Abstract
Over the past decade, arthroscopic microfracture has become increasingly popular to treat full-thickness (Outerbridge grade IV) chondral defects of the hip. This procedure borrows marrow stimulation treatment principles and techniques from knee arthroscopy, with similar mixed clinical outcomes that may be more favorable in the short term (<2 years) and poorer in the long term. Despite these varied outcomes, microfracture remains the most frequently used technique to treat small focal chondral defects because of the relative ease and cost-effectiveness of the procedure. Consequently, recent efforts have been aimed at improving or augmenting traditional microfracture to achieve more consistent success. BioCartilage (Arthrex, Naples, FL) is a biologically active scaffold containing allograft cartilage that, when combined with autologous conditioned platelet-rich plasma and placed in a defect in which microfracture was performed, may provide a superior repair that mimics native hyaline cartilage rather than the less-durable fibrocartilage that is formed with microfracture alone. This Technical Note and accompanying video review the pertinent techniques, pearls, and potential pitfalls of the microfracture procedure augmented with BioCartilage in the treatment of symptomatic full-thickness chondral defects of the hip.
Damage to the articular cartilage of the hip may be treated arthroscopically, in which case arthroscopic microfracture is a frequently used technique.1, 2 Indications for microfracture include small focal chondral lesions (typically <400 mm2) without evidence of surrounding chondromalacia, full-thickness (Outerbridge grade IV) chondral defects on weight-bearing surfaces, and unstable fragments with intact subchondral bone.3, 4 In this procedure, which borrows marrow stimulation treatment principles and techniques from knee arthroscopy, small holes are made in the subchondral bone forming the base of the defect, providing a conduit for mesenchymal stem cells (MSCs) and growth factors. The resultant surgically induced marrow clot provides an optimal environment for the mobilized MSCs to differentiate into stable chondral repair tissue that fills the articular defect.5 Rather than regenerating the organized hyaline cartilage present in the native hip joint, microfracture results in a primarily fibrocartilage healing response.6 This softer and less-durable tissue may provide a short-term (<2 years) reprieve from symptoms, but the long-term efficacy of this repair remains in question.1, 7, 8, 9 Other limitations of traditional microfracture include unpredictable fibrocartilage fill volume, poorer prognosis for larger defects, and potential formation of subchondral osteophytes within defects.8 Consequently, recent efforts have been aimed at improving or augmenting this procedure to achieve more consistent success.
The use of scaffolds containing allogeneic cartilage has shown success in stimulating chondrogenesis and the production of tissue that mimics articular cartilage.8, 9 BioCartilage (Arthrex, Naples, FL) is a scaffold containing dehydrated, micronized allogeneic cartilage extracellular matrix that is reconstituted intraoperatively with autologous conditioned leukocyte-reduced platelet-rich plasma (PRP). Indications for microfracture with cartilage allograft augmentation such as BioCartilage are similar to those for microfracture alone. The rationale for PRP use is to deliver a supraphysiological dose of growth factors that regulate key processes in tissue repair, which may promote chondrogenesis and induce chemotaxis of MSCs at the site of cartilage repair.10 When placed in prepared chondral defects after microfracture has been performed, BioCartilage potentially sequesters locally derived pluripotent stem cells and growth factors beyond what the marrow clot is able to do itself. This may allow for better integration of the repair, greater durability, and regeneration of hyaline-like cartilage with greater type II cartilage content within the defect, leading to a superior repair over microfracture alone.8, 9 Reported complications of BioCartilage include abnormal morphologic characteristics of repair tissue, bone overgrowth, detachment of the subchondral plate, and sclerosis; however, these have also been associated with microfracture alone.8 In addition, although a specific lesion size threshold has not been established for BioCartilage, its use should be avoided with uncontained or very large lesions (the senior author [A.J.S.] prefers lesions <250 mm2 with this technique), because those scenarios may result in poor integration of the graft and/or depression of the graft against abutting joint surfaces.9 In this Technical Note, we review the pertinent techniques, pearls, and potential pitfalls of the microfracture procedure augmented with BioCartilage in the treatment of symptomatic full-thickness chondral defects of the hip (Video 1).
Surgical Technique
The patient is positioned supine as described previously.11 The PRP system of choice should be readily available in the room, and a peripheral blood draw by the anesthesia team should be obtained before incision. A standard approach using anterolateral, modified anterior, and optional posterolateral portals is used.12 A thorough diagnostic arthroscopic examination of the hip is performed as described previously12 to identify and/or confirm chondral lesions that may be amenable to microfracture with BioCartilage as an adjunct (Fig 1A). Any additional intra-articular pathology is addressed before microfracture is performed to prevent loss of visualization and clot disruption at the microfracture site (Table 1). Microfracture is then performed as described previously,13 with care taken to ensure that the margins around the defect are adequate to hold the marrow clot and graft (Fig 1 B and C).
Fig 1.
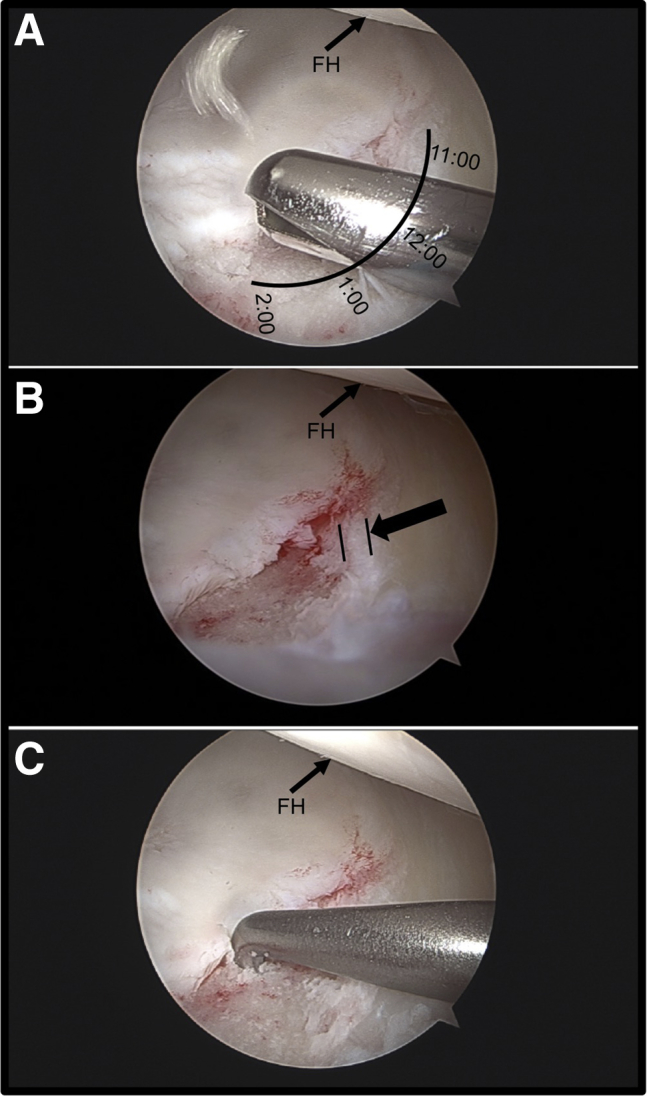
(A) Arthroscopic view from the modified anterior portal showing a full-thickness chondral lesion of the right superior acetabulum (11- to 2-o'clock position) undergoing debridement with an arthroscopic shaver in preparation for graft placement. The indications for microfracture for this lesion included Outerbridge grade IV, an area of approximately 120 mm2, and no evidence of surrounding diffuse chondromalacia. Adequate vertical margins are established with an arthroscopic biter before microfracture is performed to provide containment of the marrow clot and graft. The patient is positioned supine. (B) Arthroscopic view of the full-thickness chondral lesion in the same patient after completion of lesion preparation, showing vertical margins circumferentially (arrow). (C) Arthroscopic view of the prepared full-thickness chondral lesion in the same patient showing the use of a 90° arthroscopic awl to make microfracture holes. (FH, femoral head.)
Table 1.
Technical Pearls for Performing Arthroscopic Microfracture of Hip Augmented With BioCartilage
| Labral repair or labral reconstruction around the rim of full-thickness acetabular chondral defects provides containment of graft material and fibrin glue. |
| Before graft placement, the surgeon should turn off and remove irrigation from the arthroscopic inflow port and place the patient in Trendelenburg positioning of 30° to aid in drying of the defect and avoid fluid washout of the graft. |
| Traction should be released slowly after the fibrin glue has been allowed to set to allow the construct to contour to the opposing articular surface and avoid graft displacement. |
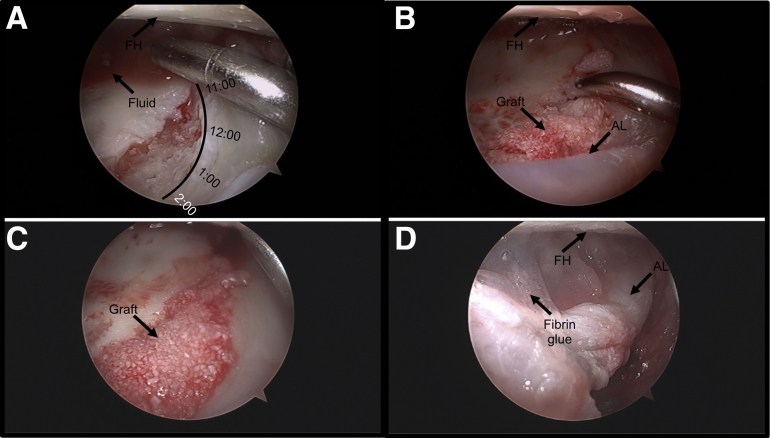
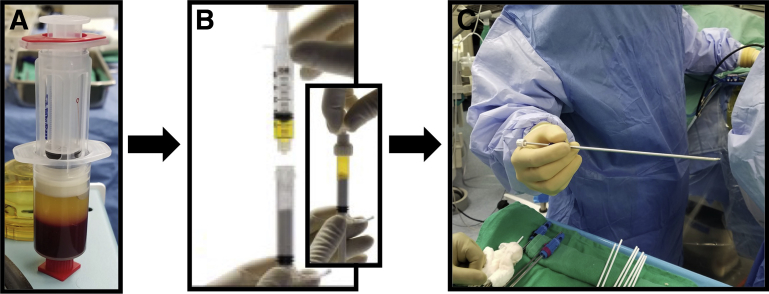
Once the microfracture is completed, the site is then prepared under traction for receipt of the graft. Irrigation is turned off and removed from the arthroscopic inflow port at this point, because the defect and central compartment must be dried thoroughly before graft placement. A 70° arthroscope is placed in the modified anterior portal, and a rounded, extended-length slotted cannula (Smith & Nephew, Andover, MA) is placed in the anterolateral portal. The joint is dried using shaver suction to aspirate fluid and long swabs (Arthrex) to wick excessive moisture from the space (Fig 2A). Furthermore, the patient is placed in Trendelenburg positioning of 30° to ensure gravity-dependent flow away from the graft site of any excess fluid within the hip joint. Autologous PRP is prepared using a sample of the patient's blood and the PRP system of choice (Arthrex) (Fig 3A). The dehydrated scaffold is then mixed with the PRP solution in a 1:0.8 scaffold–PRP volume ratio within the mixing syringe provided with the scaffold (Fig 3B). A dryer mix is preferable because over-hydration of the scaffold with PRP may make arthroscopic placement more difficult. It is important to note that blood from the microfracture will also hydrate the graft after placement. Next, the supplied arthroscopic delivery needle is attached to the mixing syringe, and the graft is transferred into the cannulated 10-gauge delivery needle (Fig 3C). By use of a slow, twisting motion, an obturator is then used to deliver the graft from the needle to the defect (Fig 2B). Once all graft material has been delivered, the rounded aspect of the slotted cannula or a polished arthroscopic elevator (Smith & Nephew) may be used to smooth the graft so that it is slightly recessed with respect to the surrounding cartilage border (Fig 2C). A layer of fibrin sealant (Evicel; Ethicon, Somerville, NJ) is then applied over the graft area only and allowed to set for 5 minutes before any manipulation of the hip or release of the traction on the joint (Fig 2D). Trendelenburg positioning should also be maintained as the sealant sets to avoid the risk of fluids disrupting the polymerizing construct.
Fig 2.
(A) Arthroscopic view from the modified anterior portal showing a full-thickness chondral lesion of the right superior acetabulum (11- to 2-o'clock position) after microfracture, showing the use of shaver suction to aspirate fluid. The defect and central compartment must be dried thoroughly before graft placement to prevent over-hydration and/or washout of the graft. Long swabs are also used to wick excessive moisture from the space, and the patient is placed in Trendelenburg positioning of 30° to ensure gravity-dependent flow away from the graft site of any excess fluid within the hip joint. The patient is positioned supine. (B) Arthroscopic view of the same lesion during delivery of the graft to the lesion using the supplied cannulated 10-gauge delivery needle. The graft is applied in strips across the lesion using a slow, twisting motion. (C) Arthroscopic view of the same lesion after delivery of BioCartilage graft. By use of the rounded aspect of a slotted cannula or a polished arthroscopic elevator, the graft is smoothed and slightly recessed with respect to the surrounding cartilage border to allow for placement of fibrin sealant. (D) Arthroscopic view of the repaired chondral lesion in the same patient after the fibrin glue has dried and as traction is slowly released. (AL, anterior labrum; FH, femoral head.)
Fig 3.
Sequence of steps in the preparation of the BioCartilage graft, beginning with preparation of autologous conditioned leukocyte-reduced platelet-rich plasma (PRP) using a sample of the patient's blood and the PRP system of choice (A). A peripheral blood draw by the anesthesia team should be obtained before incision to optimize the biological milieu of the PRP. (B) The dehydrated scaffold (bottom) is mixed with the PRP (top) in a 1:0.8 scaffold–PRP volume ratio within the mixing syringe provided with the scaffold. A dryer mix is preferable because over-hydration may impair handling and forming properties and may lead to graft washout. (C) The arthroscopic delivery needle is loaded with the prepared BioCartilage graft from the mixing syringe, and an obturator is inserted to facilitate graft delivery to the lesion.
On completion of the procedure, traction is gently removed to allow the construct to contour to the opposing articular surface while avoiding the risk of graft displacement from a rapid increase in stress across the repair site (Table 2). Postoperatively, the patient is braced and protected weight bearing is initiated. Standard microfracture postoperative rehabilitation is prescribed.7
Table 2.
Potential Pitfalls of Performing Arthroscopic Microfracture of Hip Augmented With BioCartilage
| Obtaining adequate access remains an inherent challenge of performing arthroscopic microfracture of the hip. The procedure can become even more precarious with graft placement, particularly when applying the fibrin glue. |
| Size- and location-specific indications for microfracture of the hip have yet to be defined. In the experience of the senior author (A.J.S.), anterior and superior acetabular lesions may be more amenable to BioCartilage repair than inferior or posterior lesions because of more direct access. |
| As with microfracture alone, the cartilage rim surrounding the defect must be robust and intact enough to create a pocket for the graft to sit in. Advanced degenerative lesions are generally contraindicated for traditional microfracture because of thinning around the rim of the defect. Consequently, microfracture augmented with BioCartilage may also not be appropriate for these patients. |
| It is unclear whether other contraindications for traditional microfracture (e.g., malalignment, prior surgery, and noncompliance) are necessarily contraindications for BioCartilage or whether these may be attenuated by BioCartilage. |
| The graft must be handled gently during placement because it may break apart in the defect if unstable or if mobilized too aggressively (e.g., if traction is released too quickly). Once traction is removed, reapplying traction should be avoided. |
| Delivering too much graft to the defect (“overstuffing”) can impede sealing of the repair by the fibrin glue, resulting in possible graft mobilization, poor graft integration, and/or mechanical interference within the hip joint. |
| Meticulous sterile technique must be used to avoid contamination of foreign graft materials. |
Discussion
This Technical Note presents the senior author's preferred method for the microfracture procedure augmented with BioCartilage in the treatment of symptomatic full-thickness chondral defects of the hip. Biological augmentation of traditional microfracture with stem cells, growth factors, and scaffolds (e.g., BioCartilage) has shown encouraging results in recent trials and may offer improvement in cartilage repair over microfracture alone.2, 8 Advantages of using cartilage allograft augmentation such as BioCartilage over microfracture alone may include better integration of the cartilaginous repair, greater durability, and regeneration of hyaline-like cartilage with greater type II cartilage content within the defect.8, 9 Reported downsides of BioCartilage, which have also been reported with microfracture alone, include abnormal morphologic characteristics of repair tissue, bone overgrowth, delamination of the subchondral plate, and sclerosis.8 In addition, although a specific lesion size threshold has not been established for BioCartilage, its use should be avoided with uncontained or very large lesions, because those scenarios may result in poor integration of the graft and/or depression of the graft against abutting joint surfaces.9
BioCartilage contains dehydrated, micronized allogeneic cartilage extracellular matrix that is native to articular cartilage, including type II collagen, proteoglycans, and additional growth factors. Chondral lesions of the hip joint amenable to BioCartilage repair include defects located on the acetabulum or femoral head. As an adjunct to microfracture, BioCartilage acts as a scaffold to sequester locally derived MSCs and growth factors, which may enhance cartilage repair beyond the capacity of MSCs and growth factors alone.8, 9 The micronized matrix granules provide a chondroconductive, biocompatible, resorbable material that has a consistent particle size range of 100 to 300 μm, which improves handling and delivery into the defect and provides a greater surface area for attachment of MSCs in vivo. Augmentation of microfracture marrow stimulation with BioCartilage appears to promote a more focused healing response at the site of chondral injury with subsequent repair tissue that more closely resembles native hyaline cartilage compared with microfracture alone.8, 9
In conclusion, early experience with this technique supports the concept that biological augmentation of microfracture with BioCartilage has similar indications, is equally safe, is simple to perform, and may lead to better overall restoration of the articular surface compared with microfracture alone. Future studies are needed to further delineate indications for cartilage allograft in the hip, assess longevity of the repair, reproduce results in a larger population, and compare outcomes with those of traditional microfracture and other articular cartilage repair techniques.
Footnotes
The authors report the following potential conflict of interest or source of funding: A.J.S. receives support from Smith & Nephew, AAOS, ABOS, AOSSM, AANA, ISAKOS, ISHA, MASH group, Journal of Hip Preservation Surgery, Johnson & Johnson, Bauerfeind AG. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
The patient is positioned supine with traction applied, and a standard approach with anterolateral, modified anterior, and optional posterolateral portals is used. Microfracture is performed using the Steadman technique with care taken to ensure that the margins around the defect are adequate to hold the marrow clot and graft. After completion of the microfracture, irrigation is turned off and the joint is dried. Autologous platelet-rich plasma (PRP) is prepared using a sample of the patient's blood and the PRP system of choice and is subsequently mixed with the dehydrated scaffold in a 1:0.8 scaffold–PRP volume ratio. The graft is delivered to the defect through the arthroscopic delivery needle and obturator using a slow, twisting motion. The graft is then smoothed using the rounded aspect of a slotted cannula or a polished arthroscopic elevator. A layer of fibrin sealant is applied over the graft area only and allowed to set for 5 minutes before any manipulation of the hip or release of the traction on the joint. On completion of the procedure, traction is gently removed to allow the construct to contour to the opposing articular surface while avoiding the risk of graft displacement.
References
- 1.Domb B.G., Redmond J.M., Dunne K.F., Stake C.E., Gupta A. A matched-pair controlled study of microfracture of the hip with average 2-year follow-up: Do full-thickness chondral defects portend an inferior prognosis in hip arthroscopy? Arthroscopy. 2015;31:628–634. doi: 10.1016/j.arthro.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Fontana A. A novel technique for treating cartilage defects in the hip: A fully arthroscopic approach to using autologous matrix-induced chondrogenesis. Arthrosc Tech. 2012;1:e63–e68. doi: 10.1016/j.eats.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald A.E., Bedi A., Horner N.S. Indications and outcomes for microfracture as an adjunct to hip arthroscopy for treatment of chondral defects in patients with femoroacetabular impingement: A systematic review. Arthroscopy. 2016;32:190–200.e2. doi: 10.1016/j.arthro.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Trask D.J., Keene J.S. Analysis of the current indications for microfracture of chondral lesions in the hip joint. Am J Sports Med. 2016;44:3070–3076. doi: 10.1177/0363546516655141. [DOI] [PubMed] [Google Scholar]
- 5.Salzmann G.M., Sah B., Südkamp N.P., Niemeyer P. Clinical outcome following the first-line, single lesion microfracture at the knee joint. Arch Orthop Trauma Surg. 2013;133:303–310. doi: 10.1007/s00402-012-1660-y. [DOI] [PubMed] [Google Scholar]
- 6.Oussedik S., Tsitskaris K., Parker D. Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: A systematic review. Arthroscopy. 2015;31:732–744. doi: 10.1016/j.arthro.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Abrams G.D., Mall N.A., Fortier L.A., Roller B.L., Cole B.J. BioCartilage: Background and operative technique. Oper Tech Sports Med. 2013;21:116–124. [Google Scholar]
- 8.Fortier L.A., Chapman H.S., Pownder S.L. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am J Sports Med. 2016;44:2366–2374. doi: 10.1177/0363546516648644. [DOI] [PubMed] [Google Scholar]
- 9.Hirahara A.M., Mueller K.W., Jr. BioCartilage: A new biomaterial to treat chondral lesions. Sports Med Arthrosc Rev. 2015;23:143–148. doi: 10.1097/JSA.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 10.Krüger J.P., Hondke S., Endres M., Pruss A., Siclari A., Kaps C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J Orthop Res. 2012;30:845–852. doi: 10.1002/jor.22005. [DOI] [PubMed] [Google Scholar]
- 11.Mannava S., Howse E.A., Stone A.V., Stubbs A.J. Basic hip arthroscopy: Supine patient positioning and dynamic fluoroscopic evaluation. Arthrosc Tech. 2015;4:e391–e396. doi: 10.1016/j.eats.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone A.V., Howse E.A., Mannava S., Miller B.A., Botros D., Stubbs A.J. Basic hip arthroscopy: Diagnostic hip arthroscopy. Arthrosc Tech. 2017;6:e699–e704. doi: 10.1016/j.eats.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karthikeyan S., Roberts S., Griffin D. Microfracture for acetabular chondral defects in patients with femoroacetabular impingement. Am J Sports Med. 2012;40:2725–2730. doi: 10.1177/0363546512465400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The patient is positioned supine with traction applied, and a standard approach with anterolateral, modified anterior, and optional posterolateral portals is used. Microfracture is performed using the Steadman technique with care taken to ensure that the margins around the defect are adequate to hold the marrow clot and graft. After completion of the microfracture, irrigation is turned off and the joint is dried. Autologous platelet-rich plasma (PRP) is prepared using a sample of the patient's blood and the PRP system of choice and is subsequently mixed with the dehydrated scaffold in a 1:0.8 scaffold–PRP volume ratio. The graft is delivered to the defect through the arthroscopic delivery needle and obturator using a slow, twisting motion. The graft is then smoothed using the rounded aspect of a slotted cannula or a polished arthroscopic elevator. A layer of fibrin sealant is applied over the graft area only and allowed to set for 5 minutes before any manipulation of the hip or release of the traction on the joint. On completion of the procedure, traction is gently removed to allow the construct to contour to the opposing articular surface while avoiding the risk of graft displacement.