Abstract
Clubroot is an economically important disease affecting plants in the family Cruciferae worldwide. In this study, a collection of 50 Cruciferae accessions was screened using Plasmodiophora brassicae pathotype 4 in China. Eight of these demonstrated resistance, including three Chinese cabbages, two cabbages, one radish, one kale, and one Brassica juncea. The three clubroot-resistant Chinese cabbages (1003, 1007 and 1008) were then used to transfer the clubroot resistance genes to B. napus by distant hybridization combined with embryo rescue. Three methods including morphological identification, cytology identification, and molecular marker-assisted selection were used to determine hybrid authenticity, and 0, 2, and 4 false hybrids were identified by these three methods, respectively. In total, 297 true hybrids were identified. Clubroot resistance markers and artificial inoculation were utilized to determine the source of clubroot resistance in the true hybrids. As a result, two simple sequence repeat (SSR) and two intron polymorphic (IP) markers linked to clubroot resistance genes were identified, the clubroot resistance genes of 1007 and 1008 were mapped to A03. At last, 159 clubroot-resistant hybrids were obtained by clubroot resistance markers and artificial inoculation. These intermediate varieties will be used as the ‘bridge material’ of clubroot resistance for further B. napus breeding.
Keywords: clubroot, distant hybridization, embryo rescue, molecular markers
Introduction
Clubroot is a soil-borne disease caused by Plasmodiophora brassicae (Woronin) that has been spreading rapidly worldwide and has been reported in 60 countries (Dixon 2009). In China, the area affected by clubroot disease currently accounts for 1/3 of the total area of Cruciferous crops, causing losses of 20%–30% yield, with more than 60% loss in the most seriously damaged regions, resulting in significant production constraints (Wang et al. 2012). It is difficult to control clubroot disease using traditional methods such as cultivation methods, chemical agents, biological control, because the pathogen can persist in the soil as resting spores for more than eight years, and can even survive up to 15 years in infected fields when conditions are suitable (Jubault et al. 2008).
In amphidiploid Brassica species, there are limited resistant sources available in B. napus, and no resistant genotypes were identified for the mustard species B. juncea and B. carinata (Peng et al. 2014). Germplasms resistant to a broad range of pathotypes of P. brassicae have been identified in the progenitor diploid Brassica species B. rapa, B. nigra, and B. oleracea (Hasan 2012, Peng et al. 2014), which could possibly be used for developing B. napus and mustard species with resistance to clubroot by re-synthesizing the Brassica amphidiploids. Therefore some researchers routinely use B. rapa and B. oleracea as sources for clubroot resistance genes. Clubroot resistance genes currently identified in Chinese cabbage primarily originated from European turnips, and at least eight resistance-related genes have been identified (Crute et al. 1980, Diederichsen et al. 2009, Hirai 2006, Piao et al. 2009). It have been reported that the clubroot resistance in Chinese cabbages is controlled by one or two major genes (Cho et al. 2016, Gao et al. 2014, Piao et al. 2004, Suwabe et al. 2003, Yoshikawa 1981). The clubroot resistance of B. oleracea was controlled by one or more recessive genes (Jichuan and Wang 1989, Voorrips and Visser 1993), while that of kale is controlled by one recessive or many major dominant genes (Laurens and Thomas 1993, Voorrips and Visser 1993).
In view of the current intensification of clubroot disease worldwide, traditional methods cannot effectively control the spread of clubroot. Therefore, clubroot resistance breeding is considered to be one of the most effective ways to control this disease. Studies have been reported from Japan, South Korea, Europe, North America and other countries, and many clubroot resistant vegetable varieties have been cultivated (Jichuan and Wang 1989). However, few resistant varieties of B. napus have been reported; therefore it is more feasible to transfer the clubroot resistance genes from vegetables to B. napus by interspecific hybridization. Using this method, a number of synthetic species have been reported, including B. napus, B. carinata, and B. juncea (Liu 1985). Initially, researchers utilized artificial crossing and natural fruiting to synthesize interspecific hybrids, but the rate of success was low (Liu 1992). With the emergence of embryo rescue technology, researchers began to use this method combined with conventional hybrid breeding to synthesize a range of new rapeseed varieties, concentrating primarily on oil content, quality, yield, and resistance (Zhang et al. 2001, Zhou et al. 2005). However, few of studies on clubroot-resistant germplasm synthesis of rapeseed were reported. Thus it is necessary to use resistant resources from the close relatives of rapeseed to cultivate clubroot-resistant varieties of B. napus. Therefore the purpose of this study is three folds: 1) Screening of clubroot-resistant Cruciferous varieties, 2) Transferring the clubroot resistance genes from Chinese cabbage to B. napus using distant hybridization, and 3) Verification of hybrid authenticity and clubroot resistance. It is hoped that this study will provide the ‘bridge material’ for clubroot resistance breeding in B. napus.
Materials and Methods
Sources of Brassica germplasm
A collection of 50 Brassica accessions including 42 inbred lines and eight hybrid varieties were obtained from Northwest A&F University (Yangling, Shaanxi, China) and Shanghai Academy of Agricultural Sciences (Shanghai, China) (Table 1). Species included B. rapa (13), B. juncea (3), Chinese cabbage (5, hybrid varieties), B. napus (4), cabbage (3, hybrid varieties), broccoli (7), cauliflower (8), kale (6) and radish (1).
Table 1.
Information of 50 Cruciferae accessions
| Accessions | Species | Type of plant | Source of materials |
|---|---|---|---|
| 2941 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 2944 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 2948 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 2952 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 2957 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 2968 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 2927 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 2972 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 2985 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 2990 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 2998 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 3002 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 3009 | B. rapa subsp. sylvestris | B. rapa | Northwest A&F University, Yangling |
| 833 | B. napus | B. napus | Northwest A&F University, Yangling |
| 2348 | B. napus | B. napus | Northwest A&F University, Yangling |
| 2523 | B. napus | B. napus | Northwest A&F University, Yangling |
| 2541 | B. napus | B. napus | Northwest A&F University, Yangling |
| 1010 | B. juncea subsp. juncea | B. juncea | Northwest A&F University, Yangling |
| 1011 | B. juncea subsp. juncea | B. juncea | Northwest A&F University, Yangling |
| 1012 | B. juncea subsp. juncea | B. juncea | Northwest A&F University, Yangling |
| 1003 | B. rapa subsp. pekinensis | Chinese cabbage | Northwest A&F University, Yangling |
| 1004 | B. rapa subsp. pekinensis | Chinese cabbage | Northwest A&F University, Yangling |
| 1007 | B. rapa subsp. pekinensis | Chinese cabbage | Northwest A&F University, Yangling |
| 1008 | B. rapa subsp. pekinensis | Chinese cabbage | Northwest A&F University, Yangling |
| 1009 | B. rapa subsp. pekinensis | Chinese cabbage | Northwest A&F University, Yangling |
| 1001 | B. oleracea var. capitata | Cabbage | Northwest A&F University, Yangling |
| 1005 | B. oleracea var. capitata | Cabbage | Northwest A&F University, Yangling |
| 1006 | B. oleracea var. capitata | Cabbage | Northwest A&F University, Yangling |
| 1002 | R. raphanistrum subsp. sativus | Radish | Northwest A&F University, Yangling |
| JL1 | B. oleracea var. acephala | Kale | Shanghai Academy of Agricultural Sciences, Shanghai |
| JL2 | B. oleracea var. acephala | Kale | Shanghai Academy of Agricultural Sciences, Shanghai |
| JL3 | B. oleracea var. acephala | Kale | Shanghai Academy of Agricultural Sciences, Shanghai |
| JL4 | B. oleracea var. acephala | Kale | Shanghai Academy of Agricultural Sciences, Shanghai |
| JL5 | B. oleracea var. acephala | Kale | Shanghai Academy of Agricultural Sciences, Shanghai |
| JL6 | B. oleracea var. acephala | Kale | Shanghai Academy of Agricultural Sciences, Shanghai |
| QH1 | B. oleracea var. italica | Broccoli | Shanghai Academy of Agricultural Sciences, Shanghai |
| QH2 | B. oleracea var. italica | Broccoli | Shanghai Academy of Agricultural Sciences, Shanghai |
| QH3 | B. oleracea var. italica | Broccoli | Shanghai Academy of Agricultural Sciences, Shanghai |
| QH4 | B. oleracea var. italica | Broccoli | Shanghai Academy of Agricultural Sciences, Shanghai |
| QH5 | B. oleracea var. italica | Broccoli | Shanghai Academy of Agricultural Sciences, Shanghai |
| QH6 | B. oleracea var. italica | Broccoli | Shanghai Academy of Agricultural Sciences, Shanghai |
| QH7 | B. oleracea var. italica | Broccoli | Shanghai Academy of Agricultural Sciences, Shanghai |
| BH1 | B. oleracea var. botrytis | Cauliflower | Shanghai Academy of Agricultural Sciences, Shanghai |
| BH2 | B. oleracea var. botrytis | Cauliflower | Shanghai Academy of Agricultural Sciences, Shanghai |
| BH3 | B. oleracea var. botrytis | Cauliflower | Shanghai Academy of Agricultural Sciences, Shanghai |
| BH4 | B. oleracea var. botrytis | Cauliflower | Shanghai Academy of Agricultural Sciences, Shanghai |
| BH5 | B. oleracea var. botrytis | Cauliflower | Shanghai Academy of Agricultural Sciences, Shanghai |
| BH6 | B. oleracea var. botrytis | Cauliflower | Shanghai Academy of Agricultural Sciences, Shanghai |
| BH7 | B. oleracea var. botrytis | Cauliflower | Shanghai Academy of Agricultural Sciences, Shanghai |
| BH8 | B. oleracea var. botrytis | Cauliflower | Shanghai Academy of Agricultural Sciences, Shanghai |
Inoculation and resistance test
A single-spore isolate, defined as pathotype 4 (P4) of P. brassicae (Williams 1966), was used to test clubroot resistance of each accession. Ten individuals per accession were selected to be inoculated, and two replicates were assessed. The plants were grown in 50-well multipots. The resistance tests were carried out at Northwest A&F University (Yangling, Shaanxi, China) and Shenyang Agricultural University (Shenyang, Liaoning, China).
The P. brassicae isolate was propagated and isolated from infected root tissues of susceptible plants as described by Piao et al. (2004). Seedlings were inoculated 3 days after germination by injecting 10 ml P. brassicae resting spore suspension (1 × 107 spores/ml) into each well, and seedlings were then maintained in a greenhouse under a 16 hL/8 hD photoperiod at an average temperature of 20–25°C. The soil was kept moist during the treatment period. Infection was checked after 35 days by pulling out the plants. Roots of each accession were assessed for clubroot disease severity at 5 weeks after inoculation using a standard 0 to 3 scale where: 0 = no clubbing; 1 = small clubs only; 2 = moderate clubs; and 3 = severe clubbing. For statistical analysis, two indicators, disease incidence and disease index (DI) were used. The disease incidence of each accession was calculated according to this formula: disease incidence=Number of susceptible plants/total number of investigated plants. The DI was calculated using the following formula (Strelkov et al. 2006):
The criteria for resistance classification according to the DI were as follows: DI = 0, highly resistant (HR); DI < 10, resistant (R); 10 ≤ DI ≤ 20, moderately susceptible (MS); 20 ≤ DI ≤ 50, susceptible (S); DI > 50, highly susceptible (HS). The plants with DI values of 0–10 were identified as resistant.
Analysis of resistance
The SPSS Statistics v20.0.0 software was used to analyze the standard deviation and the significance of 50 test accessions. The Euclidean cluster average method was utilized to study the cluster analysis of DI for the test materials (Huang et al. 2008). The linear regression method was used to analyze the correlation between the disease incidence and the DI of 50 accessions (Yang et al. 2011).
Distant hybridization
The resistant Chinese cabbage varieties (1003, 1007, and 1008) that were identified from the 50 accessions were used as the donors of the clubroot resistance genes, and the four susceptible B. napus varieties (833, 2348, 2523 and 2541) were used as receptors. These four B. napus varieties were used as the female parent and crossed with the three resistant Chinese cabbages. The method of distant hybridization combined with embryo rescue was used to transfer the clubroot resistance genes from Chinese cabbages to B. napus. The procedure was as follows: ten pods per combination were selected to isolate the embryos. The embryos (at 15 days after pollination) were cultured in B5 liquid medium with 2% sucrose, placed on a shaking platform with a rotating speed of 50 r/min, and a 16 hL/8 hD photoperiod at an average temperature of 25°C. When cotyledons appeared, they were transferred into B5 solid medium supplemented with 2% sucrose. When the seedlings grew main roots, they were inoculated with P4 (10 ml, 1 × 107 spores/ml), using the procedure as described by Piao et al. (2004).
Identification of F1 hybrid authenticity
In this study, morphological, cytological and molecular markers were used to identify the authenticity of F1 hybrids derived from Chinese cabbages and B. napus.
Morphological identification
The morphological characteristics at the seedling and flowering stages of F1 hybrids and their parents were compared to determine whether the hybrids had similar characteristics to their parents. These characteristics included leaf shape, leaf margin, bud, flower size and flower color.
Cytological identification
Flower buds of 2–3 mm diameter were picked for chromosome count. These were then placed in 0.002 M 8-hydroxyquinoline under darkness at room temperature for 3–4 hours. The pistil was then transferred to Kano fixed liquid (ethanol: glacial acetic acid = 3:1) overnight, and then placed at −20°C for long-term storage. Chromosomes were visualized using the method reported by Li et al. (1995). In brief, the pistils were first placed in 1 M HCl for 6–8 minutes at 60°C and then soaked in distilled water for 1 minute, followed by addition of one drop of magenta dye for 30 seconds. Chromosome preparations were then observed under an optical microscope.
Molecular marker identification
Genomic DNA was extracted from young leaves of parents and F1 individuals using the CTAB method (Doyle 1990). The final DNA concentration was adjusted to 50 ng/μl. The SSR amplification was performed as described by Lowe et al. (2002). Sequences of all SSR markers were obtained from public sources including the databases on http://ukcrop.net/perl/ace/search/BrassicaDB (Lowe et al. 2004) and http://www.brassica.info/resource/markers.php (for those with the prefixes: Ra, Na, BN, and BRMS), as well as the electronic supplementary material of Piquemal et al. (2005) (for those primer pairs with the prefixes ‘BRAS’ and ‘CB’). Silver staining was performed according to the procedures described by Lu et al. (2001). Eighty one pairs of SSR primers that amplified multiply bands in the previous studies were used to amplify both parents and F1 individuals. The F1 individuals that were found to be consistent with the parental male bands after amplification were identified as true hybrids.
Clubroot resistance identification
Clubroot resistant markers assisted selection and artificial inoculation using P4 were used to identify the resistance of F1. Initially, 25 SSR markers linked to seven clubroot resistance genes, such as Crr1, Crr2, Crr3, Crr4, CRk, CRc and CRb (Hirai et al. 2004, Piao et al. 2003, Sakamoto et al. 2008, Suwabe et al. 2003, 2006) were selected and 12 pairs of IP primers around CRa were designed to amplify the parents and F1 individuals. The individuals that were identified as resistant by both methods were selected for future study.
Results
Analysis of disease resistance for test materials
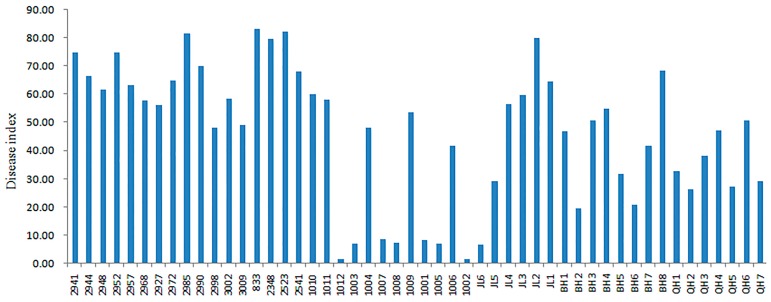
The results showed that the disease incidence of the 50 accessions was between 5.00% and 100.00%, with an average incidence of 76.93%. The DI was between 1.67 and 83.34, with an average of 46.58. The disease incidence of these materials is significantly different, and the DI also has a significant difference (Table 2). Among these, DI of eight accessions were less than 10 (Fig. 1), including three Chinese cabbages (1003, 1007 and 1008), two cabbages (1001, 1005), one radish (1002), one B. juncea (1012) and one kale (JL6). These accessions were confirmed as resistant materials according to the criteria for resistance classification, accounting for 16% of the test materials. The DI of other test materials was greater than 10, showing different degrees of disease; among these, two were moderately susceptible to disease, 14 were susceptible to disease and 26 showed high sensitivity, accounting for 4%, 28% and 52% of the test materials, respectively.
Table 2.
Significance analysis of 50 accessions on clubroot resistance
| Names | Disease incidence | Disease index |
|---|---|---|
| 1012 | 5.00 ± 0.07aA | 1.67 ± 2.35aA |
| 1002 | 5.00 ± 0.07aA | 1.67 ± 2.35aA |
| JL6 | 17.50 ± 0.06abA | 6.67 ± 4.72aA |
| 1003 | 21.00 ± 0.01abA | 7.04 ± 0.52aAB |
| 1005 | 15.50 ± 0.06abA | 7.04 ± 0.52aAB |
| 1008 | 16.50 ± 0.05abA | 7.50 ± 1.17aAB |
| 1001 | 15.00 ± 0.07abA | 8.34 ± 2.35aAB |
| 1007 | 26.00 ± 0.06bA | 8.71 ± 1.83aAB |
| BH2 | 59.50 ± 0.05cdBC | 19.68 ± 1.63bBC |
| BH6 | 52.00 ± 0.11ghijB | 19.91 ± 3.40bCD |
| QH2 | 66.00 ± 0.13cdefBCD | 26.49 ± 3.79bcCDE |
| QH5 | 69.00 ± 0.03defgBCDE | 27.32 ± 3.27bcCDE |
| JL5 | 82.00 ± 0.10efghijCDEFG | 29.17 ± 5.89bcdCDEF |
| QH7 | 69.00 ± 0.27defgBCDE | 29.17 ± 5.89bcdCDEF |
| BH5 | 75.00 ± 0.07defghCDEF | 31.67 ± 2.35cdCDEF |
| QH1 | 82.00 ± 0.10efghijCDEFG | 32.92 ± 5.30cdeDEF |
| QH3 | 94.50 ± 0.08jFG | 38.34 ± 2.35defEFG |
| 1006 | 65.00 ± 0.07cdeBCD | 41.67 ± 2.35efgFGH |
| BH7 | 84.00 ± 0.08ghijDEFG | 41.86 ± 6.81efgFGH |
| BH1 | 82.00 ± 0.10efghijCDEFG | 46.99 ± 1.64fghGHI |
| QH4 | 84.50 ± 0.06ghijDEFG | 47.22 ± 3.93fghGHI |
| 1004 | 75.00 ± 0.07defghCDEF | 48.15 ± 5.24ghiGHIJ |
| 2998 | 95.00 ± 0.07jFG | 48.34 ± 2.35ghiGHIJ |
| 3009 | 95.00 ± 0.07jFG | 49.08 ± 1.31ghiGHIJ |
| BH3 | 83.00 ± 0.07fghijDEFG | 50.70 ± 6.88ghijGHIJk |
| QH6 | 89.50 ± 0.01hijEFG | 50.74 ± 3.66ghijGHIJk |
| 1009 | 76.00 ± 0.08defghiCDEF | 53.71 ± 2.62hijkHIJkL |
| BH4 | 95.00 ± 0.07jFG | 55.00 ± 2.36hijklHIJkLM |
| 2927 | 100.00 ± 0.00jG | 56.30 ± 4.19hijklIJkLM |
| JL4 | 84.50 ± 0.06ghijDEFG | 56.67 ± 4.72hijklmIJkLMN |
| 2968 | 100.00 ± 0.00jG | 57.78 ± 3.14ijklmIJkLMN |
| 1011 | 95.00 ± 0.07jFG | 58.15 ± 6.81ijklmnIJkLMN |
| 3002 | 100.00 ± 0.00jG | 58.34 ± 2.35ijklmnIJkLMN |
| JL3 | 94.50 ± 0.08jFG | 59.63 ± 0.52jklmnIJkLMN |
| 1010 | 100.00 ± 0.00jG | 60.00 ± 4.71klmnoIJkLMN |
| 2948 | 95.00 ± 0.07jFG | 61.67 ± 7.07klmnoJkLMN |
| 2957 | 95.00 ± 0.07jFG | 63.34 ± 9.43klmnoKLMNO |
| JL1 | 93.00 ± 0.10ijFG | 64.59 ± 2.95lmnoLMNO |
| 2972 | 100.00 ± 0.00jG | 65.00 ± 2.36lmnoLMNO |
| 2944 | 100.00 ± 0.00jG | 66.67 ± 4.72mnopLMNO |
| 2541 | 100.00 ± 0.00jG | 68.15 ± 7.33nopMNOP |
| BH8 | 95.00 ± 0.07jFG | 68.34 ± 2.35nopMNOP |
| 2990 | 100.00 ± 0.00jG | 70.00 ± 4.71opNOPQ |
| 2941 | 100.00 ± 0.00jG | 75.00 ± 2.36pqOPQR |
| 2952 | 100.00 ± 0.00jG | 75.00 ± 2.36pqOPQR |
| 2348 | 100.00 ± 0.00jG | 79.63 ± 2.62qPQR |
| JL2 | 100.00 ± 0.00jG | 80.00 ± 9.43qPQR |
| 2985 | 100.00 ± 0.00jG | 81.67 ± 2.35qQR |
| 2523 | 100.00 ± 0.00jG | 82.23 ± 6.29qQR |
| 833 | 100.00 ± 0.00jG | 83.34 ± 4.72qR |
Fig. 1.
Comparison of disease index (DI) of 50 Cruciferae accessions inoculated with pathotype 4 of Plasmodiophora brassicae. The horizontal axis represents the accessions name, and the vertical axis is DI.
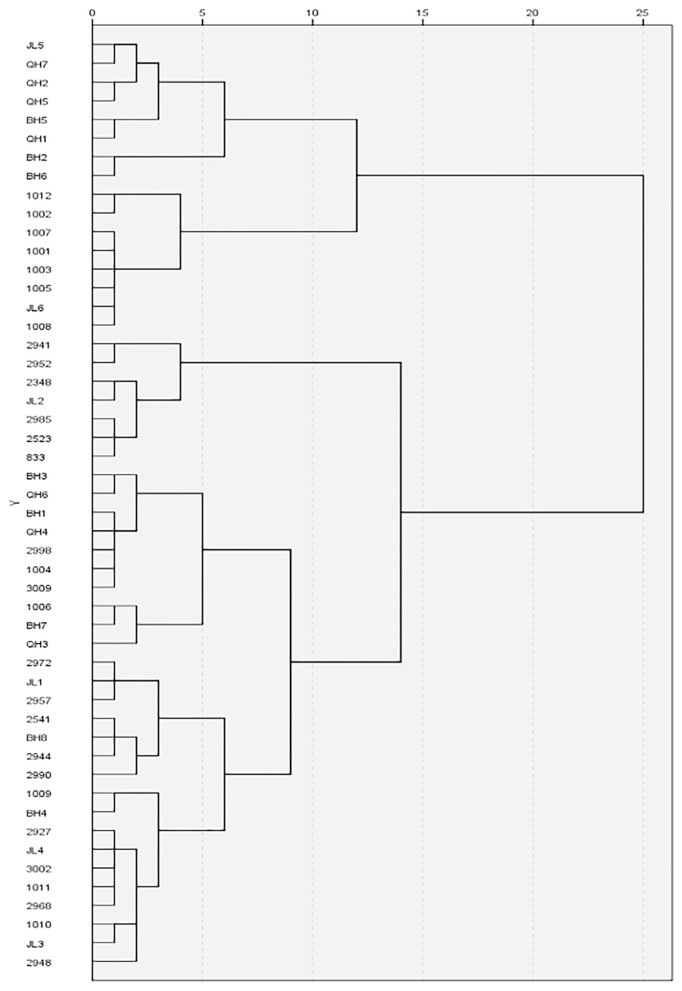
Euclidean cluster analysis of the DI of test materials
The cluster analysis of the DI of the 50 accessions showed that when the Euclidean distance was 9.12, the 50 accessions could be classified into 4 groups: resistant, moderately susceptible, susceptible and highly susceptible. Eight resistant materials (1001, 1002, 1003, 1005, 1007, 1008, 1012 and JL6) clustered into one group with a Euclidean distance of 3.98. The moderately susceptible materials BH2 and BH6 clustered together with a Euclidean distance of 0.97; six susceptible materials (JL5, QH7, QH2, and others) clustered with a Euclidean distance of 3.11. Eight susceptible materials (BH1, QH3, QH4, and others) clustered together with a Euclidean distance of 5.00 (Fig. 2).
Fig. 2.
Cluster analysis with Euclidean distance of 50 Cruciferae accessions. The horizontal axis and vertical axis represent Euclidean distance and the accessions name, respectively.
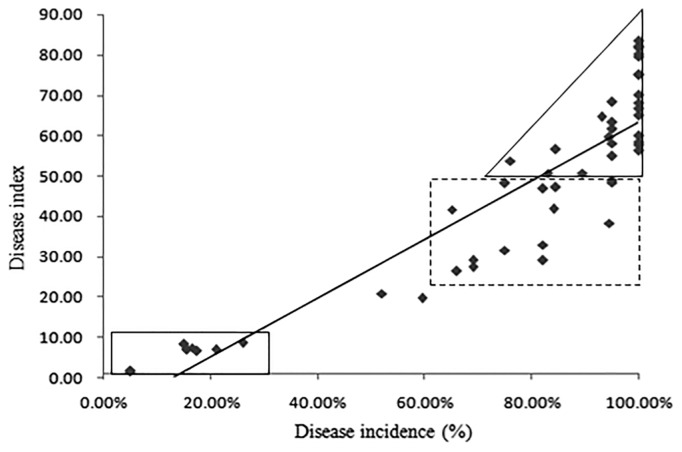
Correlation analysis between disease incidence and DI
The correlation between the disease incidence and the DI of different accessions showed that the DI increased with the increase of disease incidence, showing a significant correlation between them (P = 0.05, r = 0.906). The linear regression equation was Y = −9.603x + 72.953 (Fig. 3). The DI of the eight disease-resistant accessions in the rectangle was lower than 10, and the incidence was lower than 30%. The DI of 26 highly-sensitive accessions in the triangle frame was greater than 50, and the disease incidence was also higher than 75%. In the dotted rectangular box, the DI of 14 susceptible accessions was between 20 and 50, and the disease incidence was higher than 60%. The other two were moderately susceptible (Fig. 3).
Fig. 3.
Relation analysis between disease incidence and disease index of 50 Cruciferae accessions. Rectangles and triangle represent coverage similar individuals together.
Comparison of embryo rescue for different hybridizations
The results showed that the average number of embryos per 10 pods was 53.88. Using the embryo rescue method, the average number of embryos that survived (per 10 pods) was 37.63. The germination rate of embryos in eight hybridizations ranged from 50% to 84.48%, with an average of 69.29%. A total of 112 embryos derived from two cross-hybridizations, 2348 × 1003 and 2348 × 1008, were selected, and the number of surviving embryos from these two hybridizations was 49 and 44, respectively, and the embryo germination rates were 84.48% and 81.48%, respectively. When the female parent was B. napus (2348), the rate of embryo germination per 10 pods was higher than those of other hybridizations (Table 3).
Table 3.
Comparison of embryo rescue for different cross combinations
| Cross combination | No. of siliqua | No. of embryo culture | No. of developing embryo | Rate of embryo germination |
|---|---|---|---|---|
| 833 (S) × 1007-5 (R) | 10 | 52 | 26 | 50.00% |
| 833 (S) × 1008-2 (R) | 10 | 48 | 24 | 50.00% |
| 2523 (S) × 1007-4 (R) | 10 | 55 | 39 | 70.91% |
| 2523 (S) × 1008-1 (R) | 10 | 56 | 44 | 78.57% |
| 2348 (S) × 1003-7 (R) | 10 | 58 | 49 | 84.48% |
| 2348 (S) × 1008-1 (R) | 10 | 54 | 44 | 81.48% |
| 2541 (S) × 1007-5 (R) | 10 | 53 | 37 | 69.81% |
| 2541 (S) × 1008-1 (R) | 10 | 55 | 38 | 69.09% |
| Mean | 10 | 53.88 | 37.63 | 69.29% |
Identification of F1 hybrid authenticity
After preliminary evaluation, the distant hybrids were found to have similar characteristics to both parents, with phenotypes between the two parents, though some characteristics derived from only one parent. For example, for the cross-hybridization 2348 × 1003, the leave margin of the female parent 2348 is sharp, while the leaves of F1 hybrids and male parent 1003 were round. Thus, the leaf characteristics of the hybrid derived wholly from the male parent, but the long petiole traits were similar to the female parent. For the buds, the hybrids were yellow-green and similar to the male parent. The plant height and branch number of this hybrid were similar to those of the male parent (Fig. 4). Finally, morphological identification of all hybrids revealed no false hybrids (Table 4).
Fig. 4.
Morphological identification of F1, a, b, c and d represent the flowers, leaves, buds and mature plants of F1 and two parents (2348 and 1003).
Table 4.
Identification of F1 authenticity using three methods
| Cross combination | No. of developing embryo | No. of false hybrids using morphological identification | No. of false hybrids using cytological identification | No. of false hybrids using molecular markers identification |
|---|---|---|---|---|
| 833 (S) × 1007-5 (R) | 26 | 0 | 0 | 0 |
| 833 (S) × 1008-2 (R) | 24 | 0 | 0 | 0 |
| 2523 (S) × 1007-4 (R) | 39 | 0 | 1 | 1 |
| 2523 (S) × 1008-1 (R) | 44 | 0 | 0 | 0 |
| 2348 (S) × 1003-7 (R) | 49 | 0 | 1 | 2 |
| 2348 (S) × 1008-1 (R) | 44 | 0 | 0 | 1 |
| 2541 (S) × 1007-5 (R) | 37 | 0 | 0 | 0 |
| 2541 (S) × 1008-1 (R) | 38 | 0 | 0 | 0 |
| Total | 301 | 0 | 2 | 4 |
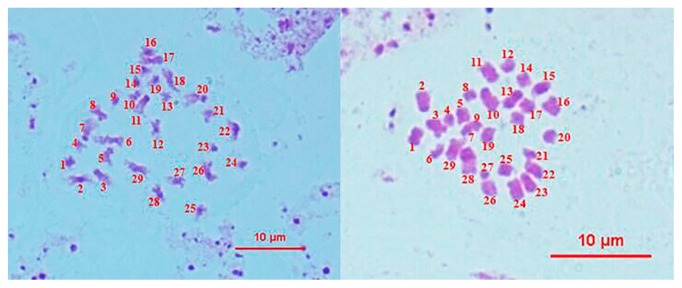
Since Chinese cabbage has 20 chromosomes and B. napus has 38 chromosomes, the interspecific hybrids should have 29 chromosomes. Cytological identification of 301 hybrid seedlings showed that there were two individuals without the 29 chromosomes; therefore they were identified as false hybrids, while the plants with the 29 chromosomes were regarded as true hybrids (Fig. 5). The two false hybrids were from the hybrid combinations 2523 × 1007 and 2348 × 1003 (Table 4).
Fig. 5.
Cytological identification of F1 hybridization 2541 × 1008, both right and left ones has 29 chromosomes, indicating that they were the true hybrids. Each chromosome is numbered by 1, 2, 3, … 29.
Eighty pairs of SSR primers were used to amplify distant hybrids and their parents. Seven pairs of SSR primers showed polymorphism between F1 hybrids and their parents (Table 5), accounting for 9% of the total primers. These polymorphic primers were used to screen F1 individuals, which revealed that four individuals did not have the same banding pattern as those of the male parents. These were from three combinations (2348 × 1003, 2348 × 1008 and 2523 × 1007). Thus, the numbers of false hybrids were two, one and one, respectively (Table 4), and the remaining 297 true hybrids were used for clubroot resistance identification.
Table 5.
Sequences of SSR markers used in markers identification
| Markers | Sequences (5′ to 3′) |
|---|---|
| BrgMS225 | CGGCAGAAAGAAAGAAAGAGAG ACCAAACCAAAAGGAGAGTCAA |
| BrgMS321 | CCTCTGTCCTCTGTAGTCCCAT GCTTACTCTAATCAGGCCCATC |
| BnGMS43 | TTTGATGGGTCTTCATCTTC GAGGTTAAGGGTTTGGAGTT |
| CB10504 | GGTGTCCCAACTGTTGAA CATTGGCATAGGAACAGG |
| CB10347 | ATCTGAACACTTTCGGCA GGAAGCACCATGTCAGC |
| CB10524 | ATGGAAGGCAACGATTCT TTCTGTGCTAGGTCTGCC |
| Na10-E08 | TCGGGGTTTGTTGTGAGG GAGGAGGATGCTAAGAGTGAGC |
Clubroot resistance identification
Thirty seven pairs of primers around eight clubroot resistance genes of B. rapa were selected to screen the parents, and as a result, two SSR primers (TCR108 and MS1) and two IP primers (IPr1 and IPr2) close to CRa and CRb were found to have the ability to amplify the polymorphic clubroot resistant fragment between the resistant parents 1007 and 1008 (Fig. 6, Table 6). TCR108 and MS1 were in 23.77 Mb and 24.05 Mb on A03, respectively. IPr1 and IPr2 were in 25.36 Mb and 25.56 Mb on A03, respectively. However, the susceptible bands were also amplified in the resistant parent 1003 and susceptible parents, which suggested that the resistance genes of 1007 and 1008 are linked to each other or the same, and the resistance gene of 1003 was different. The two molecular markers TCR108 and MS1 were used to screen all combinations except 2348 × 1003, and the results indicated that 140 clubroot resistant individuals derived from seven combinations (Fig. 6). The percentage of resistant plants per combination was generally between 36.36% and 72.97% (Table 7). Two hundred and ninety-seven individuals from eight combinations were inoculated using P4 (Fig. 7), with the results indicating that 165 plants were resistant to P4. The percentage of resistant plants per combination was between 30.77% and 63.63% (Table 8). Furthermore, the results of most combinations were in agreement regardless of which of the two identification methods were used. Two plants from combination 2523 × 1007 were classified as resistant using clubroot resistance markers but were susceptible to clubroot disease after inoculation with P4. One individual from combination 2348 × 1008 was identified as susceptible using clubroot resistance markers, but was resistant when inoculated with P4. After removal of these three individuals that were deemed inconsistent depending on the method used, 159 resistant plants from eight combinations were selected to be used for future cultivation of clubroot resistant B. napus.
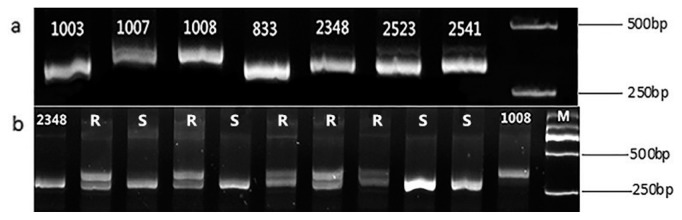
Fig. 6.
a: PCR amplification of seven parents used in distant hybridization using MS1marker. b: Partial PCR amplification of cross hybridization 2348 × 1008 with MS1marker, 1–9 represent F1 hybrids, R and S are the resistant and susceptible plants respectively, M is DNA2000 marker.
Table 6.
Information of molecular markers linked to the clubroot resistance
| Markers | Sequences (5′ to 3′) | Location on A03 (Mb) |
|---|---|---|
| MS1 | AAAACAAATATCCACCACG/CTCAATCCCACAAACCTG | 24.05 |
| TCR108 | CGGATATTCGATCTGTGTTCA/AAAATGTATGTGTTTATGTGTTTCTGG | 23.77 |
| IPr1 | GAGGCCTCCTTTTCTGGTTT/CCGGAGAAGTTTGATTCGAG | 25.36 |
| IPr2 | TGGAAGCATTGGGAGGATAG/TGGGGGTTTTCACATTCATT | 25.56 |
Table 7.
Clubroot resistance identification of F1 using molecular marker
| Cross combination | No. of hybrids seedlings | No. of susceptible materials | No. of resistant materials | Rate of susceptible materials | Rate of resistant materials |
|---|---|---|---|---|---|
| 833 (S) × 1007-5 (R) | 26 | 8 | 18 | 30.77% | 69.23% |
| 833 (S) × 1008-2 (R) | 24 | 12 | 12 | 50.00% | 50.00% |
| 2523 (S) × 1007-4 (R) | 38 | 15 | 23 | 39.47% | 60.53% |
| 2523 (S) × 1008-1 (R) | 44 | 28 | 16 | 63.64% | 36.36% |
| 2348 (S) × 1008-1 (R) | 43 | 20 | 23 | 46.51% | 53.49% |
| 2541 (S) × 1007-5 (R) | 37 | 10 | 27 | 27.03% | 72.97% |
| 2541 (S) × 1008-1 (R) | 38 | 17 | 21 | 44.74% | 55.26% |
| Total | 250 | 110 | 140 |
Fig. 7.
Performance of cross hybridization 2523 × 1008 six weeks later after inoculation using pathtype 4.
Table 8.
Clubroot resistance identification of F1 using inoculation
| Cross combination | No. of hybrids seedlings | Class of disease | Disease incidence | |||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 | 1 | 2 | 3 | |||
| 833 (S) × 1007-5 (R) | 26 | 18 | 2 | 0 | 6 | 30.77% |
| 833 (S) × 1008-2 (R) | 24 | 12 | 3 | 0 | 9 | 50.00% |
| 2523 (S) × 1007-4 (R) | 38 | 25 | 1 | 2 | 10 | 34.21% |
| 2523 (S) × 1008-1 (R) | 44 | 16 | 2 | 2 | 24 | 63.63% |
| 2348 (S) × 1003-7 (R) | 47 | 22 | 1 | 0 | 24 | 53.19% |
| 2348 (S) × 1008-1 (R) | 43 | 24 | 0 | 0 | 19 | 44.19% |
| 2541 (S) × 1007-5 (R) | 37 | 27 | 0 | 0 | 10 | 27.03% |
| 2541 (S) × 1008-1 (R) | 38 | 21 | 2 | 0 | 15 | 44.74% |
| Total | 297 | 165 | 11 | 4 | 117 | |
Discussion
Synthesis of clubroot-resistant B. napus using embryo rescue
Clubroot disease is caused by the biotrophic soil-borne pathogen P. brassicae. In recent years, this disease has been rapidly spreading among the areas where rapeseed is grown in China, causing huge losses in rapeseed production. However, there are few clubroot-resistant strains of B. napus in China. Fortunately, many clubroot-resistant materials have been found in species that are closely related to rapeseed, such as B. rapa, B. oleracea, B. nigra, and others. In this study, 50 Cruciferae accessions were analyzed to identify the clubroot-resistant germplasms. Eight clubroot-resistant accessions were obtained, of which only one rapeseed (B. juncea) germplasm was identified as clubroot resistant, and no clubroot-resistant B. napus were identified. Similar results have been reported in other studies (Peng et al. 2014), where 955 Brassica accessions were screened using pathotype 3 in Canada, but only one resistant individual out of 94 B. napus sources was identified, and the other resistant materials were primarily from B. rapa, B. oleracea, and B. nigra. Therefore, it is difficult to obtain resistant materials from existing B. napus sources, and thus the only alternative is to obtain the resistance genes from closely-related species.
Since clubroot disease is spreading widely in many countries, rapeseed production has been affected worldwide. One of the most effective ways to prevent the spread of clubroot disease is to cultivate B. napus clubroot-resistant varieties. Therefore, the resistant genes of B. rapa or B. olerecea have been transferred to B. napus to cultivate clubroot-resistant B. napus varieties by means of distant hybridization. However, interspecific hybrids between B. napus and other species are difficult to obtain due to misogamy. Therefore, the embryo rescue technique has been used to overcome the incompatibility between interspecific hybridization. This method has been successfully applied in several studies; for example, interspecific hybridization between radish and Chinese Cabbage (Zhao 1983), distant hybridization between B. napus and kale (Chen et al. 2000), and interspecific hybridization between radish and cabbage (Fang et al. 1983). In this study, distant hybrid seedlings were also successfully obtained by the embryo rescue technique. We also compared the two methods of natural seed setting and embryo rescue, and it was found that embryo rescue technology can improve the embryo germination rate of cross combinations (data not show). In addition, we also found that when B. napus 2348 was used as a female parent, it was relatively easy to obtain hybrids, indicating that the success of distant hybridization is closely related to the selection of the female parent.
Identification of F1 hybrid authenticity
Because false hybrids are likely to appear when using the distant hybridization technique, it is necessary to verify the authenticity of distant hybrids. Currently, morphological observation, cell observation, and molecular marker identification are commonly used for verification of hybrid authenticity. In this study, these three methods were used in combination, allowing identification of 297 true hybrids and four false hybrids. These results indicate that verification of distant hybrids is necessary as false hybrids will appear in the offspring. In addition, morphological identification is likely to be influenced by environmental and other factors. Therefore, a variety of identification methods should be utilized in combination to increase the reliability and authenticity of the identified hybrids for credible results.
Identification of F1 resistance
Resistance identification is an integral part of clubroot resistance breeding, and currently, artificial inoculation at the seedling stage is the most commonly used method. However, artificial inoculation is easily influenced by temperature, light, pH and other factors, affecting identification of resistant individuals. Thus, molecular markers linked to resistance genes are a more sensitive method that can be used to screen for clubroot-resistant plants. In this study, two methods, artificial inoculation and markers assisted selection were used to identify the resistance of the F1 generation. The results obtained from the two methods were similar; however, molecular marker-assisted selection is obviously much simpler. The artificial inoculation method is not suitable for screening a large number of disease-resistant materials, and the procedures are relatively complex. In addition, when inoculation conditions are modified, some individuals may not be inoculated successfully, resulting in an incorrect identification of the phenotype. Therefore, in large-scale identification of resistant individuals, the molecular marker method would be the preferred method to save labour and cost.
Analysis of clubroot resistant genes
In the current study, four molecular markers associated with clubroot resistance were identified, which were located in a region from 23.77 Mb to 25.56 Mb on A03 of B. rapa. In the adjacent area of this region, two clubroot resistant genes CRa (Ueno et al. 2012) and CRb (Hatakeyama et al. 2017) have been cloned, in which both genes encode the TIR-NBS-LRR (TNL) protein. Through analyzing the genes structure of the region surrounding the CRa and CRb, it was found that six genes Bra012540, Bra012541, Bra019409, Bra019410, Bra019412 and Bra019413 have the structure of TNL. It is difficult to determine which TNL gene is the candidate gene for this study based on the present results. Therefore, it is necessary to clone these six TNL genes in our materials to analyze the gene structures, and this work is ongoing.
Acknowledgment
The research was supported by the National key research and development program (2016YFD0100300; 2016YFD0101200), National Natural Science Foundation of China (31771835), Tang Scholar, Shaanxi Science and Technology Program (2017NY-020) and Special Project of Guizhou Academy of Agricultural Sciences [2014] 015.
Literature Cited
- Chen, S.Z., Yin, J.M., Tang, Z. and Li, J.N. (2000) A preliminary study on interspecific hybridization between Brassica napus and B. oleracea var. aceaphala. J. Southwest Agric. Univ. 22: 208–210. [Google Scholar]
- Cho, K.H., Kim, K.T., Park, S.Y., Kim, S., Do, K.R., Woo, J.G. and Lee, H.J. (2016) Evaluation of clubroot resistance in Chinese cabbage and its inheritance in the European turnip line “IT033820”, a new genetic resource. Kor. J. Hor. Sci. Technol. 34: 433–441. [Google Scholar]
- Crute, I.R., Gray, A.R., Crisp, P. and Buczacki, S.T. (1980) Variation in Plasmodiophora brassicae and resistance to clubroot disease in Brassicae and allied crops—a critical review. Plant Breed Abstr. 50: 91–104. [Google Scholar]
- Diederichsen, E., Frauen, M., Linders, E.G.A., Hatakeyama, K. and Hirai, M. (2009) Status and perspectives of clubroot resistance breeding in crucifer crops. J. Plant Growth Regul. 28: 265–281. [Google Scholar]
- Dixon, G.R. (2009) The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. J. Plant Growth Regul. 28: 194–202. [Google Scholar]
- Doyle, J. (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13–15. [Google Scholar]
- Fang, Z.Y., Sun, P.T. and Liu, Y.M. (1983) Study on distant hybridization between Raphanus Sativus and Brassica oleracea. Acta Hortic. Sin. 10: 187–191. [Google Scholar]
- Gao, F., Hirani, A.H., Liu, J., Liu, Z., Fu, G.H., Wu, C.R., McVetty, P.B.E. and Li, G.Y. (2014) Fine mapping a clubroot resistance locus in Chinese cabbage. J. Am. Soc. Hortic. Sci. 139: 247–252. [Google Scholar]
- Hasan, M.J. (2012) Screening of Brassica germplasm for resistance to Plasmodiophora brassicae pathotypes prevalent in Alberta, Canada. Can. J. Plant Sci. 92: 501–515. [Google Scholar]
- Hatakeyama, K., Niwa, T., Kato, T., Ohara, T., Kakizaki, T. and Matsumoto, S. (2017) The tandem repeated organization of NB-LRR genes in the clubroot-resistant CRb locus in Brassica rapa L. Mol. Genet. Genomics 292: 397–405. [DOI] [PubMed] [Google Scholar]
- Hirai, M., Harada, T., Kubo, N., Tsukada, M., Suwabe, K. and Matsumoto, S. (2004) A novel locus for clubroot resistance in Brassica rapa and its linkage markers. Theor. Appl. Genet. 108: 639–643. [DOI] [PubMed] [Google Scholar]
- Hirai, M. (2006) Genetic analysis of clubroot resistance in Brassica crops. Breed. Sci. 56: 223–229. [Google Scholar]
- Huang, X.Q., Ke, S.Y. and Liu, Y. (2008) Screening for resistance of oilseed rape to Plasmodiophora brassicae. Southwest China J. Agric. Sci. 21: 349–352. [Google Scholar]
- Jichuan, H.Z. and Wang, S. (1989) Breeding for clubroot resistance of crucifer in Japan. China Vegetables 3: 55–56. [Google Scholar]
- Jubault, M., Hamon, C., Gravot, A., Lariagon, C., Delourme, R., Bouchereau, A. and Manzanares-Dauleux, M.J. (2008) Differential regulation of root arginine catabolism and polyamine metabolism in clubroot-susceptible and partially resistant Arabidopsis genotypes. Plant Physiol. 146: 2008–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens, F. and Thomas, G. (1993) Inheritance of resistance to clubroot (Plasmodiophora brassicae Wor.) in Kale (Brassica oleracea ssp. acephala). Hereditas 119: 253–262. [Google Scholar]
- Li, Z., Liu, H.L. and Luo, P. (1995) Production and cytogenetics of intergeneric hybrids between Brassica napus and Orychophragmus violaceus. Theor. Appl. Genet. 91: 131–136. [DOI] [PubMed] [Google Scholar]
- Liu, H.L. (1985) Genetics and breeding in rapeseed. Shanghai: Shanghai Scientific and Technical Publishers. [Google Scholar]
- Liu, H.L. (1992) Genetic study on yellow-seeded rapeseed (Brassica napus L.). Acta Agron. Sin. 18: 241–249. [Google Scholar]
- Lowe, A.J., Jones, A.E., Raybould, A.F., Trick, M., Mole, C.L. and Edwards, K.J. (2002) Transferability and genome specificity of a new set of microsatellite primers among Brassica species of the U triangle. Mol. Ecol. Notes 2: 7–11. [Google Scholar]
- Lowe, A.J., Moule, C., Trick, M. and Edwards, K.J. (2004) Efficient large-scale development of microsatellites for marker and mapping applications in Brassica crop species. Theor. Appl. Genet. 108: 1103–1112. [DOI] [PubMed] [Google Scholar]
- Lu, G.Y., Yang, G.S. and Fu, T.D. (2001) Silver stained AFLP a novel assay for DNA finger printing in Brassica napus. J. Huazhong Agric. Univerisity 20: 413–415. [Google Scholar]
- Peng, G., Falk, K.C., Gugel, R.K., Franke, C., Yu, F.Q., James, B., Strelkow, S.E., Hwang, S.-F. and Mcgregor, L. (2014) Sources of resistance to Plasmodiophora brassicae (clubroot) pathotypes virulent on canola. Can. J. Plant Pathol. 36: 89–99. [Google Scholar]
- Piao, Z.Y., Su, R.C., Deng, Y. and Yong, P.L. (2003) SCAR and CAPS mapping of CRb gene and their application to Chinese cabbage cultivars. Kor. J. Hor. Sci. Technol. 21: 54. [Google Scholar]
- Piao, Z.Y., Deng, Y.Q., Choi, S.R., Park, Y.J. and Lim, Y.P. (2004) SCAR and CAPS mapping of CRb, a gene conferring resistance to Plasmodiophora brassicae in Chinese cabbage (Brassica rapa ssp. pekinensis). Theor. Appl. Genet. 108: 1458–1465. [DOI] [PubMed] [Google Scholar]
- Piao, Z.Y., Ramchiary, N. and Lim, Y.P. (2009) Genetics of clubroot resistance in brassica species. J. Plant Growth Regul. 28: 252–264. [Google Scholar]
- Piquemal, J., Cinquin, E., Couton, F., Rondeau, C., Seignoret, E., doucet, I., Perret, D., Villeger, M.-J., Vincourt, P. and Blanchard, P. (2005) Construction of an oilseed rape (Brassica napus L.) genetic map with SSR markers. Theor. Appl. Genet. 111: 1514–1523. [DOI] [PubMed] [Google Scholar]
- Sakamoto, K., Saito, A., Hayashida, N., Taguchi, G. and Matsumoto, E. (2008) Mapping of isolate-specific QTLs for clubroot resistance in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Theor. Appl. Genet. 117: 759–767. [DOI] [PubMed] [Google Scholar]
- Strelkov, S.E., Tewari, J.P. and Smith-Degenhardt, E. (2006) Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Can. J. Plant Pathol. 28: 467–474. [Google Scholar]
- Suwabe, K., Tsukazaki, H., Iketani, H., Hatakeyama, K., Fujimura, M., Nunome, T., Fukuoka, H., Matsumoto, S. and Hirai, M. (2003) Identification of two loci for resistance to clubroot (Plasmodiophora brassicae Woronin) in Brassica rapa L. Theor. Appl. Genet. 107: 997–1002. [DOI] [PubMed] [Google Scholar]
- Suwabe, K., Tsukazaki, H., Iketani, H., Hatakeyama, K., Kondo, M., Fujimura, M., Nunome, T., Fukuoka, H., Hirai, M. and Matsumoto, S. (2006) Simple sequence repeat-based comparative genomics between Brassica rapa and Arabidopsis thaliana: the genetic origin of clubroot resistance. Genetics 173: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, H., Matsumoto, E., Aruga, D., Kitagawa, S., Matsumura, H. and Hayashida, N. (2012) Molecular characterization of the CRa gene conferring clubroot resistance in Brassica rapa. Plant Mol. Biol. 80: 621–629. [DOI] [PubMed] [Google Scholar]
- Voorrips, R.E. and Visser, D.L. (1993) Examination of resistance to clubroot in accessions of Brassica oleracea using a glasshouse seedling test. Netherlands J. Plant Pathol. 99: 269–276. [Google Scholar]
- Wang, J., Huang, Y., Li, X.L. and Li, H.Z. (2012) Research progress in clubroot of crucifers. Plant Prot. 37: 153–158. [Google Scholar]
- Williams, P.H. (1966) A system for the determination of races of Plasmodiophora brassicae that infect cabbage and rutabaga. Phytopathology 56: 624–626. [Google Scholar]
- Yang, Y.Z., Zhang, X.L., Yang, W.G., Tian, W.W. and Wang, Q. (2011) Studies on the resistance of rape variety to Plasmodiophora brassica and screening. Chinese Agric. Sci. Bull. 27: 278–282. [Google Scholar]
- Yoshikawa, H. (1981) Breeding for clubroot resistance in Chinese cabbage. In: “Chinese cabbage” Talekar, N.S. and Griggs T.D. (eds.) AVRDC, Tainan, pp. 405–413. [Google Scholar]
- Zhang, X.W., Gao, M.Q., Yuan, Y.X., Gen, J.F., Wen, Y.C., Zhang, S.F. and Li, G.Y. (2001) Studies on artificially synthesized B. napus L. J. Henan Agric. Sci. 2: 7–10. [Google Scholar]
- Zhao, D.P. (1983) Production of intergeneric hybrids between Raphanus sativus and Brassica rapa. Acta Biol. Exp. Sin. 16: 21–26. [Google Scholar]
- Zhou, Q.Y., Li, J.N., Cui, C., Yin, J.M., Chen, L. and Tang, Z.L. (2005) Obtaining and character of the interspecfic hybrids between B. juncea and B. oleracea var. aceaphala. Acta Agron. Sin. 31: 1058–1063. [Google Scholar]