Dunnett’s multiple comparison and BayesC model regression analysis (Fig. 2, Table 1, Supplemental Fig. 2). Four QTLs on chromosomes 6 (qGL6-1 and qGL6-2), 10 (qGL10), and 11 (qGL11) were identified (Table 2). All these QTLs increased GL in the CSSLs carrying the Yamadanishiki allele. However, we did not identify any QTLs in the Yamadanishiki allele that decreased GL (Table 2, Supplemental Fig. 2B). CSSL11-3, which harbors qGL11, showed the longest GL among the CSSLs carrying the Yamadanishiki allele in the target QTL regions (CSSL6-3, 6-4, 6-5, 10-1, and 11-3). Thus, we assumed that qGL11 has the largest effect on GL (Table 1). qGL4-2 on chromosome 4, which was one of the major QTLs for GL as reported by Okada et al. (2017) was unstable in the CSSLs.
Table 1.
The phenotypic average values of each CSSL and parents and comparison between Koshihikari and each CSSL in 2015 and 2016
| Line | Grain length (mm) | Grain width (mm) | Grain thickness (mm) | 100-grain weight (g) | Days to heading (days) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | |
| CSSL1-1 | 5.34 *** | 5.27 | 3.04 | 2.99 | 2.01 *** | 2.04 *** | 2.37 *** | 2.26 *** | 92 ** | 91 *** |
| CSSL1-2 | 5.25 | 5.18 ** | 3.10 *** | 3.01 | 2.02 *** | 2.04 *** | 2.37 *** | 2.25 *** | 92 ** | 90 *** |
| CSSL1-3 | 5.28 | 5.23 | 3.05 | 2.96 | 2.01 *** | 2.01 *** | 2.34 *** | 2.19 | 92 * | 91 *** |
| CSSL1-4 | 5.23 | 5.17 *** | 3.04 | 3.00 | 2.02 *** | 2.05 *** | 2.30 *** | 2.21 | 94 | 93 |
| CSSL1-5 | 5.24 | 5.23 | 3.06 * | 2.98 | 2.02 *** | 2.01 *** | 2.32 *** | 2.20 | 92 ** | 91 *** |
| CSSL2-1 | 5.22 | 5.21 | 3.04 | 2.97 | 2.00 *** | 2.01 *** | 2.24 | 2.18 | 92 * | 91 *** |
| CSSL2-2 | 5.24 | 5.23 | 3.05 | 2.97 | 2.02 *** | 2.03 *** | 2.31 *** | 2.23 * | 92 ** | 91 *** |
| CSSL2-3 | 5.32 *** | 5.19 | 2.93 *** | 2.90 *** | 1.98 *** | 2.04 *** | 2.26 | 2.22 | 102 *** | 101 *** |
| CSSL3-1 | 5.30 | 5.29 * | 3.05 | 2.99 | 2.03 *** | 2.10 *** | 2.37 *** | 2.34 *** | 99 *** | 91 *** |
| CSSL3-2 | 5.21 | 5.14 *** | 3.09 *** | 3.00 | 2.05 *** | 2.04 *** | 2.38 *** | 2.19 | 92 ** | 91 *** |
| CSSL3-3 | 5.28 | 5.24 | 3.05 | 2.97 | 2.02 *** | 2.03 *** | 2.34 *** | 2.21 | 92 *** | 91 *** |
| CSSL3-4 | 5.22 | 5.20 | 3.11 *** | 3.00 | 1.98 *** | 1.97 | 2.30 *** | 2.21 | 117 *** | 113 *** |
| CSSL4-1 | 5.26 | 5.20 | 3.03 | 2.96 | 2.02 *** | 2.02 *** | 2.31 *** | 2.18 | 92 ** | 91 *** |
| CSSL4-2 | 5.26 | 5.18 ** | 3.08 *** | 3.00 | 2.02 *** | 2.02 *** | 2.40 *** | 2.23 * | 91 *** | 89 *** |
| CSSL4-3 | 5.19 * | 5.16 *** | 3.12 *** | 3.07 *** | 2.04 *** | 2.03 *** | 2.40 *** | 2.28 *** | 91 *** | 90 *** |
| CSSL4-4 | 5.36 *** | 5.29 * | 3.04 | 2.97 | 2.02 *** | 2.01 *** | 2.40 *** | 2.24 ** | 91 *** | 91 *** |
| CSSL5-1 | 5.28 | 5.23 | 3.04 | 2.94 * | 1.98 *** | 2.01 *** | 2.33 *** | 2.16 | 93 | 92 ** |
| CSSL5-2 | 5.27 | 5.19 | 3.06 * | 2.95 | 2.02 *** | 2.03 *** | 2.37 *** | 2.18 | 92 *** | 91 *** |
| CSSL5-3 | 5.25 | 5.20 | 3.06 * | 2.96 | 2.00 *** | 2.01 *** | 2.33 *** | 2.16 | 92 ** | 91 *** |
| CSSL5-4 | 5.28 | 5.23 | 3.07 *** | 2.98 | 2.02 *** | 2.00 ** | 2.38 *** | 2.20 | 91 *** | 90 *** |
| CSSL5-5 | 5.22 | 5.18 ** | 3.11 *** | 3.03 ** | 2.05 *** | 2.06 *** | 2.39 *** | 2.25 *** | 91 *** | 90 *** |
| CSSL6-1 | 5.16 *** | 5.07 *** | 3.13 *** | 3.03 ** | 2.05 *** | 2.03 *** | 2.41 *** | 2.19 | 89 *** | 86 *** |
| CSSL6-2 | 5.33 *** | 5.24 | 3.09 *** | 3.01 | 2.00 *** | 2.01 *** | 2.41 *** | 2.24 ** | 93 | 93 |
| CSSL6-3 | 5.44 *** | 5.34 *** | 3.14 *** | 3.01 | 2.06 *** | 2.04 *** | 2.58 *** | 2.32 *** | 93 | 94 |
| CSSL6-4 | 5.40 *** | 5.30 *** | 3.05 | 2.96 | 2.01 *** | 1.99 | 2.42 *** | 2.20 | 92 ** | 92 ** |
| CSSL6-5 | 5.36 *** | 5.30 ** | 3.10 *** | 2.99 | 2.04 *** | 2.00 * | 2.44 *** | 2.24 ** | 92 *** | 92 ** |
| CSSL6-6 | 5.34 *** | 5.26 | 3.16 *** | 3.02 | 2.06 *** | 2.00 * | 2.52 *** | 2.23 * | 91 *** | 92 ** |
| CSSL7-1 | 5.29 | 5.20 | 3.08 *** | 3.00 | 2.01 *** | 2.02 *** | 2.38 *** | 2.25 *** | 92 * | 92 ** |
| CSSL7-2 | 5.27 | 5.15 *** | 3.12 *** | 3.05 *** | 2.04 *** | 2.06 *** | 2.46 *** | 2.28 *** | 98 *** | 91 *** |
| CSSL7-3 | 5.26 | 5.19 | 3.10 *** | 2.99 | 1.98 *** | 2.00 ** | 2.40 *** | 2.19 | 92 * | 93 |
| CSSL7-4 | 5.20 * | 5.15 *** | 3.06 ** | 3.00 | 1.95 * | 2.04 *** | 2.30 *** | 2.21 | 93 | 92 * |
| CSSL8-1 | 5.29 | 5.21 | 3.01 | 2.88 *** | 1.94 | 1.99 | 2.26 | 2.05 *** | 92 ** | 92 *** |
| CSSL8-2 | 5.27 | 5.22 | 3.08 *** | 2.98 | 2.03 *** | 2.05 *** | 2.39 *** | 2.21 | 91 *** | 90 *** |
| CSSL8-3 | 5.26 | 5.18 ** | 3.08 *** | 2.99 | 2.00 *** | 2.03 *** | 2.35 *** | 2.18 | 92 ** | 91 *** |
| CSSL9-1 | 5.30 | 5.28 | 3.03 | 3.01 | 1.98 *** | 2.03 *** | 2.30 *** | 2.24 ** | 92 ** | 92 *** |
| CSSL9-2 | 5.48 *** | 5.43 *** | 3.00 | 2.97 | 1.97 *** | 2.00 ** | 2.38 *** | 2.25 *** | 93 | 91 *** |
| CSSL9-3 | 5.43 *** | 5.38 *** | 3.06 | 3.00 | 1.96 *** | 2.03 *** | 2.39 *** | 2.26 *** | 92 *** | 91 *** |
| CSSL9-4 | 5.20 * | 5.15 *** | 3.17 *** | 3.06 *** | 2.03 *** | 2.05 *** | 2.42 *** | 2.26 *** | 90 *** | 86 *** |
| CSSL10-1 | 5.32 ** | 5.28 * | 3.09 *** | 3.02 | 2.02 *** | 2.06 *** | 2.46 *** | 2.31 *** | 92 ** | 92 * |
| CSSL10-2 | 5.25 | 5.18 * | 3.11 *** | 3.05 *** | 2.00 *** | 2.05 *** | 2.38 *** | 2.25 *** | 89 *** | 86 *** |
| CSSL10-3 | 5.26 | 5.21 | 3.14 *** | 3.04 ** | 2.05 *** | 2.08 *** | 2.47 *** | 2.30 *** | 91 *** | 90 *** |
| CSSL11-1 | 5.30 | 5.24 | 3.08 *** | 2.99 | 1.99 *** | 2.04 *** | 2.39 *** | 2.23 * | 93 | 90 *** |
| CSSL11-2 | 5.28 | 5.22 | 3.08 *** | 2.96 | 2.02 *** | 2.02 *** | 2.36 *** | 2.18 | 92 *** | 91 *** |
| CSSL11-3 | 5.44 *** | 5.36 *** | 3.06 * | 3.01 | 1.97 *** | 2.01 *** | 2.39 *** | 2.26 *** | 93 | 93 |
| CSSL11-4 | 5.36 *** | 5.28 | 3.03 | 2.98 | 2.01 *** | 2.03 *** | 2.38 *** | 2.23 * | 92 ** | 91 *** |
| CSSL12-1 | 5.30 | 5.30 ** | 3.09 *** | 3.05 *** | 2.02 *** | 2.04 *** | 2.41 *** | 2.31 *** | 92 * | 90 *** |
| CSSL12-2 | 5.35 *** | 5.28 * | 3.04 | 2.97 | 1.99 *** | 2.02 *** | 2.36 *** | 2.21 | 93 | 90 *** |
| CSSL12-3 | 5.35 *** | 5.20 | 3.04 | 2.96 | 1.96 *** | 2.00 ** | 2.34 *** | 2.14 | 93 | 91 *** |
| CSSL12-4 | 5.28 | 5.22 | 3.01 | 2.98 | 1.99 *** | 2.03 *** | 2.30 *** | 2.21 | 92 * | 90 *** |
| Koshihikari | 5.25 | 5.24 | 3.02 | 2.98 | 1.92 | 1.97 | 2.20 | 2.16 | 95 | 94 |
| Yamadanishiki | 5.59 | 5.54 | 3.27 | 3.23 | 2.07 | 2.07 | 2.80 | 2.72 | 110 | 108 |
Dunnett’s multiple comparison test was conducted for each trait to compare Koshihikari with each CSSL, and “*”, “**” and “***” represented significance at P < 0.05, P < 0.01 and P < 0.001, respectively.
Table 2.
Identified QTL for grain traits
| Trait | QTL | Position (Mb) | Marker interval | Allelic effecta | Refferenceb | Representitive CSSLc |
|---|---|---|---|---|---|---|
| GL | qGL6-1 | 18.9–23.3 | ac06000665-RM1340 | ↑ | 12 | CSSL6-3 |
| qGL6-2 | 27.39–27.88 | aa06001119-aa06001139 | ↑ | 6 | CSSL6-4, 6-5 | |
| qGL10 | 2.15 | aa10000749 | ↑ | CSSL10-1 | ||
| qGL11 | 16.96–21.06 | aa11003403-aa11004494 | ↑ | 5, 16 | CSSL11-3 | |
|
| ||||||
| GWh | qGWh2 | 25.59 | aa02002928 | ↓ | 2 | CSSL2-3 |
| qGWh4 | 20.03–23.13 | RM1359-ab04001157 | ↑ | 5 | CSSL4-3 | |
| qGWh5 | 28.22–28.99 | ac05000341-aa05001022 | ↑ | 5, 14, 16 | CSSL5-5 | |
| qGWh10 | 18.01–20.36 | RM6704-aa10003274 | ↑ | 2, 8, 15, 16 | CSSL10-2, 10-3 | |
|
| ||||||
| GT | qGT3 | 9.29–18.88 | ac03000229-aa03002121 | ↑ | 9 | CSSL3-1, 3-2 |
| qGT10-1 | 12.45–13.06 | aa10002652-ac10000368 | ↑ | CSSL10-1, 10-2 | ||
| qGT10-2 | 18.55 | ac10000429 | ↑ | 7 | CSSL10-2, 10-3 | |
|
| ||||||
| GWt | qGWt6-1 | 9.14–11.84 | ac06000397-ac06000592 | ↑ | 1 | CSSL6-2, 6-3 |
| qGWt6-2 | 30.97 | RM5753 | ↑ | 13, 16 | CSSL6-6 | |
| qGWt7 | 4.77–7.18 | aa07001807-aa07001842 | ↑ | 3 | CSSL7-1, 7-2 | |
| qGWt10-1 | 2.15–10.26 | aa10000749-aa10001539 | ↑ | CSSL10-1 | ||
| qGWt10-2 | 18.55–21.00 | ac10000429-aa10003332 | ↑ | 15, 16 | CSSL10-2, 10-3 | |
|
| ||||||
| DTH | qDTH3 | 29.09–36.35 | aa03002463-aa03002773 | ↑ | 4, 11 | CSSL3-4 |
| qDTH6 | 0.78–6.09 | aa06000024-ac06000103 | ↓ | 10 | CSSL6-1 | |
↑ and ↓ represented increase and decrease of trait values at Yamadanishiki allele, respectively.
The number of refference report followed as 1: Lu et al. 1996, 2: Huang et al. 1997, 3: Li et al. 2000, 4: Takahashi et al. 2001, 5: Yoshida et al. 2002, 6: Aluko et al. 2004, 7: Bai et al. 2010, 8: Nelson et al. 2011, 9: Lu et al. 2013, 10: Matsubara et al. 2012, 11: Hori et al. 2013, 12: Huang et al. 2013, 13: Dang et al. 2015, 14: Nagata et al. 2015, 15: Zhen et al. 2017 and 16: Okada et al. 2017.
Representitive CSSL indicated CSSL having a listed QTL in the foreground region.
The GWh of CSSL2-3 was significantly smaller than that of Koshihikari, whereas eight CSSLs exhibited greater grain width (Table 1). Four QTLs located on chromosomes 2 (qGWh2), 4 (qGWh4), 5 (qGWh5), and 10 (qGWh10; Table 2) were identified. The latter three increased GWh in the CSSLs carrying the Yamadanishiki allele, while qGWh2 decreased GWh (Tables 1, 2, Supplemental Fig. 2B). Of these QTLs, it appears that qGWh10 has the largest effect on GWh (Tables 1, 2).
Most of the CSSLs had greater GT than Koshihikari, and GT in CSSL10-3 was similar to Yamadanishiki (Table 1). Three QTLs were identified on chromosomes 3 (qGT3) and 10 (qGT10-1 and qGT10-2; Table 2). The CSSLs carrying the Yamadanishiki allele increased GT (Tables 1, 2, Supplemental Fig. 2B).
The GWt of half the CSSLs was significantly greater than that of Koshihikari plants, but CSSL8-1 had significantly lower GWt than Koshihikari in 2016 (Table 1). Five QTLs were identified on chromosomes 6 (qGWt6-1 and qGWt6-2), 7 (qGWt7) and 10 (qGWt10-1 and qGWt10-2; Table 2). The CSSLs carrying the Yamadanishiki allele increased GWt (Tables 1, 2, Supplemental Fig. 2B). Interestingly, qGWt10-2 was located in a similar region to qGWh10 and qGT10-2 (Table 2).
Most of the CSSLs had slightly shorter DTH than Koshihikari (Table 1). In particular, the DTH of both CSSL6-1 and CSSL10-2 were notably shorter than that of Koshihikari, by approximately six days in 2015 and eight days in 2016 (Table 1). In contrast, the DTHs of CSSL2-3 and CSSL3-4 were longer than that of Koshihikari (Table 1). Although four chromosomal substituted regions in the four CSSLs were found on chromosomes 2, 3, 6, and 10, we confirmed that CSSL3-4 and CSSL2-3 carried the Yamadanishiki allele at Hd6 (Takahashi et al. 2001), and CSSL6-1 and CSSL10-2 carried the Yamadanishiki allele at Hd17 (Matsubara et al. 2012, Supplemental Table 2). Therefore, two QTLs on chromosomes 3 (qDTH3) and 6 (qDTH6) were verified (Table 2). These results corresponded with the report by Okada et al. (2017).
QTL reanalysis for GWt using RILs derived from a Koshihikari/Yamadanishiki cross
Okada et al. (2017) reported that qGWt5, which is in a similar region of qGWh5 that was identified in the present study, was detected as a major QTL in F2 and in RILs derived from a Koshihikari/Yamadanishiki cross. However, qGWt5 was not detected in the present CSSL analyses (Tables 1, 2, Supplemental Fig. 2). We therefore conducted QTL reanalysis for GWt in the RIL population, divided into eRILs and lRILs based on flowering date (Supplemental Fig. 1). We only obtained significant logarithm of odds values for qGWt10-2 in the eRILs and for qGWt5 in the lRILs (Table 3, Supplemental Fig. 3). In addition, the linkage maps for chromosomes 5 and 10 of the eRILs and lRILs were almost identical (Supplemental Fig. 4).
Table 3.
QTLs detected by QTL reanalysis for GWt of eRILs and lRILs
| Population | QTL | Year | Peak (cM) | LOD | AEa (g) | PVEb (%) |
|---|---|---|---|---|---|---|
| eRILs | qGWt5c | 2013 | 0.4 | −0.016 | 0.9 | |
| 2014 | 0.5 | −0.018 | 1.1 | |||
|
| ||||||
| qGWt10-2 | 2013 | 61.6 | 8.5 | −0.078 | 25.7 | |
| 2014 | 58.9 | 4.5 | −0.057 | 13.8 | ||
|
| ||||||
| lRILs | qGWt5 | 2013 | 126.7 | 4.5 | −0.057 | 14.6 |
| 2014 | 126 | 10.5 | −0.086 | 31.4 | ||
|
| ||||||
| qGWt10-2c | 2013 | 1.4 | −0.028 | 3.5 | ||
| 2014 | 0.4 | −0.016 | 1.1 | |||
Additive effect.
Phenotypic variance expressed.
The data of non-significant QTLs represented the values at peak positions of qGWt5 detected in lRILs and qGWt10-2 detected in eRILs.
Discussion
A set of CSSLs carries genomic segment(s) from a donor parent placed in the genetic background of a recipient parent, and manipulating these facilitates the comprehension of the whole genome of the donor parent by allowing the precise assessment of the genetic effects of the segments from the donor parent. In this study, we developed novel CSSLs in the genetic background of Koshihikari, a cooking-rice cultivar, with substituted chromosomal fragments from Yamadanishiki, a brewing-rice cultivar. Koshihikari and Yamadanishiki are distantly related Japanese cultivars (Yamasaki and Ideta 2013) and differ in many traits, such as grain size and heading date (Okada et al. 2017). In this study, we evaluated the grain size and heading date of the CSSLs that we developed, which enabled us to identify relevant QTLs.
We identified a total of 16 QTLs for grain traits: four QTLs for GL, four QTLs for GWh, three QTLs for GT, and five QTLs for GWt (Table 2). Of these, 15 QTLs caused an increase in the corresponding grain traits in the CSSLs carrying the Yamadanishiki allele, and only qGWh2 caused a decrease in its trait (Tables 1, 2, Supplemental Fig. 2B). This suggests that the grain size of Yamadanishiki is controlled by complex genetic mechanisms. Of the 13 QTLs identified for GL, GWh, and GWt, five (qGL11, qGWh5, qGWh10, qGWt6-2, and qGWt10-2) were similarly detected in RILs from the same crossing combination, whereas eight were newly identified in the CSSL-QTL analysis. The QTLs for GT were not identified in this manner because GT was not included in the RIL analysis. The results indicate that CSSLs can be used to identify QTLs that have relatively small genetic effects (Howell et al. 1996, Nagata et al. 2015). However, qGL4-2, one of the major QTLs on chromosome 4 that was detected using the RILs (Okada et al. 2017), was detected in only one of the two years of the present study using the CSSLs. Therefore, qGL4-2 may be affected by the environment; for example, inter-year variation in mean temperature or rainfall patterns may modify its effect. We propose that the combination of the previously identified major QTLs and the newly identified QTLs results in the large grain size of Yamadanishiki. In addition, many of the QTLs identified in the present study might overlap to some extent with previously reported QTLs (Table 2), because the QTLs associated with grain size have been detected in large loci (Huang et al 2013, Nagata et al. 2015).
Two QTLs (qDTH3 and qDTH6) for DTH that were identified on chromosomes 3 and 6 correspond to the known genes Hd6 and Hd16 on chromosome 3 and Hd17 on chromosome 6, respectively (Hori et al. 2013, Matsubara et al. 2012, Takahashi et al. 2001). In this study, we validated and confirmed the effects of 15 previously identified QTLs (Table 2), and identified three novel QTLs (qGL10, qGT10-1, and qGWt10-1). Of the QTLs identified in this study, seven (qGL11, qGWh5, qGWh10, qGWt6-2, qGWt10-2, qDTH3 and qDTH6) had previously been detected by using F2 and RILs derived from Koshihikari/Yamadanishiki (Okada et al. 2017). These QTLs had relatively large genetic effects, suggesting that they were particularly important in regulating the grain size and heading date of Yamadanishiki.
In previous studies associated with grain size, GS5 (3.4 Mb) and GW5/qSW5 (5.3 Mb) on chromosome 5, and TGW6 (25.1 Mb) and GW6a (26.6 Mb) on chromosome 6 have been cloned (Ishimaru et al. 2013, Li et al. 2011, Shomura et al. 2008, Song et al. 2015, Weng et al. 2008). However, qGWh5 and qGWt6-2 were clearly different from the cloned genes, because they were located in the distal regions of the long arms of chromosomes 5 and 6, respectively (Table 2). Using Yamadanishiki as a crossing parent, Yoshida et al. (2002) and Okada et al. (2017) detected a major QTL for GWh that was in the same region as qGWh5. In addition, based on an advanced backcrossed population with substituted IR64 genomic segments in a Koshihikari background, Nagata et al. (2015) reported that this QTL has a very small decreasing effect on GWh in the IR64 allele, and is located at 29.54 Mb on chromosome 5. We can therefore conclude that the QTL reported by Nagata et al. (2015) is identical to qGWh5 (Table 2), and we infer that the alleles of Koshihikari, Yamadanishiki, and IR64 exhibit different genetic effects.
qGWh10 is located in the same region as qGT10-2 and qGWt10-2 (Table 2), suggesting that these QTLs are associated with a single gene. Recently, Zhen et al. (2017) confirmed that qGS10 is associated with grain size, and that it affects GL, GWh, and grain weight. They suggested that qGS10 was identical to qGWh10; in the present study, the latter affected GT, but had no genetic effect on GL. The region around qGWh10 has also been detected via QTL analysis of populations derived from japonica × indica and indica × indica crosses (Huang et al. 1997, Nelson et al. 2011, Zhen et al. 2017), suggesting that this QTL is highly conserved across Asian rice cultivars. We found that qGL11 had the largest effect on GL (Table 2), which was consistent with previous research (Okada et al. 2017, Yoshida et al. 2002). The present study presents the first validation of this QTL.
Genotype-by-environment interaction and QTL-by-environment interaction are observed in many crops; in the context of breeding, it is important to understand how QTLs respond environmental conditions (Moreau et al. 2004, Nelson et al. 2011, Wang et al. 2016, Zheng et al. 2010). Okada et al. (2017) reported that qGWh5 and qGWh10 were major QTLs for not only GWh but also GWt. However, CSSL-QTL analysis in the present study did not detect qGWt5 as a robust QTL for GWt, but instead detected qGWt10-2 (Table 2). Three hypotheses were considered: the effect of the genetic background, QTL-by-QTL interaction, and QTL-by-environment interaction. We then performed a reanalysis of these QTLs, using two RIL populations divided according to flowering date, to examine QTL-by-environment interaction (Okada et al. 2017, Supplemental Figs. 1, 3, 4). The results reveal that qGWt5 and qGWt10-2 have different responses to the environment (Table 3, Supplemental Fig. 3). qGWt5 had an important genetic effect in the late-flowering plants (lRILs: 11 August–1 September), whereas qGWt10-2 had a large effect in the early-flowering plants (eRILs: 23 July–9 August). qDTH3, a QTL for DTH, would have impacted these results, and CSSL3-4, which carries the Yamadanishiki allele, showed an approximately 20-day increase in DTH compared to Koshihikari (Table 1). Because most CSSLs exhibited early flowering, the CSSL-QTL analysis was unlikely to have detected qGWt5. Since the average air temperature of the 30 days of the ripening term was 27.3°C (2013) and 25.9°C (2014) for the eRILs, and 24.1°C (2013) and 23.5°C (2014) for the lRILs, it is possible that the different effects of these two QTLs were caused by differences in the ripening temperature. Nevertheless, the effect of genetic background and QTL-by-QTL interactions should be also considered. Okada et al. (2017) reported that qGWh10 corresponded to the QTL for white-core expression, but no QTL for white core was detected around qGWh5. Therefore, these two QTLs may have different functions in grain development during ripening. Evaluation of the effect of these QTLs under environmental changes, e.g., changes in flowering date, is essential for future breeding. It is also important to understand the effect of qGWh5 on GWt in Yamadanishiki, since this cultivar flowers around 20 August.
In conclusion, 18 QTLs for grain size and DTH were identified using CSSLs, and six major QTLs for grain trait and two QTLs for DTH that were previously detected by Okada et al. (2017) appear to be particularly important for Yamadanishiki. In addition, qGWt5 and qGWt10-2 appear to have different functions and exhibit different responses to the environment. This information could potentially be used not only to improve the breeding of brewing-rice cultivars, but also to increase the yield of cooking-rice cultivars.
Supplementary Information
Acknowledgments
We thank Prof. Shigeo Takumi (Kobe University, Japan) for use of the epMotion 5070 pipetting machine. This work was supported by JSPS KAKENHI Grant Number 17J01082 and Council for Science, Technology and Innovation (CSTI), Cross-ministerial Strategic Innovation Promotion Program (SIP), “Technologies for creating next-generation agriculture, forestry and fisheries” (funding agency: Bio-oriented Technology Research Advancement Institute, NARO).
Literature Cited
- Aluko, G., Martinez, C., Tohme, J., Castano, C., Bergman, C. and Oard, J.H. (2004) QTL mapping of grain quality traits from the interspecific cross Oryza sativa × O. glaberrima. Theor. Appl. Genet. 109: 630–639. [DOI] [PubMed] [Google Scholar]
- Ando, T., Yamamoto, T., Shimizu, T., Ma, X.F., Shomura, A., Takeuchi, Y., Lin, S.Y. and Yano, M. (2008) Genetic dissection and pyramiding of quantitative traits for panicle architecture by using chromosomal segment substitution lines in rice. Theor. Appl. Genet. 116: 881–890. [DOI] [PubMed] [Google Scholar]
- Aramaki, K., Ogawa, K., Yamamoto, K., Suzuki, J., Kanno, M., Kizaki, Y. and Okazaki, N. (1995) Polishing properties of white-core and non-white-core grains fractionated from the same variety of rice. Seibutsu-kogaku 73: 381–386. [Google Scholar]
- Bai, X., Luo, L., Yan, W., Kovi, M.R., Zhan, W. and Xing, Y. (2010) Genetic dissection of rice grain shape using a recombinant inbred line population derived from two contrasting parents and fine mapping a pleiotropic quantitative trait locus qGL7. BMC Genet. 11: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, J.M., Jiang, L., Liu, L.L., Wei, X.J., Xiao, Y.H., Zhang, L.J., Zhao, Z.G., Zhai, H.Q. and Wan, J.M. (2010) Construction of a new set of rice chromosome segment substitution lines and identification of grain weight and related traits QTLs. Breed. Sci. 60: 305–313. [Google Scholar]
- Churchill, G.A. and Doerge, R.W. (1994) Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, X., Thi, T.G.T., Edzesi, W.M., Liang, L., Liu, Q., Liu, E., Wang, Y., Qiang, S., Liu, L. and Hong, D. (2015) Population genetic structure of Oryza sativa in east and southeast Asia and the discovery of elite alleles for grain traits. Sci. Rep. 5: 11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellaporta, S.L., Wood, J. and Hicks, J.B. (1983) A plant DNA minipreparation version II. Plant Mol. Biol. Rep. 1: 19–21. [Google Scholar]
- Ebitani, T., Takeuchi, Y., Nonoue, Y., Yamamoto, T., Takeuchi, K. and Yano, M. (2005) Construction and evaluation of chromosome segment substitution lines carrying overlapping chromosome segments of indica rice cultivar ‘Kasalath’ in a genetic background of japonica elite cultivar ‘Koshihikari’. Breed. Sci. 55: 65–73. [Google Scholar]
- Fan, C., Xing, Y., Mao, H., Lu, T., Han, B., Xu, C., Li, X. and Zhang, Q. (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112: 1164–1171. [DOI] [PubMed] [Google Scholar]
- Furuta, T., Uehara, K., Angeles-Shim, R.B., Shim, J., Ashikari, M. and Takashi, T. (2014) Development and evaluation of chromosome segment substitution lines (CSSLs) carrying chromosome segments derived from Oryza rufipogon in the genetic background of Oryza sativa L. Breed. Sci. 63: 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habier, D., Fernando, R.L., Kizilkaya, K. and Garrick, D.J. (2011) Extension of the Bayesian alphabet for genomic selection. BMC Bioinformatics 12: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, K., Ogiso-Tanaka, E., Matsubara, K., Yamanouchi, U., Ebana, K. and Yano, M. (2013) Hd16, a gene for casein kinase I, is involved in the control of rice flowering time by modulating the day-length response. Plant J. 76: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horigane, K.A., Suzuki, K. and Yoshida, M. (2014) Moisture distribution in rice grains used for sake brewing analyzed by magnetic resonance imaging. J. Cereal Sci. 60: 193–201. [Google Scholar]
- Howell, P.M., Lydiate, D.J. and Marshall, D.F. (1996) Towards developing intervarietal substitution lines in Brassica napus using marker-assisted selection. Genome 39: 348–358. [DOI] [PubMed] [Google Scholar]
- Huang, N., Parco, A., Mew, T., Magpantay, G., McCouch, S., Guiderdoni, E., Xu, J., Subudhi, P., Angeles, E.R. and Khush, G.S. (1997) RFLP mapping of isozymes, RAPD and QTLs for grain shape, brown planthopper resistance in a doubled haploid rice population. Mol. Breed. 3: 105–113. [Google Scholar]
- Huang, R., Jiang, L., Zheng, J., Wang, T., Wang, H., Huang, Y. and Hong, Z. (2013) Genetic bases of rice grain shape: so many genes, so little known. Trends in Plant Sci. 18: 218–226. [DOI] [PubMed] [Google Scholar]
- Ishimaru, K., Hirotsu, N., Madoka, Y., Murakami, N., Hara, N., Onodera, H., Kashiwagi, T., Ujiie, K., Shimizu, B., Onishi, A. et al. (2013) Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 45: 707–711. [DOI] [PubMed] [Google Scholar]
- Kaji, R., Sakai, M., Tamura, K., Tamura, Y., Okamoto, M., Mizobuchi, R., Hirabayashi, H., Nishimura, M. and Fukaura, S. (2013) “Ginnosato”, a new sake-brewing rice variety. NARO Kyushu Okinawa Agricultural Research Center report 60: 13–28. [Google Scholar]
- Li, J.X., Yu, S.B., Xu, C.G., Tan, Y.F., Gao, Y.J., Li, X.H. and Zhang, Q. (2000) Analyzing quantitative trait loci for yield using a vegetatively replicated F2 population from a cross between the parents of an elite rice hybrid. Theor. Appl. Genet. 101: 248–254. [Google Scholar]
- Li, Y., Fan, C., Xing, Y., Jiang, Y., Luo, L., Sun, L., Shao, D., Xu, C., Li, X., Xiao, J. et al. (2011) Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 43: 1266–1269. [DOI] [PubMed] [Google Scholar]
- Lu, B., Yang, C., Xie, K., Zhang, L., Wu, T., Li, L., Liu, X., Jiang, L. and Wan, J. (2013) Quantitative trait loci for grain-quality traits across a rice F2 population and backcross inbred lines. Euphytica 192: 25–35. [Google Scholar]
- Lu, C., Shen, L., Tan, Z., Xu, Y., He, P., Chen, Y. and Zhu, L. (1996) Comparative mapping of QTLs for agronomic traits of rice across environments using a doubled haploid population. Theor. Appl. Genet. 93: 1211–1217. [DOI] [PubMed] [Google Scholar]
- Matsubara, K., Ogiso-Tanaka, E., Hori, K., Ebana, K., Ando, T. and Yano, M. (2012) Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol. 53: 709–716. [DOI] [PubMed] [Google Scholar]
- McCouch, S.R., Cho, Y.G., Yano, M., Paul, E., Blinstrub, M., Morishima, H. and Kinoshita, T. (1997) Report on QTL nomenclature. Rice Genet. Newsl. 14: 11–13. [Google Scholar]
- Moreau, L., Charcosset, A. and Gallais, A. (2004) Use of trial clustering to study QTL × environment effects for grain yield and related traits in maize. Theor. Appl. Genet. 110: 92–105. [DOI] [PubMed] [Google Scholar]
- Morinaka, Y., Sakamoto, T., Inukai, Y., Agetsuma, M., Kitano, H., Ashikari, M. and Matsuoka, M. (2006) Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol. 141: 924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata, K., Iyama, Y., Yamaguchi, T., Ozaki, H., Kidani, Y. and Ebitani, T. (2014) Identification of a novel gene (Apq1) from the indica rice cultivar ‘Habataki’ that improves the quality of grains produced under high temperature stress. Breed. Sci. 64: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki, H., Ebana, K., Shibaya, T., Yonemaru, J. and Yano, M. (2010) Core single-nucleotide polymorphisms—a tool for genetic analysis of the Japanese rice population. Breed. Sci. 60: 648–655. [Google Scholar]
- Nagata, K., Ando, T., Nonoue, Y., Mizubayashi, T., Kitazawa, N., Shomura, A., Matsubara, K., Ono, N., Mizobuchi, R., Shibaya, T. et al. (2015) Advanced backcross QTL analysis reveals complicated genetic control of rice grain shape in a japonica × indica cross. Breed. Sci. 65: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagato, K. and Ebata, M. (1959) Studies on white-core rice kernel II. On the physical properties of the kernel. Jpn. J. Crop Sci. 28: 46–50. [Google Scholar]
- Nelson, J.C., McClung, A.M., Fjellstrom, R.G., Moldenhauer, K.A.K., Boza, E., Jodari, F., Oard, J.H., Linscombe, S., Scheffler, B.E. and Yeater, K.M. (2011) Mapping QTL main and interaction influences on milling quality in elite US rice germplasm. Theor. Appl. Genet. 122: 291–309. [DOI] [PubMed] [Google Scholar]
- Okada, S., Suehiro, M., Ebana, K., Hori, K., Onogi, A., Iwata, H. and Yamasaki, M. (2017) Genetic dissection of grain traits in Yamadanishiki, an excellent sake-brewing rice cultivar. Theor. Appl. Genet. 130: 2567–2585. [DOI] [PubMed] [Google Scholar]
- Onogi, A. and Iwata, H. (2016) VIGoR: Variational bayesian inference for genome-wide regression. Journal of Open Research Software 4: e11. [Google Scholar]
- R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/ [Google Scholar]
- Shomura, A., Izawa, T., Ebana, K., Ebitani, T., Kanegae, H., Konishi, S. and Yano, M. (2008) Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 40: 1023–1028. [DOI] [PubMed] [Google Scholar]
- Song, X.J., Huang, W., Shi, M., Zhu, M.Z. and Lin, H.X. (2007) A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 39: 623–630. [DOI] [PubMed] [Google Scholar]
- Song, X.J., Kuroha, T., Ayano, M., Furuta, T., Nagai, K., Komeda, N., Segami, S., Miura, K., Ogawa, D., Kamura, T. et al. (2015) Rare allele of a previously unidentified histone H4 acetyltrainsferase enhances grain weight, yield, and plant biomass in rice. Proc. Natl. Acad. Sci. USA 112: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y., Shomura, A., Sasaki, T. and Yano, M. (2001) Hd6, a rice quantitative trait locus involved photoperiod sensitivity, encodes the α subunit of protein kinase CK2. Proc. Natl. Acad. Sci. USA 98: 7922–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano-Kai, N., Jiang, H., Kubo, T., Sweeney, M., Matsumoto, T., Kanamori, H., Padhukasahasram, B., Bustamante, C., Yoshimura, A., Doi, K. et al. (2009) Evolutionary history of GS3, a gene conferring grain length in rice. Genetics 182: 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Sun, G., Ren, X., Li, C., Liu, L., Wang, Q., Du, B. and Sun, D. (2016) QTL underlying some agronomic traits in barley detected by SNP markers. BMC Genet. 17: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Wu, K., Yuan, Q., Liu, X., Liu, Z., Lin, X., Zeng, R., Zhu, H., Dong, G., Qian, Q. et al. (2012a) Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 44: 950–954. [DOI] [PubMed] [Google Scholar]
- Wang, S., Basten, C.J. and Zeng, Z.B. (2012b) Windows QTL Cartographer 2.5. Department of Statistics. North Carolina State University, Raleigh, NC. [Google Scholar]
- Weng, J., Gu, S., Wan, X., Gao, H., Guo, T., Su, N., Lei, C., Zhang, X., Cheng, Z., Guo, X. et al. (2008) Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res. 18: 1199–1209. [DOI] [PubMed] [Google Scholar]
- Yamamoto, T., Nagasaki, H., Yonemaru, J., Ebana, K., Nalajima, M., Shibaya, T. and Yano, M. (2010) Fine definition of the pedigree haplotypes of closely related rice cultivars by means of genome-wide discovery of single-nucleotide polymorphisms. BMC Genomics 11: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, M. and Ideta, O. (2013) Population structure in Japanese rice population. Breed. Sci. 63: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagiuchi, T., Yamamoto, H., Miyazaki, N., Nagano, T., Mizuma, T. and Wakai, Y. (1997) Influence of grain type on suitability of rice for sake brewing. Seibutsu-kogaku 75: 169–176. [Google Scholar]
- Yoshida, S., Ikegami, M., Kuze, J., Sawada, K., Hashimoto, Z., Ishii, T., Nakamura, C. and Kamijima, O. (2002) QTL analysis for plant and grain characters of Sake-brewing rice using a doubled haploid population. Breed. Sci. 52: 309–317. [Google Scholar]
- Zeng, Z.B. (1994) Precision mapping of quantitative trait loci. Genetics 136: 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen, W., Jun-yu, C., Yu-jun, Z., Ye-yang, F. and Jie-yun, Z. (2017) Validation of qGS10, a quantitative trait locus for grain size on the long arm of chromosome 10 in rice (Oryza sativa L.). J. Integr. Agric. 16: 16–26. [Google Scholar]
- Zheng, B.S., Gouis, J.L., Leflon, M., Rong, W.Y., Laperche, A. and Brancourt-Hulmel, M. (2010) Using probe genotypes to dissect QTL × environment interactions for grain yield components in winter wheat. Theor. Appl. Genet. 121: 1501–1517. [DOI] [PubMed] [Google Scholar]
- Zheng, J., Zhang, Y. and Wang, C. (2015) Molecular functions of genes related to grain shape in rice. Breed. Sci. 65: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, J. and Li, J. (2014) Molecular genetic dissection of quantitative trait loci regulating rice grain size. Annu. Rev. Genet. 48: 99–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.