Summary
The research project entitled “rapid prototyping of custom-made bone-forming tissue engineering constructs” (RAPIDOS) is one of the three unique projects that are the result of the first coordinated call for research proposals in biomaterials launched by the European Union Commission and the National Natural Science Foundation of China in 2013 for facilitating bilateral translational research. We formed the RAPIDOS European and Chinese consortium with the aim of applying technologies creating custom-made tissue engineered constructs made of resorbable polymer and calcium phosphate ceramic composites specifically designed by integrating the following: (1) imaging and information technologies, (2) biomaterials and process engineering, and (3) biological and biomedical engineering for novel and truly translational bone repair solutions. Advanced solid free form fabrication technologies, precise stereolithography, and low-temperature rapid prototyping provide the necessary control to create innovative high-resolution medical implants. The use of Chinese medicine extracts, such as the bone anabolic factor icaritin, which has been shown to promote osteogenic differentiation of stem cells and enhance bone healing in vivo, is a safe and technologically relevant alternative to the intensely debated growth factors delivery strategies. This unique initiative driven by a global consortium is expected to accelerate scientific progress in the important field of biomaterials and to foster strong scientific cooperation between China and Europe.
Keywords: additive manufacturing, biomaterials, bone repair
Background on the European Union–China joint project
The genesis
The launching of the biomaterials cocoordinated research call by the European Commission and the National Natural Science Foundation of China (NSFC) originated from the undertakings of European and Chinese scientists represented by the European Society for Biomaterials (2007–2013 president: Professor Luigi Ambrosio) and the Chinese Committee for Biomaterials (president: Professor Xingdong Zhang) together with the representatives of the respective funding agencies. Following initiatives such as the China–Europe Symposiums on Biomaterials in Regenerative Medicine started in 2006, and held alternatively in Europe and China promoting collaboration between European and Chinese researchers and fostering the exchange of talent, trends in biomaterials research and applications were identified [1], [2]. This collective endeavour targeted the enhancement of international collaboration in translational research and development where the needs to develop innovative biomaterial products for clinical applications in a truly international effort are desirable in order to reach the markets in a short to medium term [3], [4].
Among the research directions that were expected to lead to consolidation and extension of the knowledge and competitiveness of Europe and China in biomaterials in regenerative medicine, rapid prototyping (RP) technology for custom-made scaffolds was recognised for its strong potential. This led to the first coordinated call for research proposals in biomaterials by the European Commission and the NSFC, launched in 2013 [5], [6].
The call
The proposed call from the European Union (EU) commission was entitled “Biomaterials: imaging and rapid precise prototyping technology for custom made scaffolds” [7].
The expected impacts were targeting the development of technologies for the production of custom-made structures for the repair or regeneration of human tissues, and the improved manufacturing and performance of custom-made scaffolds for tissue repair or regeneration in the medium to long term. Additionally, a more robust European–Chinese research cooperation as well as successful joint research activities, publications, contributions to scientific events, and more intensive exchange and training of researchers were anticipated.
Formation of the RAPIDOS consortium
History of a successful application
The 9th World Biomaterials Congress organised in Chengdu, China, during the summer of 2012, which attracted biomaterial scientists from around the globe, was a catalyst for the project genesis on biomaterials—imaging and rapid precise prototyping technology for custom-made scaffolds—as most of the partners to be were united in this single location. Back from the world congregation, e-mail exchanges and discussion between the initial groups were started for the definition of an exciting research project and integration of partners with complementary expertise (Fig. 1). The proposals were submitted in October 2012 to the European Commission and the NSFC. The project was positively evaluated in February 2013 and effectively started on 1 July 2013 for both Chinese and European partners, less than 1 year after the launch of the call.
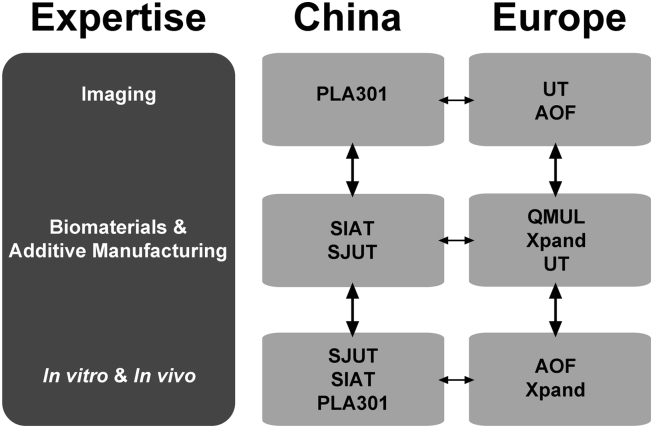
Figure 1.
Structure and interactions of the RAPIDOS consortium and partners' expertise. AOF = AO Forschungs Institute Davos (Switzerland); PLA 301 = General Hospital of People's Liberation Army–Beijing 301 Hospital (People's Republic of China); QMUL = Queen Mary, University of London (United Kingdom); RAPIDOS = rapid prototyping of custom-made bone-forming tissue engineering constructs; SIAT = Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences (People's Republic of China); SJUT = Shanghai Jiao Tong University (People's Republic of China); UT = University of Twente (The Netherlands); Xpand = Xpand Biotechnology B.V. (The Netherlands).
RAPIDOS project structure and management
The project consortium is an integrated collaborative project involving four partners from three EU countries together with three Chinese partners from different locations in a multidisciplinary collaboration. The strength of this team relies on its composition: a translational research institute with a strong track record, three academic universities, a clinic, and a small- and medium-sized enterprise and their integrated capability, which encompass the whole spectrum of biomaterials and bioengineering disciplines. The focus on relevant clinical problems (blow-out fracture of the walls of the orbit for EU partners and long bone defect for Chinese partners) will ensure the long-term success of our custom-made tissue engineered approach. Thus, this consortium is aiming to establish a benchmark in the development and transfer of innovative and performance-orientated biomaterials RP at an international scale.
Additionally, an information technology platform has been created comprising a portal linking the EU–CN partners, the partners with the AO Foundation orthopaedic surgeons network (more than 10,000 surgeons worldwide), the biomaterials and tissue engineering (TE) scientific community, and the lay community [8].
Scientific scope of the RAPIDOS project
Bone is a biological tissue with a robust capacity to heal and regenerate. Indeed, most bone fractures will, when appropriately treated, heal without any complication. However, there are still numbers of cases when the current surgical techniques together with bone grafts such as the autologous bone gold standard graft are insufficient. Examples of such impaired bone regeneration are large trauma with infections (e.g., road accidents) and bone metabolic disorders including avascular necrosis [9], [10]. In March 2010, the United Nations General Assembly proclaimed the period 2011–2020 as the Decade of Action for Road Safety [11]. Road traffic injuries kill nearly 1.3 million people annually. If current trends continue, road crashes are predicted to rise from ninth leading cause of death to fifth by 2030. About 50 million individuals sustain nonfatal injuries that represent an important cause of disability worldwide. Road traffic injuries are the leading cause of death among young people, aged between 15 and 29. Until recently, the extent of the road safety situation around the world was unclear. In 2009, the World Health Organization published the global status report on road safety as the first assessment of the road safety situation at the global level.
Bone is the most often transplanted tissue after blood, and the need for bone graft substitute materials is enormous. Worldwide, the number of bone grafts used in surgical procedures has been estimated at more than 24 million in 2010. About one-third represents the European market, whereas the total Asian markets (China and India) for bone graft substitutes and other biomaterials increased by 53.2% from 2009 to 2010. The bone graft sales are forecasted to reach a total of $3.3 billion worldwide in 2017 [12]. Therefore, the global increase of needs for bone graft substitutes and emergence of large healthcare providers in Asia support the necessities for better bone repair solutions based on biomaterial scaffolds.
Among the pertinent nonhealing bone fractures, the region of the head is a major target for development of precise custom-made bone constructs. In craniomaxillofacial surgery, large blow-out orbital floor fractures have still mitigated outcomes, and improved scaffold solutions are needed [13]. The reconstruction of large bone defects in proximal femur or proximal tibia in orthopaedic trauma surgery is also an enormous challenge for biomaterial devices owing to the requirements for both complex shape and partial load-bearing ability, but also because of the risk of incidence of steroid-associated osteonecrosis or infection, which may exceed 30% for large open fractures [14].
Biomaterials and bone Tissue Engineering (TE) have failed until now in facilitating reliable bone repair [15]. The technical issues for the engineering of a scaffold for bone TE are as follows: (1) fabrication of biomaterial scaffolds with anatomical fit of complex three-dimensional (3D) large bone defects; (2) fabrication of biomaterials with adequate mechanical and structural stability/degradation kinetics; (3) controlling the behaviour (adhesion, proliferation, differentiation, and matrix remodelling) of cells and tissues at the interface with biomaterials; and (4) fabrication of biomaterials with optimised macroarchitecture for improved mass transport and perfusion for delivery of biological effectors. These effectors could be antibacterial agents [e.g., magnesium (Mg)] compounded with the biomaterials to decrease infection in large trauma due to road traffic accidents, growth factors, nutrients, or other cells. However, the main desirable biological capacities of a biomaterial scaffold for bone are osteoconductivity, osteoinductivity, and osteogenicity. Calcium phosphate (CaP) ceramics and incorporated growth factors can provide these properties [16], [17]. The use and efficacy of growth factor containing bone graft substitutes (e.g., Infuse; recombinant bone human bone morphogenetic protein-2 or rhBMP-2) is intensely debated nowadays [18], and use of Chinese medicine extracts such as the bone anabolic compound icaritin, which has been shown to promote osteogenic differentiation of stem cells and enhance bone healing in vivo, is a promising alternative [19], [20].
For bone repair, patient-specific tissue engineered biomaterials can address several drawbacks of the current orthopaedic devices: optimised shape for best cosmetic, mechanical and anatomical stability, no thermosensitivity associated with the use of metallic implants, optimised internal architecture in accordance to the delivered biological effectors (e.g., cells, antibacterial agent, Chinese medicine extract, magnesium), and mass transport for vascularisation and bone ingrowth. However, there are also other clinical considerations to be taken into account. Definitely, a macroscopic precision above 0.5 mm for the considered biomaterial scaffolds is unnecessary for bone repair as surgeons would not be able to achieve construct positioning with a better accuracy in challenging surgical situations [21]. Additionally, clinical available 3D imaging instruments [e.g., computed tomography (CT)] have a precision not lower than 0.2 mm, and sometimes precision less than 0.4 mm can be achieved, like in the orbit. Hence, the clinical focus is necessary to achieve real and relevant progresses toward fitting custom-made biomaterial products.
A high resolution for scaffold TE is needed to create biomaterial scaffolds with correct external shape, adequate mechanical and resorption properties, and optimised internal architecture and biofunctionalities in combination with a clinically relevant cell source: human mesenchymal stromal cells (hMSCs) in the case of this consortium. Advanced solid free form fabrication, also called rapid prototyping, could provide the necessary control to create such innovative medical devices. Stereolithography (SLA) offers the high resolution necessary to create controlled architecture and anatomically well-fitting devices. The low-temperature rapid prototyping (LT-RP) is a unique technique in its ability to incorporate temperature-sensitive active compounds into the scaffolds [19], [22], [23].
Thus, the goal of this European and Chinese consortium is to apply RP technologies to create custom-made tissue engineered biomaterial constructs by integrating (1) imaging and information technologies, (2) biomaterials and process engineering, and (3) biological and biomedical engineering for novel and truly translational bone repair solutions [24].
There are major challenges to our approaches [25]. First, we need to integrate patient-specific clinical images together with relevant architecture design for optimal mass transport and delivery of biological stimuli (hMSCs and icaritin) and develop a valid surgical procedure. Second, we need to develop adequate biomaterial composites with controlled nanostructure and precise scaffold fabrication techniques. Third, it is necessary to create adaptive and biologically active TE constructs through the addition of osteogenic biological stimuli such as hMSCs isolated from the patient bone marrow (endogenous growth factor) and/or small anabolic molecule extract from Chinese medicine (e.g., icaritin). Finally, we need to combine all of the above advanced stages for the delivery and possibly product registration of the custom-made biomaterial tissue-engineered construct.
Concept and objectives of the RAPIDOS project
Imaging custom-made scaffold design
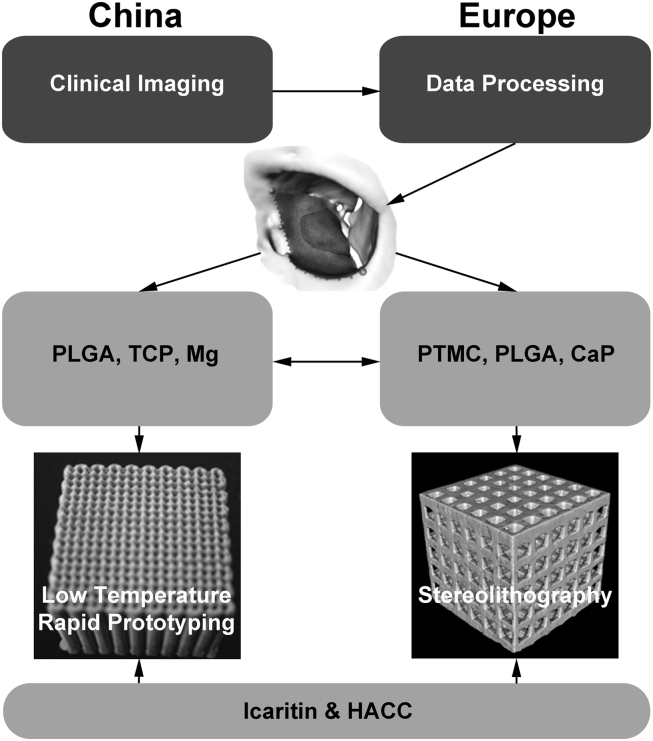
Image processing software and computer graphic technologies can now be semiautomated to provide radiologists and surgeons with tools to analyse clinical imaging data such as CT images and exploit this original dataset to create, for example, a 3D stereolithographic model of an implant that can be transferred as a set of coordinates for the printing system to create a custom-made scaffold implant (Fig. 2). In this consortium, we will develop a computer workflow based on clinical CT data in which it is envisioned that the surgeons will have an interactive tool to decide which scaffold implant design is suitable for the reconstruction of the bony defect with taking into account boundaries such as the biomaterials' mechanical properties and fabrication parameters.
Figure 2.
Scheme of the RAPIDOS approach toward custom-made scaffolds: from standard clinical computed tomography data collection and processing to create specific implant design to development of composite biomaterials for fabrication of implants using additive manufacturing technologies and inclusion of bioactive products. CaP = calcium phosphate particles; HAAC = hydroxypropyltrimethyl ammonium chloride chitosan; Mg = magnesium; PLGA = poly(l-lactide-co-glycolide); PTMC = poly(trimethyl carbonate); RAPIDOS = rapid prototyping of custom-made bone-forming tissue engineering constructs; TCP = β-tricalcium phosphate.
Biomaterials
A poly(trimethylene carbonate) (PTMC)-based resin will be used in combination with osteoinductive CaP [i.e., biphasic calcium phosphate (BCP)] particles for the preparation of the SLA scaffold. PTMC resins can be prepared and photocrosslinked to form a solid material [26], [27]. PTMC is an amorphous polymer with a relatively low elastic modulus of 5–7 MPa at room temperature. PTMC degrades enzymatically in vivo via a surface erosion process without the formation of acidic degradation products [28], [29]. The polymer has not been shown to calcify or lead to the formation of new bone upon implantation. However, its combination with BCP provides osteoinductive properties to the scaffold as the blended BCP becomes exposed upon in vivo degradation of the PTMC matrix. Moreover, the combination of bone anabolic icaritin loaded polymeric microspheres or nanofibres prepared from poly(lactic acid) (PLA) or poly(lactic-co-glycolic acid) (PLGA) within the PTMC resin will provide additional biofunctionality to the scaffold through the sustained release of the Chinese medicine extract provided by the Chinese partners [30], [31]. In addition, such polyester fibres will strengthen the mechanical properties of the resulting polymer–polymer composites, owing to the high modulus of PLA and derivatives. The nanofibres will also provide a simple mean to texture the interface of the composites, which is a well-established factor impacting on cell adhesion and bone engineering [32], [33]. PLGA/CaP composites will be the alternative biomaterial compositions for the direct comparison of several RP technologies. PLGA and CaP [i.e., tricalcium phosphate (TCP)] particles will be combined to form osteoinductive scaffolds. PLGA is biocompatible and possesses a range of degradation rates that can be adjusted to the new bone tissue growth rate, which results in a natural replacement of tissue without the long-term complications associated with none or very slow resorbable implants [34], [35]. However, some drawbacks still exist in its applications, including notably the generation of acidic species, resulting in a decrease in local pH, as it could cause tissue inflammation in the region adjacent to the implants [36]. To overcome these drawbacks, pH neutralisation with basic compounds such as TCP and other biodegradable and bioactive ceramics has been shown to improve the dissolution properties (neutral pH upon degradation of PLGA) and enhance cell adhesion and bone formation [37], [38]. TCP is used commercially in clinical orthopaedics as material for bone fillers, and as a local delivery system for drug and cells [39]. PLGA/TCP scaffolds prepared using LT-RP as a TE construct and drug release device have already been reported [40]. The different degradation mechanisms of PLGA and PTMC will influence the availability of the biological stimuli (e.g., icaritin, magnesium) included in the bulk of the composite matrices of the scaffolds allowing for improved release and activity of the biologics [41], [42], [43], [44], [45], [46] and provide a simple tool to control the remodelling of the matrix and its mechanical behaviour. Furthermore, Chinese partners have synthesised a new quaternised chitosan derivative [hydroxypropyltrimethyl ammonium chloride chitosan (HACC)] that contains a series of substitution of quaternary ammonium, and demonstrated that HACC with 26% degree of substitution had a strong antibacterial activity and simultaneously good cytocompatibility with osteogenic cells [47]. An additional strategy of the consortium will be to impregnate the chitosan derivatives into the PLGA/TCP and PTMC/CaP composites, which would provide dual functions to promote bone regeneration as well as prevent bacterial colonization.
RP technologies
In our consortium, high-resolution SLA will be used and developed by the EU partners (Fig. 2). It is a precise RP manufacturing method, in which objects are constructed in a layer-by-layer fashion by photopolymerisation [48]. By using SLA to directly prepare the TE scaffolds, the drawbacks of conventional scaffold preparation methods can be overcome: cell support structures with predetermined architectures can be prepared that not only precisely fit a (bone) defect, but also create accurate macrostructures down to a cell size (∼20 μm) [49]. It has already been shown that scaffolds prepared via SLA fabrication can have adaptable mechanical stiffness and permeability that, in turn, can stimulate mesenchymal stromal cell (MSC) responses and potentially promote faster bone tissue regeneration [50]. LT-RP technology will be used and developed to yield biological effectors (e.g., cells, antibacterial agent, Chinese medicine extract, magnesium) incorporated into PLGA/TCP scaffolds by the Chinese partners (Fig. 2) [51]. PTMC/CaP composites prepared by LT-RP with a different degradation profile compared to PLGA/CaP will be prepared for direct comparison to the planned European study using SLA fabrication [52].
Tissue engineering for bone repair
This consortium aims to modulate and control the biological response of hMSCs seeded into scaffolds via (1) the optimisation of the scaffold architecture and (2) biofunctionality (icaritin and Mg-loaded microspheres and particles). The anti-infection and antibacterial effects of PLGA/TCP/Mg and HACC incorporated into PLGA/TCP scaffolds will also be investigated. This specific objective will be coordinated with the Chinese partners to develop common experimental protocols in order to standardise the evaluation of the biological performance of the prepared TE constructs. Typically, MSC seeding efficiency, proliferation, alkaline phosphatase activity, osteogenic differentiation, calcium deposition in several scaffold architectures, PTMC/CaP (and PLGA/TCP) compositions and in the presence of icaritin and Mg-loaded microparticles at different concentrations will be assessed. The final goal will be the selection of scaffold characteristics (e.g., porosity, pore size and design, mechanics) that allow for improved cell response. Then, the in vivo assessment of the selected candidate custom-made bone TE construct(s) will be performed in a relevant preclinical model in parallel by the European and Chinese partners.
In vivo efficacy investigation will be designed to evaluate osteogenesis, for example, in a bilateral ulna bone segmental defect model implanted with composite scaffold in rabbits, with radiography and in vivo micro-CT for studying new bone regeneration and histology for host tissue and scaffold material interactions. Finally, a large animal pilot study has been performed to assess the whole chain of concepts from imaging to bone TE in order to demonstrate clinical feasibility of the custom-made biomaterial scaffold-based therapy prior to clinical testing.
Impact and perspectives on the RAPIDOS project
To date, a clinical CT imaging process technology workflow for the development of anatomically relevant and precise custom-made macrostructured designed scaffolds has been created. The goal of this workflow is to allow the surgeons to design and self-assess patient-specific implants taking into account the constraints of the biomaterial and fabrication process. The optimisation of composite formulations—poly(trimethylcarbonate)/CaP and PLGA/TCP/Mg for SLA and low-temperature rapid manufacturing, respectively—is well advanced, and already composite scaffolds can be fabricated via both SLA and LT-RP. PLA nanofibres loaded with icaritin have been prepared for incorporation into the photopolymerisable resin formulation for SLA. In vitro and in vivo studies have shown the osteopromotive effect of icaritin loaded into scaffolds, and magnesium was shown to influence biofilm formation onto the surface of PLGA/TCP/Mg scaffolds. We expect the combined approach of the project to give rise to additional and multiple innovations to be exploited by the networks of partners.
Finally, we hope that through our (EU–China) collaboration, we can advance therapeutic solutions to ease suffering from nonhealing bone fractures/defects in the future and help achieve faster patient recovery through the development of custom-made implant and patient-specific therapy.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors acknowledge the funding provided by the NSFC-DG-RTD Joint Scheme (Project No. 51361130034) and the European Union's 7th Framework Program under grant agreement no. NMP3-SL-2013-604517. The authors also thank Mr Adriano Rucci, Mr Peter Smith, and Dr Zhen Li for help with the RAPIDOS web platform setup, design, and Chinese translation, respectively.
Contributor Information
R. Geoff Richards, Email: geoff.richards@aofoundation.org.
Ling Qin, Email: qin@ort.cuhk.edu.hk.
References
- 1.European Society for Biomaterials. Available at: http://www.esbiomaterials.eu [accessed 11.12.14].
- 2.Chinese Society for Biomaterials. Available at: http://www.csbm.org.cn [accessed 11.12.14].
- 3.Qin L. Translation medicine in orthopedics (Editorial) J Orthop Transl. 2013;1:3–5. [Google Scholar]
- 4.Bian L., Mak A.F.T., Wu C., Cheng C., Gu Z., Zhang X. A Model for facilitating translational research and development in China: call for establishing Hong Kong Branch of Chinese National Engineering Research Centre for Biomaterials. J Orthop Transl. 2014;2:170–176. [Google Scholar]
- 5.European Research Commission. Available at: www.http://cordis.europa.eu [accessed 11.12.14].
- 6.National Natural Science Foundation of China. Available at: http://www.nsfc.gov.cn [accessed 11.12.14].
- 7.RAPIDOS project description (European Commision web site). Available at: http://cordis.europa.eu/project/rcn/108972_en.html [accessed 11.12.14].
- 8.RAPIDOS. Available at: http://www.rapidos-project.eu [accessed 11.12.14].
- 9.Darouiche R.O. Treatment of infections associated with surgical implants. New Engl J Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg M.E. Core decompression of the femoral head for avascular necrosis: indications and results. Can J Surg. 1995;38:S18–S24. [PubMed] [Google Scholar]
- 11.Ki-moon B. United Nations Decade of Action for Road Safety 2011–2020. Available at: http://www.un.org/en/roadsafety/2011 [accessed 11.12.14]. p. http://www.un.org/en/roadsafety/.
- 12.Medipoint Bone Grafts and Substitutes — EU Analysis and Market Forecasts. Medipoint; 2014. pp. 1–185.http://www.reportlinker.com/p02027473-summary/MediPoint-Bone-Grafts-and-Substitutes-EU-Analysis-and-Market-Forecasts.html Available at: [Google Scholar]
- 13.Giannoudis P.V., Dinopoulos H., Tsiridis E. Bone substitutes: an update. Injury. 2005;36:S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Trampuz A., Widmer A.F. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19:349–356. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- 15.Hollister S.J., Murphy W.L. Scaffold translation: barriers between concept and clinic. Tissue Eng B Rev. 2011;17:459–474. doi: 10.1089/ten.teb.2011.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao C., Zhou H., Ge S., Tang T., Hou H., Luo M. Repair of orbital wall defects using biocoral scaffolds combined with bone marrow stem cells enhanced by human bone morphogenetic protein-2 in a canine model. Int J Mol Med. 2010;26:517–525. doi: 10.3892/ijmm_00000494. [DOI] [PubMed] [Google Scholar]
- 17.Yuan H., Fernandes H., Habibovic P., de Boer J., Barradas A.M., de Ruiter A. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci U S A. 2010;107:13614–13619. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carragee E.J., Hurwitz E.L., Weiner B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Wang X.L., Xie X.H., Zhang G., Chen S.H., Yao D., He K. Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair. J Orthop Res. 2013;31:164–172. doi: 10.1002/jor.22188. [DOI] [PubMed] [Google Scholar]
- 20.Huang J., Yuan L., Wang X., Zhang T.L., Wang K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007;81:832–840. doi: 10.1016/j.lfs.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 21.D'Haese J., Van De Velde T., Komiyama A., Hultin M., De Bruyn H. Accuracy and complications using computer-designed stereolithographic surgical guides for oral rehabilitation by means of dental implants: a review of the literature. Clin Implant Dent Rel Res. 2012;14:321–335. doi: 10.1111/j.1708-8208.2010.00275.x. [DOI] [PubMed] [Google Scholar]
- 22.Xie X.H., Wang X.L., Zhang G., He Y.X., Wang X.H., Liu Z. Structural and degradation characteristics of an innovative porous PLGA/TCP scaffold incorporated with bioactive molecular icaritin. Biomed Mater. 2010;5:054109. doi: 10.1088/1748-6041/5/5/054109. [DOI] [PubMed] [Google Scholar]
- 23.Chen S.-H., Zheng L.-Z., Xie X.-H., Wang X.-L., Lai Y.-X., Chen S.-K. Comparative study of PLGA/TCP scaffolds incorporated or coated with osteogenic growth factors for enhancement of bone regeneration. J Orthop Transl. 2014;2:91–104. [Google Scholar]
- 24.Sun W., Darling A., Starly B., Nam J. Computer-aided tissue engineering: overview, scope and challenges. Biotechnol Appl Biochem. 2004;39:29–47. doi: 10.1042/BA20030108. [DOI] [PubMed] [Google Scholar]
- 25.Kim K., Yeatts A., Dean D., Fisher J.P. Stereolithographic bone scaffold design parameters: osteogenic differentiation and signal expression. Tissue Eng B Rev. 2010;16:523–539. doi: 10.1089/ten.teb.2010.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin Q.M., Takita H., Kohgo T., Atsumi K., Itoh H., Kuboki Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J Biomed Mater Res. 2000;52:491–499. [PubMed] [Google Scholar]
- 27.van Leeuwen A.C., Bos R.R., Grijpma D.W. Composite materials based on poly(trimethylene carbonate) and beta-tricalcium phosphate for orbital floor and wall reconstruction. J Biomed Mater Res B Appl Biomater. 2012;100:1610–1620. doi: 10.1002/jbm.b.32729. [DOI] [PubMed] [Google Scholar]
- 28.Schuller-Ravoo S., Feijen J., Grijpma D.W. Preparation of flexible and elastic poly(trimethylene carbonate) structures by stereolithography. Macromol Biosci. 2011;11:1662–1671. doi: 10.1002/mabi.201100203. [DOI] [PubMed] [Google Scholar]
- 29.Pego A.P., Van Luyn M.J., Brouwer L.A., van Wachem P.B., Poot A.A., Grijpma D.W. In vivo behavior of poly(1,3-trimethylene carbonate) and copolymers of 1,3-trimethylene carbonate with d,l-lactide or epsilon-caprolactone: degradation and tissue response. J Biomed Mater Res A. 2003;67:1044–1054. doi: 10.1002/jbm.a.10121. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z., Grijpma D.W., Feijen J. Trimethylene carbonate-based polymers for controlled drug delivery applications. J Control Rel. 2006;116:e28–e29. doi: 10.1016/j.jconrel.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z., Grijpma D.W., Feijen J. Poly(trimethylene carbonate) and monomethoxy poly(ethylene glycol)-block-poly(trimethylene carbonate) nanoparticles for the controlled release of dexamethasone. J Control Rel. 2006;111:263–270. doi: 10.1016/j.jconrel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Gautrot J.E., Malmstrom J., Sundh M., Margadant C., Sonnenberg A., Sutherland D.S. The nanoscale geometrical maturation of focal adhesions controls stem cell differentiation and mechanotransduction. Nano Lett. 2014;14:3945–3952. doi: 10.1021/nl501248y. [DOI] [PubMed] [Google Scholar]
- 33.Biggs M., Jonathan P., Richards R.G., Dalby M.J. Nanotopographical modification: a regulator of cellular function through focal adhesions. Nanomed Nanotechnol Biol Med. 2010;6:619–633. doi: 10.1016/j.nano.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kai H., Wang X., Madhukar K.S., Qin L., Yan Y., Zhang R. Fabrication of a two-level tumor bone repair biomaterial based on a rapid prototyping technique. Biofabrication. 2009;1:025003. doi: 10.1088/1758-5082/1/2/025003. [DOI] [PubMed] [Google Scholar]
- 35.Pan Z., Ding J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus. 2012;2:366–377. doi: 10.1098/rsfs.2011.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ge Z., Tian X., Heng B.C., Fan V., Yeo J.F., Cao T. Histological evaluation of osteogenesis of 3D-printed poly-lactic-co-glycolic acid (PLGA) scaffolds in a rabbit model. Biomed Mater. 2009;4:021001. doi: 10.1088/1748-6041/4/2/021001. [DOI] [PubMed] [Google Scholar]
- 37.Bostman O., Hirvensalo E., Vainionpaa S., Makela A., Vihtonen K., Tormala P. Ankle fractures treated using biodegradable internal fixation. Clin Orthop Rel Res. 1989:195–203. [PubMed] [Google Scholar]
- 38.Wang D.-X., He Y., Bi L., Qu Z.-H., Zou J.-W., Pan Z. Enhancing the bioactivity of poly(lactic-co-glycolic acid) scaffold with a nano-hydroxyapatite coating for the treatment of segmental bone defect in a rabbit model. Int J Nanomed. 2013;8:1855–1865. doi: 10.2147/IJN.S43706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehrenfried L.M., Patel M.H., Cameron R.E. The effect of tri-calcium phosphate (TCP) addition on the degradation of polylactide-co-glycolide (PLGA) J Mater Sci Mater Med. 2008;19:459–466. doi: 10.1007/s10856-006-0061-6. [DOI] [PubMed] [Google Scholar]
- 40.Liu G., Zhao L., Cui L., Liu W., Cao Y. Tissue-engineered bone formation using human bone marrow stromal cells and novel beta-tricalcium phosphate. Biomed Mater. 2007;2:78–86. doi: 10.1088/1748-6041/2/2/004. [DOI] [PubMed] [Google Scholar]
- 41.Chen S.H., Wang X.L., Xie X.H., Zheng L.Z., Yao D., Wang D.P. Comparative study of osteogenic potential of a composite scaffold incorporating either endogenous bone morphogenetic protein-2 or exogenous phytomolecule icaritin: an in vitro efficacy study. Acta Biomater. 2012;8:3128–3137. doi: 10.1016/j.actbio.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 42.Li J., Song Y., Zhang S., Zhao C., Zhang F., Zhang X. In vitro responses of human bone marrow stromal cells to a fluoridated hydroxyapatite coated biodegradable Mg–Zn alloy. Biomaterials. 2010;31:5782–5788. doi: 10.1016/j.biomaterials.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Janning C., Willbold E., Vogt C., Nellesen J., Meyer-Lindenberg A., Windhagen H. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010;6:1861–1868. doi: 10.1016/j.actbio.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 44.Yang C., Yuan G., Zhang J., Tang Z., Zhang X., Dai K. Effects of magnesium alloys extracts on adult human bone marrow-derived stromal cell viability and osteogenic differentiation. Biomed Mater. 2010;5:045005. doi: 10.1088/1748-6041/5/4/045005. [DOI] [PubMed] [Google Scholar]
- 45.Witte F., Kaese V., Haferkamp H., Switzer E., Meyer-Lindenberg A., Wirth C.J. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26:3557–3563. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 46.Wu F., Wei J., Guo H., Chen F., Hong H., Liu C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008;4:1873–1884. doi: 10.1016/j.actbio.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Tan H.L., Lin W.T., Tang T.T. The use of antimicrobial-impregnated PMMA to manage periprosthetic infections: controversial issues and the latest developments. Int J Artif Organs. 2012;35:832–839. doi: 10.5301/ijao.5000163. [DOI] [PubMed] [Google Scholar]
- 48.Peltola S.M., Melchels F.P., Grijpma D.W., Kellomaki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann Med. 2008;40:268–280. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 49.Melchels F.P., Feijen J., Grijpma D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 50.Kim K., Dean D., Wallace J., Breithaupt R., Mikos A.G., Fisher J.P. The influence of stereolithographic scaffold architecture and composition on osteogenic signal expression with rat bone marrow stromal cells. Biomaterials. 2011;32:3750–3763. doi: 10.1016/j.biomaterials.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao H., Walboomers X.F., Habraken W.J., Zhang Z., Li Y., Grijpma D.W. Injectable calcium phosphate cement with PLGA, gelatin and PTMC microspheres in a rabbit femoral defect. Acta Biomater. 2011;7:1752–1759. doi: 10.1016/j.actbio.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 52.Zeng N., van Leeuwen A., Yuan H., Bos R.R., Grijpma D.W., Kuijer R. Evaluation of novel resorbable membranes for bone augmentation in a rat model. Clin Oral Implants Res. 2014;7 doi: 10.1111/clr.12519. [DOI] [PubMed] [Google Scholar]