Summary
Background/Objective
We have previously shown that an extracellular matrix (ECM) bioscaffold derived from porcine small intestine submucosa (SIS) enhanced the healing of a gap injury of the medial collateral ligament as well as the central third defect of the patellar tendon. With the addition of a hydrogel form of SIS, we found that a transected goat anterior cruciate ligament (ACL) could also be healed. The result begs the research question of whether SIS hydrogel has positive effects on ACL fibroblasts (ACLFs) and thus facilitates ACL healing.
Methods
In the study, ECM-SIS hydrogel was fabricated from the digestion of decellularised and sterilised sheets of SIS derived from αGal-deficient (GalSafe) pigs. As a comparison, a pure collagen hydrogel was also fabricated from commercial collagen type I solution. The morphometrics of hydrogels was assessed with scanning electron microscopy. The ECM-SIS and collagen hydrogels had similar fibre diameters (0.105 ± 0.010 μm vs. 0.114 ± 0.004 μm), fibre orientation (0.51 ± 0.02 vs. 0.52 ± 0.02), and pore size (0.092 ± 0.012 μm vs. 0.087 ± 0.008 μm). The preservation of bioactive properties of SIS hydrogel was assessed by detecting bioactive molecules sensitive to processing and enzyme digestion, such as growth factors fibroblast growth factor-2 (FGF-2) and transforming growth factor-beta 1 (TGF-β1), with enzyme-linked immunosorbent assay. ACLFs were isolated and expanded in culture from explants of rat ACLs (n = 3). The cells were then seeded on the hydrogels and cultured with 0%, 1%, and 10% foetal bovine serum (FBS) for 3 days and 7 days. Cell attachment was observed using a light microscope and scanning electron microscopy, whereas cell proliferation and matrix production (collagen types I and III) were examined with bromodeoxyuridine assays and reverse transcription-polymerase chain reaction, respectively.
Results
The results showed that FGF-2 and TGF-β1 in the SIS hydrogel were preserved by 50% (65.9 ± 26.1 ng/g dry SIS) and 90% (4.4 ± 0.6 ng/g dry SIS) relative to their contents in ECM-SIS sheets, respectively. At Day 3 of culture, ACLFs on the SIS hydrogel were found to proliferate 39%, 31%, and 22% more than those on the pure collagen hydrogel at 0%, 1%, and 10% FBS, respectively (p < 0.05). Collagen type I mRNA expression was increased by 150%, 207%, and 100%, respectively, compared to collagen hydrogel (p < 0.05), whereas collagen type III mRNA expression was increased by 123% and 132% at 0% and 1% FBS, respectively (all p < 0.05) but not at 10% FBS. By Day 7, collagen type I mRNA expression was still elevated by 137% and 100% compared to collagen hydrogel at 1% and 10% FBS, respectively (p < 0.05). Yet, collagen type III mRNA levels were not significantly different between the two groups at any FBS concentrations.
Conclusion
Our data showed that the ECM-SIS hydrogel not only supported the growth of ACLFs, but also promoted their proliferation and matrix production relative to a pure collagen hydrogel. As such, ECM-SIS hydrogel has potential therapeutic value to facilitate ACL healing at the early stage after injury.
Keywords: ACL fibroblasts, fibre morphology, growth factors, hydrogel, porcine small intestine submucosa
Introduction
The anterior cruciate ligament (ACL) in the knee joint is an important structure to maintain knee stability. Unlike extra-articular ligaments such as the medial collateral ligament, which have a high propensity for healing even without surgical management [1], [2], the ACL has a limited capability to heal once injured, and the outcomes of nonsurgical management of its midsubstance rupture have been poor [3], [4]. The literature has attributed the causes to detrimental factors such as high residual strain resulting in a significant retraction of ruptured ends that left a large gap between the torn ends, relative poor circulation making it difficult to form localized hematoma to bridge the gap, as well as lower activity of ACL fibroblasts (ACLFs) [5], [6], [7], [8], [9], [10]. Although surgical reconstruction using soft-tissue autografts/allografts has been the major treatment regimen to restore knee stability, graft-associated complications are not uncommon, resulting in a high rate of less-than-satisfactory outcomes in the long term [11], [12], [13]. With the advancements in functional tissue engineering, various strategies have been vigorously investigated in order to improve the healing of ACL with minimal surgical interference.
Extracellular matrix (ECM) bioscaffolds, such as those derived from the porcine small intestine submucosa (SIS), have been successfully used in a number of clinical applications to accelerate healing and repair of hernia and injuries in other soft connective tissues [14], [15], [16]. They have also been approved by the Food and Drug Administration for urethral reconstruction, pelvic prolapse, soft tissue repair (both connective and cardiac), etc. [17]. In our research centre, we have shown that ECM-SIS enhanced the healing of a central third defect of the patellar tendon after it was harvested as an autograft for ACL reconstruction. Furthermore, ECM-SIS improved the healing of a 6-mm-wide gap of a torn medial collateral ligament and, more importantly, improved the mechanical properties of the neoligament [18], [19], [20], [21].
Recently, methods have become available to create a hydrogel form of the ECM that can be injected to fill a wound in situ [22]. These ECM hydrogels have shown the potential to promote regeneration of various tissues and organs ranging from bone to central nervous system [23], [24]. In an in vivo animal study, porcine ECM-SIS hydrogel in conjunction with a sheet form of ECM-SIS has been successfully used to heal a surgically transected ACL in a goat model [25]. The structural properties of the healing femur–ACL–tibia complex with this treatment compared favourably to those of the ACL reconstruction [26], [27], which demonstrated that therapy with ECM bioscaffolds can potentially be an alternative to ACL reconstruction in the clinical arena.
Previous studies revealed that the extracts from ECM-SIS have positive bioactive effects on the cellular activities of the healing ACLFs, such as increased cell proliferation [28]. Even the degradation products of an ECM (prehydrogel solution after enzyme digestion) have been shown to possess chemotactic and mitogenic effects [29]. As the major cell population residing in the ligament, the fibroblasts are critical in orchestrating the healing response when a ligament is injured (e.g., by shifting phenotypes) and helping restore the continuity and function of ligaments by active synthesis and remodelling of the matrix [6], [7], [8]. Thus, the research question was whether the SIS hydrogel has promoting effects on the activity of ACLFs including cell proliferation and matrix formation, and thus the potential to facilitate ACL healing. If so, the hydrogel would be a desirable scaffold material for ACL healing because of its ease of administration through injections and its ability to fill irregular shapes and gaps. We hypothesised that the bioactive ECM-SIS hydrogel [17] can enhance ACLFs to proliferate as well as to produce more collagen and thus has the potential to improve ACL healing.
To test our hypothesis, we performed in vitro experiments using ACLFs derived from the rat. For comparison, we used a pure collagen type I hydrogel so that the potential advantages of ECM hydrogels can be elucidated. We also studied the possible loss of bioactive molecules from the ECM-SIS sheet in the hydrogel preparation by quantifying growth factors that are sensitive to material processing and enzyme digestion. Two of the most abundant growth factors in ECM-SIS (fibroblast growth factor-2 (FGF-2) and transforming growth factor-beta 1 (TGF-β1)) were selected based on what we have found in our previous study [28]. The ACLFs were cultured on hydrogels for 3 days and 7 days at low and high levels of concentration of foetal bovine serum (FBS). The different concentrations of FBS were chosen to test whether the effects of ECM-hydrogel on ACLFs would be impacted when the blood supply to a ruptured ACL varies [5], [6], [9]. The cell proliferation and gene expressions of collagen matrix were examined and compared.
Materials and methods
Hydrogel preparation
The small intestine from an αGal-deficient pig (GalSafe; ∼13 months, 180 kg) was obtained from Revivicor Inc. (Blacksburg, VA, USA). These αGal-deficient (αGal(−)) transgenic pigs have been engineered to be free of αGal epitope, which has been linked to acute rejection of xenogenic implants [30]. Previously, we have obtained the ECM-SIS from these transgenic pigs and confirmed that growth factors, such as FGF-2, TGF-β1, vascular endothelial growth factor (VEGF), and platelet-derived growth factor BB (PDGF-BB), were present in the αGal-deficient ECM-SIS sheets, and their levels were comparable to those derived from the wild-type ECM-SIS [28].
Sheets of the ECM-SIS were obtained using a previously described method [22], [25]. The small intestine was first manually cleaned. The αGal(−) SIS (tunica submucosa and basilar portion of the tunica mucosa) was separated from the whole intestine and decellularised in a 0.1% peracetic acid/4% ethanol solution, rinsed twice each in phosphate-buffered saline (PBS) and deionized water, and lyophilised. The ECM-SIS sheets were then sterilized with ethylene oxide and comminuted to a powder using a Wiley Mini Mill (Thomas Scientific, Swedesboro, NJ, USA) with a size 40 mesh to produce particles of an optimal size for digestion [31]. One gram of the SIS powder and 50 mg of pepsin (Sigma, St. Louis, MO, USA; ∼2000–2300 U/mg) were added to 100 mL of 0.01M HCl and stirred for 48 hours at room temperature. The resultant solution (Figure 1A) of digested SIS (10 mg/mL) was kept frozen at −20°C until used.
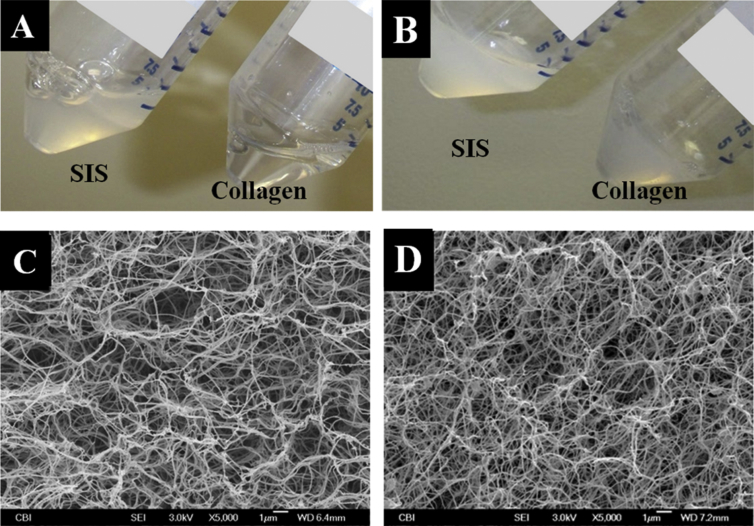
Figure 1.
(A) Appearance of solutions for making ECM-SIS hydrogel and pure collagen hydrogel prior to gelation. (B) Appearance of SIS hydrogel and pure collagen hydrogel after gelation. (C). Electron micrograph of SIS hydrogel. (D) Electron micrograph of pure collagen hydrogel showing fibre morphology. ECM = extracellular matrix; SIS = small intestine submucosa.
To make the ECM-SIS hydrogel, the digested SIS solution was diluted with 1 × PBS to 6 mg/mL from the initial concentration of 10 mg/mL, and the pH was adjusted to 7.4 with 0.1N NaOH. The final mixture was incubated at 37°C for 1 hour to form a hydrogel. A pure collagen hydrogel (3.2 mg/mL; Inamed Biomaterials, Fremont, CA, USA) was prepared as per the manufacturer's instructions and served as a comparison in the experiments. Eight parts of the purified collagen solution were mixed with 1 part of 10× PBS. The solution's pH was adjusted to 7.4 using 0.1M NaOH. One part of deionised water was added, and the resulting solution was incubated at 37°C for 90 minutes (see Figure 1B).
Scanning electron microscopy and microscopic morphology
The morphology of the SIS and pure collagen hydrogels was then studied using scanning electron microscopy (SEM) as previously described [31]. After fixing with 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) at 4°C for 24 hours, a series of washes in 1 × PBS and dehydrations in 30%, 50%, 70%, 90%, and 100% ethanol were performed. The hydrogels were cut to 2 mm × 2 mm pieces and dried using a Critical Point Dryer (Leica EM CPD030; Leica Microsystems, Buffalo Grove, IL, USA) and coated with a 4.5-nm-thick gold/palladium alloy using a sputter coater (108 Auto; Cressington Scientific Instruments, Watford, UK). Five samples were imaged with a scanning electron microscope (JSM 6330F; JEOL, Peabody, MA, USA), and micrographs were taken. The micrographs were then analyzed using a customized Matlab software as described previously [32] to obtain fibre diameter and orientation, porosity, and pore size.
Quantification of growth factors with enzyme-linked immunosorbent assay
To determine if the growth factors were still present in the ECM-SIS hydrogel after the processing and enzyme digestion, FGF-2 and TGF-β1, the two most abundant growth factors in the sheet form of ECM-SIS [28], were quantified through enzyme-linked immunosorbent assay. Briefly, 1 mL of the SIS digest (10 mg dry SIS) was mixed with 1 mL of PBS to dilute the digest because of its high viscosity. With the diluted digests, enzyme-linked immunosorbent assay solid-phase detection kits for FGF-2 (Quantikine DFB50; R&D Systems, Minneapolis, MN, USA) and TGF-β1 (QuantikineDB100B; R&D Systems) were used as per the manufacturers' instructions. The optical density at 450 nm, with a reference wavelength at 540 nm, was measured using a microplate reader (Infinite M200; Tecan, Morrisville, NC, USA).
ACLF isolation and culture
Rat ACLFs were harvested from the ACLs of rats (Sprague Dawley from Charles River, 3 to 4 months, male; n = 3) humanely euthanised as outlined and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. From these primary cell lines, third-passage cells were used in all experiments. For the cell proliferation and gene expression experiments, we used 0%, 1%, and 10% FBS to examine the effects of ECM-SIS hydrogel on the behaviours of ACLFs, and thus to estimate the function of hydrogel when the bloody supply to the injured ACL varies [5], [33].
Cell attachment observation and cell proliferation assay with bromodeoxyuridine incorporation
ACLFs from different rats (n = 3) were separately cultured on the surface of the SIS hydrogel (n = 3) and the pure collagen hydrogel (n = 3) in 96-well plates at 105 cells/well with Dulbecco's modified Eagle's medium and the different concentrations of FBS described above for 3 days and 7 days. In addition, for the purpose of SEM study, 2 × 106 cells/well were seeded on both hydrogels with 10% FBS in Dulbecco's modified Eagle's medium in six-well plates for 3 days. Cell attachment and morphology were observed with light microscopy and SEM at Day 3. For the cell proliferation assay, a bromodeoxyuridine (BrdU) incorporation assay (EMD Millipore, Billerica, MA, USA) was performed to measure cell proliferation as previously described [28]. Briefly, after incubation for 4 hours with BrdU, collagenase was added for hydrogel digestion. Cells were isolated through centrifugation and followed by incubation with anti-BrdU antibodies for BrdU detection. The plate was read on a microplate reader (Infinite M200; Tecan) with excitation at 325 nm and emission at 420 nm.
Collagen type I and III gene expressions with reverse transcription-polymerase chain reaction
ACLFs from different rats (n = 3) embedded in 2 mL SIS hydrogel (n = 3) and pure collagen hydrogel (n = 3) were cultured on six-well plates at 2 × 106 cells/well with culture medium for 3 days and 7 days with the three different concentrations of FBS. The ACLFs were isolated through collagenase treatment of the cell–hydrogel mixtures. The RNAs were then extracted using TriZol Reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription-polymerase chain reaction was performed as previously described [34]. Levels of mRNA for collagen type I, collagen type III, and a housekeeping gene, glyceradehyde-3-phosphate dehydrogenase (GAPDH; n = 3), were measured using an Opticon System Thermal Cycler (MJ Research, Waltham, MA, USA). The primer sequences are presented in Table 1. The cDNA amount (R) of each gene was calculated and normalized to that of GAPDH [35].
Table 1.
Primer sequences used in the study for mRNA quantification with RT-PCR in rat ACLFs.
| Gene | Sequence | Annealing temperature (°C) | Refs. |
|---|---|---|---|
| Collagen type I | 5′-TCA CCT ACA GCA CGC TTG-3′ 5′-GGT CTG TTT CCA GGG TTG-3′ |
55 | [45] |
| Collagen type III | 5′-ATA TCA AAC ACG CAA GGC-3′ 5′-GAT TAA AGC AAG AGG AAC AC-3′ |
52 | [45] |
| TGF-β1 | 5′-TGC TAA TGG TGG ACC G-3′ 5′-ACG TCA AAA GAC AGC C-3′ |
52 | [46] |
| GAPDH | 5′-ACA TCA TCC CTG CAT CCA CT-3′ 5′-GGG AGT TGC TGT TGA AGT CA-3′ |
55 | [45] |
ACLFs = anterior cruciate ligament fibroblasts; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; RT-PCR = reverse transcription-polymerase chain reaction; TGF-β1 = transforming growth factor-β1.
Data analysis
For statistical analysis, an independent t test was performed on fibre diameter, fibre orientation, pore size, and porosity obtained from SEM images. Significance was set at p < 0.05. On the effect of hydrogel type (SIS hydrogel vs. pure collagen hydrogel) on cell proliferation, a three-way analysis of variance (ANOVA) was first done to test the interactions between FBS concentration (0%, 1%, and 10%) and culture period (3 days and 7 days). Then t tests were performed at each FBS concentration or between time points to determine whether there were statistically significant differences. The same procedure was repeated for collagen type I mRNA expression and collagen type III mRNA expression.
Results
SEM and microscopic morphology
Electron micrographs revealed similar fibre morphologies for ECM-SIS and pure collagen hydrogels (Figures 1C and 1D). Quantitative image analysis [32] further confirmed this observation. The parameters describing the morphology of ECM-SIS hydrogel and pure collagen hydrogel were similar including average fibre diameters (0.105 ± 0.010 vs. 0.114 ± 0.004 μm; p > 0.05), fibre orientation index (0.51 ± 0.02 vs. 0.52 ± 0.02; p > 0.05), and pore sizes (0.092 ± 0.012 μm vs. 0.087 ± 0.008 μm; p > 0.05). Further analysis showed that the porosity of ECM-SIS hydrogel (88.8 ± 0.7%) was slightly higher than that of collagen hydrogel (88.0 ± 0.2%; p < 0.05). However, the percentage difference was less than 1% despite the statistical significance.
Quantification of growth factors
The presence of FGF-2 and TGF-β1 in the SIS digest was confirmed, and their contents were 66 ± 26 ng/g and 4 ± 1 ng/g dry SIS, respectively. These values represented 50% of the amount of FGF-2 and 90% of the amount of TGF-β1 found in the sheet form of ECM-SIS [28].
Cell attachment observation and cell proliferation assay
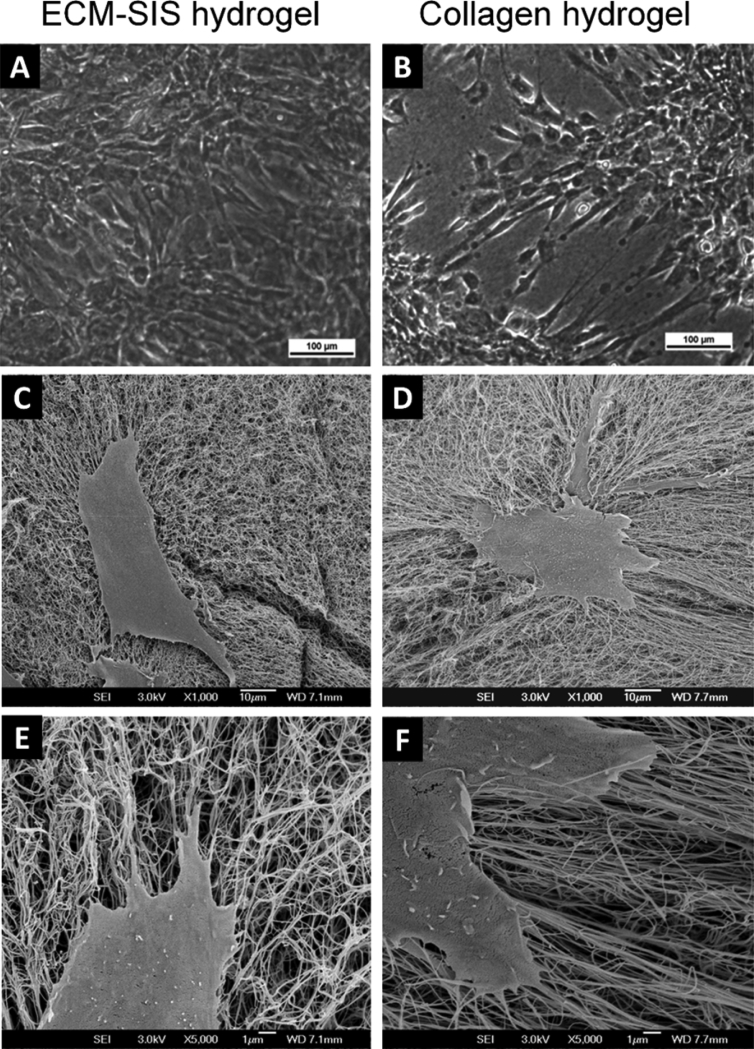
After 3 days of cell culture, ACLFs were found attached on the surface of both the ECM-SIS hydrogel and the pure collagen hydrogel even without the supply of FBS (0% FBS in medium; Figures 2A and 2B). Under light microscope and SEM, cells were observed to attach well to both hydrogels and manifest similar spindle-shaped appearance (Figure 2). It was observed that the cells on the ECM-SIS hydrogel seemed to have reached higher confluency than those on the collagen hydrogel at this time point regardless of the concentration of FBS that was applied.
Figure 2.
Cell attachment on the ECM-SIS hydrogel and pure collagen hydrogel after 3 days of cell culture. (A, B) Images from light microscopy showing more confluency of cells on the ECM-SIS hydrogel at 0% FBS (100×). (C, D). Images from SEM showing similar cell morphology upon attachment at 10% FBS (1000×). (E, F) Images from SEM showing similar cell morphology upon attachment at 10% FBS (5000×). ECM = extracellular matrix; FBS = foetal bovine serum; SEM = scanning electron microscopy; SIS = small intestine submucosa.
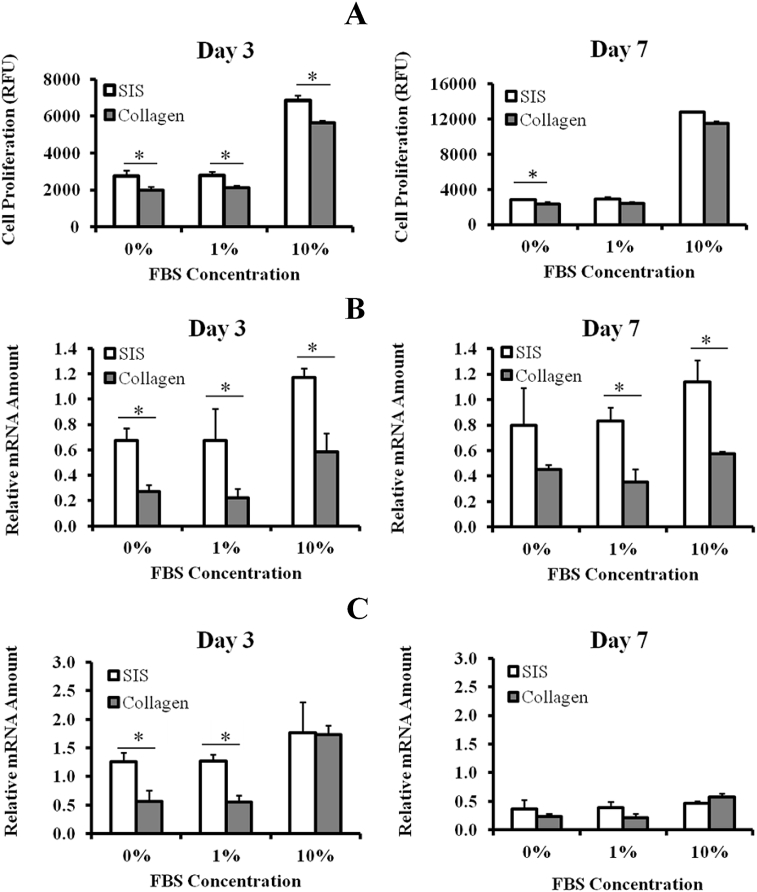
Results of the three-way ANOVA indicated that gel type, FBS concentration, and culture period all had significant effects on cell proliferation without significant interactions. At Day 3, the relative fluorescence unit values from the BrdU assay for the ECM-SIS hydrogel group were 2762 ± 286, 2771 ± 206, and 6848 ± 237 with 0%, 1%, and 10% FBS, respectively. For the pure collagen hydrogel group, the values were 1985 ± 177, 2109 ± 143, and 5623 ± 28, respectively (Figure 3A). Compared to the collagen hydrogel, the ECM-SIS hydrogel increased the cell proliferation by 39%, 31%, and 22% at 0%, 1%, and 10% FBS, respectively (all p < 0.05), with the most increase being observed at 0% FBS. At Day 7, the relative fluorescence unit values for the SIS hydrogel group were 2798 ± 115, 2872 ± 293, and 12,783 ± 27, whereas those for the pure collagen hydrogel group were 2317 ± 231, 2392 ± 177, and 11,514 ± 868 at 0%, 1%, and 10% FBS, respectively. At this time point, the significant difference between the ECM-SIS hydrogel and the collagen hydrogel was that cell proliferation at 0% FBS—but not 1% and 10% FBS—was still higher in the former (p < 0.05).
Figure 3.
Impact of ECM-SIS hydrogel on (A) cell proliferation; (B) collagen type I mRNA expression; and (C) collagen type III mRNA expression of rat ACLFs as compared with pure collagen hydrogel at different FBS concentrations (n = 3 for all data sets). Results are expressed as mean ± standard deviation. * Significant difference (p < 0.05). ACLFs = anterior cruciate ligament fibroblasts; ECM = extracellular matrix; FBS = foetal bovine serum; SIS = small intestine submucosa.
Collagen type I and III gene expressions
For collagen type I mRNA expression, the three-way ANOVA indicated the SIS hydrogel, FBS concentration, and culture period had significant effects without significant interaction. At Day 3, relative to the collagen hydrogel, the ECM-SIS hydrogel significantly increased the mRNA expression of collagen type I by 150%, 207%, and 100% at 0%, 1%, and 10% FBS, respectively (p < 0.05; Figure 3B). At Day 7, significant differences were still observed at 1% and 10% FBS between the two groups (137% and 100% higher in the ECM-SIS hydrogel, respectively; p < 0.05).
For collagen type III mRNA expression, the three-way ANOVA indicated the ECM-SIS hydrogel, FBS concentration, and culture period had significant effects. At Day 3, the significant percent increases for the SIS hydrogel group over the pure collagen gel group were 123% and 132% at 0% and 1% of FBS, respectively (p < 0.05; Figure 3C). At Day 7, no significant differences in the collagen type III mRNA levels were found between the two hydrogels at all concentrations of FBS (p > 0.05; Fig. 3C).
Discussion
The results of the current study confirmed our hypothesis that ECM hydrogel can promote the proliferation and matrix producing activity of ACLFs, particularly in the context of low serum concentration that is common when the blood supply was impaired at the initial stage of ACL rupture. Among the multiple components derived from the ECM hydrogels that benefits wound healing and tissue remodelling [17], [36], we confirmed the partial preservation of growth factors in the ECM-SIS hydrogel after processing and enzyme digestion, i.e., FGF-2 by 50% and TGF-β1 by 90% of those found in the sheet form of ECM-SIS [28]. In addition, our results on the mRNA expressions of fibroblasts are comparable with those of a previous study with cells seeding on a sheet of SIS bioscaffold [37].
Furthermore, our results showed that the ECM-SIS hydrogel and a pure collagen hydrogel possessed similar macro- and micromorphology. The morphometric parameters defining the mechanical properties of a fibrous material [38] including fibre diameter, fibre orientation, and pore size were not significantly different between the two types of hydrogels except the porosity, for which the ECM-SIS hydrogel was slightly higher than the collagen hydrogel. Although it is arguable that the higher porosity may facilitate better cell penetration in the ECM-SIS hydrogel, their overall morphological similarity indicates that the more superior beneficial effects of the ECM-SIS hydrogel on cellular activities may not be solely derived from the architecture of the material.
That the ECM-SIS hydrogel had more positive effects on cell proliferation and matrix gene expressions in comparison to those by collagen hydrogel could also be attributed to the bioactive properties of ECM bioscaffolds. In the literature, it has been shown that the ECM bioscaffolds could promote tissue formation and remodelling by limiting destructive inflammatory responses, recruiting stem cells, facilitating cell attachment and cell proliferation, as well as matrix production [36]. ECM hydrogel derived from urinary bladder matrix (UBM) has also been shown to increase the proliferation of rat aorta smooth muscle cells after 2 days of culture, which is similar to our findings [22]. Although the exact mechanisms are not yet known, it has been suggested that there are multiple bioactive factors involved in the function of ECM derived products, e.g., fibronectin [39], cryptic peptides after digestion, and other degradation products [40]. Furthermore, a panel of growth factors has been identified in the ECM-SIS and ECM-UBM sheets and they may also play a role [28]. In the current study, we confirmed that FGF-2 and TGF-β1 were retained in the ECM-SIS hydrogel, albeit in decreased amounts as compared to those in the ECM-SIS sheet. Nevertheless, because growth factors have a wide range of functions on cell activities and are concentration-dependent, it will be of interest to isolate the function of those that dwell in the ECM-SIS hydrogel in the future.
The significant improvement in cell proliferation and mRNA expression of both collagen type I and type III with ECM-SIS hydrogel at lower concentrations of serum protein and at early time point are particularly encouraging because the ruptured ACL lacks ample blood supply [5], [41]. In fact, a clinical procedure to improve the ‘healing response’ of a ruptured ACL is to make microfracture holes in its femur insertion to introduce blood to encourage hematoma formation [9], [33]. The procedure has been successful in the healing of proximal ACL injuries of patients older than 40 years. With the recent advance in tissue engineering, more and more studies on healing a transected ACL with the use of exogenous bioactive factors, such as hyaluronic acid and FGF-2, have been published [42]. Others have used bioscaffolds in combination with platelet-rich plasma or hydrogels [25], [43], [44]. Thus, ECM-SIS hydrogel could be another promising agent as it could facilitate cell attachment and growth as well as active matrix production to bridge the gap early and provide the needed structural support for healing to proceed.
It is noticeable that the positive effect of ECM-SIS hydrogel on the collagen type I mRNA expression persisted until Day 7 even at high levels of serum proteins, indicating that even at the later stage of ACL healing, when the blood supply to the ligament is restored to some extent, the ECM-SIS gel may still assist in the healing by improving collagen type I production, which is the major matrix molecule to maintain ligament structure and function.
A limitation to the current study is that the ACLFs were those isolated from normal ACLs, instead of healing ACL. Whether the positive effects of the SIS hydrogel would remain on fibroblasts in the ruptured ACL will be confirmed in follow-up studies. Nevertheless, it is necessary to perform the current study in order to clearly demonstrate that the ECM-SIS hydrogel can indeed facilitate the activities of ACLFs, which are intrinsically low in their ability to proliferate and to produce matrix in comparison to those from the extra-articular ligaments. In addition, ECM materials derived from animals are not autologous like platelet-rich plasma and may induce host foreign body reactions. However, our procedures have diminished α-Gal epitopes and followed strict standardised decellularisation that largely eliminated the risk. Considering that porcine ECMs are Food and Drug Administration approved and are used clinically, we believe that the ECM-SIS hydrogel could also be translated to clinical applications to promote the healing of ligaments and tendons.
In conclusion, the ECM-SIS hydrogel has demonstrated superior properties in promoting ACLF proliferation and matrix production. As such, the ECM-SIS hydrogel has the potential therapeutic value to facilitate ACL healing, particularly at the early stage of the injury. In the future, the exact mechanisms will be explored and its positive effects further verified in vivo.
Conflicts of interest
The authors have no conflicts of interest related to this study to disclose.
Funding/support
Financial support from the Commonwealth of Pennsylvania, NIH EB003392 (T-32 Biomechanics in Regenerative Medicine Predoctoral Training Fellowship), and Ri. MED Foundation is gratefully acknowledged. The authors also thank Revivicor Inc. for providing the αGal-deficient porcine small intestine submucosa tissue, Dr ZhaichuanMi for providing the rat ACL tissues, and Dr Steven Badylak for the generous support of materials and protocols.
References
- 1.Indelicato P.A. Non-operative treatment of complete tears of the medial collateral ligament of the knee. J Bone Joint Surg Am. 1983;65:323–329. [PubMed] [Google Scholar]
- 2.Weiss J.A., Woo S.L.Y., Ohland K.J., Horibe S., Newton P.O. Evaluation of a new injury model to study medial collateral ligament healing: primary repair versus nonoperative treatment. J Orthop Res. 1991;9:516–528. doi: 10.1002/jor.1100090407. [DOI] [PubMed] [Google Scholar]
- 3.Andersson A.C. Knee laxity and function after conservative treatment of anterior cruciate ligament injuries. A prospective study. Int J Sports Med. 1993;14:150–153. doi: 10.1055/s-2007-1021159. [DOI] [PubMed] [Google Scholar]
- 4.Meunier A., Odensten M., Good L. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: a randomized study with a 15-year follow-up. Scand J Med Sci Sports. 2007;17:230–237. doi: 10.1111/j.1600-0838.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 5.Murray M.M., Martin S.D., Martin T.L., Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82-A:1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Smith B.A., Livesay G.A., Woo S.L.Y. Biology and biomechanics of the ACL. Clinics in Sports Medicine. 1993;12A:637–670. [PubMed] [Google Scholar]
- 7.Nagineni C.N., Amiel D., Green M.H., Berchuck M., Akeson W.H. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10:465–475. doi: 10.1002/jor.1100100402. [DOI] [PubMed] [Google Scholar]
- 8.McKean J.M., Hsieh A.H., Sung K.L. Epidermal growth factor differentially affects integrin-mediated adhesion and proliferation of ACL and MCL fibroblasts. Biorheology. 2004;41:139–152. [PubMed] [Google Scholar]
- 9.Steadman J.R., Cameron-Donaldson M.L., Briggs K.K., Rodkey W.G. A minimally invasive technique (“healing response”) to treat proximal ACL injuries in skeletally immature athletes. J Knee Surg. 2006;19:8–13. doi: 10.1055/s-0030-1248070. [DOI] [PubMed] [Google Scholar]
- 10.Woo S.L.Y., Hildebrand K., Watanabe N., Fenwick J.A., Papageorgiou C.D., Wang J.H.C. Tissue engineering of ligament and tendon healing. Clin Orthop Rel Res. 1999;367:S312–S323. doi: 10.1097/00003086-199910001-00030. [DOI] [PubMed] [Google Scholar]
- 11.Anderson A.F., Snyder R.B., Lipscomb A.B., Jr. Anterior cruciate ligament reconstruction. A prospective randomized study of three surgical methods. Am J Sports Med. 2001;29:272–279. doi: 10.1177/03635465010290030201. [DOI] [PubMed] [Google Scholar]
- 12.Drogset J.O., Grontvedt T., Robak O.R., Molster A., Viset A.T., Engebretsen L.A. 16-year follow-up of three operative techniques for the treatment of acute ruptures of the anterior cruciate ligament. J Bone Jt Surg Am. 2006;88:944–952. doi: 10.2106/JBJS.D.02876. [DOI] [PubMed] [Google Scholar]
- 13.Jomha N.M., Borton D.C., Clingeleffer A.J., Pinczewski L.A. Long-term osteoarthritic changes in anterior cruciate ligament reconstructed knees. Clin Orthop Relat Res. 1999;358:188–193. [PubMed] [Google Scholar]
- 14.Franklin M.E., Jr., Trevino J.M., Portillo G., Vela I., Glass J.L., Gonzalez J.J. The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: long-term follow-up. Surg Endosc. 2008;22:1941–1946. doi: 10.1007/s00464-008-0005-y. [DOI] [PubMed] [Google Scholar]
- 15.Mostow E.N., Haraway G.D., Dalsing M., Hodde J.P., King D. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. 2005;41:837–843. doi: 10.1016/j.jvs.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Sicari B.M., Rubin J.P., Dearth C.L., Wolf M.T., Ambrosio F., Boninger M. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008085. 234ra58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 18.Karaoglu S., Fisher M.B., Woo S.L.Y., Fu Y.C., Liang R., Abramowitch S.D. Use of a bioscaffold to improve healing of a patellar tendon defect after graft harvest for ACL reconstruction: a study in rabbits. J Orthop Res. 2008;26:255–263. doi: 10.1002/jor.20471. [DOI] [PubMed] [Google Scholar]
- 19.Liang R., Woo S.L.Y., Nguyen T.D., Liu P.C., Almarza A. Effects of a bioscaffold on collagen fibrillogenesis in healing medial collateral ligament in rabbits. J Orthop Res. 2008;26:1098–1104. doi: 10.1002/jor.20616. [DOI] [PubMed] [Google Scholar]
- 20.Woo S.L.Y., Takakura Y., Liang R., Jia F., Moon D.K. Treatment with bioscaffold enhances the fibril morphology and the collagen composition of healing medial collateral ligament in rabbits. Tissue Eng. 2006;12:159–166. doi: 10.1089/ten.2006.12.159. [DOI] [PubMed] [Google Scholar]
- 21.Musahl V., Abramowitch S.D., Gilbert T.W., Tsuda E., Wang J.H., Badylak S.F. The use of porcine small intestinal submucosa to enhance the healing of the medial collateral ligament — a functional tissue engineering study in rabbits. J Orthop Res. 2004;22:214–220. doi: 10.1016/S0736-0266(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 22.Freytes D.O., Martin J., Velankar S.S., Lee A.S., Badylak S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630–1637. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Medberry C.J., Crapo P.M., Siu B.F., Carruthers C.A., Wolf M.T., Nagarkar S.P. Hydrogels derived from central nervous system extracellular matrix. Biomaterials. 2013;34:1033–1040. doi: 10.1016/j.biomaterials.2012.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawkins M.J., Bowen W., Dhadda P., Markides H., Sidney L.E., Taylor A.J. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 2013;9:7865–7873. doi: 10.1016/j.actbio.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher M.B., Liang R., Jung H.J., Kim K.E., Zamarra G., Almarza A.J. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surg Sports Traumatol Arthrosc. 2012;20:1357–1365. doi: 10.1007/s00167-011-1800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng G.Y., Oakes B.W., Deacon O.W., McLean I.D., Lampard D. Biomechanics of patellar tendon autograft for reconstruction of the anterior cruciate ligament in the goat: three-year study. J Orthop Res. 1995;13:602–608. doi: 10.1002/jor.1100130416. [DOI] [PubMed] [Google Scholar]
- 27.Papageorgiou C.D., Ma C.B., Abramowitch S.D., Clineff T.D., Woo S.L.Y. A multidisciplinary study of the healing of an intraarticular anterior cruciate ligament graft in a goat model. Am J Sports Med. 2001;29:620–626. doi: 10.1177/03635465010290051501. [DOI] [PubMed] [Google Scholar]
- 28.Liang R., Fisher M., Yang G., Hall C., Woo S.L.Y. Alpha1,3-galactosyltransferase knockout does not alter the properties of porcine extracellular matrix bioscaffolds. Acta Biomater. 2011;7:1719–1727. doi: 10.1016/j.actbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Reing J.E., Zhang L., Myers-Irvin J., Cordero K.E., Freytes D.O., Heber-Katz E. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605–614. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 30.Kuwaki K., Tseng Y.L., Dor F.J., Shimizu A., Houser S.L., Sanderson T.M. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 31.Wolf M.T., Daly K.A., Brennan-Pierce E.P., Johnson S.A., Carruthers C.A., D'Amore A. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33:7028–7038. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Amore A., Stella J.A., Wagner W.R., Sacks M.S. Characterization of the complete fiber network topology of planar fibrous tissues and scaffolds. Biomaterials. 2010;31:5345–5354. doi: 10.1016/j.biomaterials.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steadman J.R., Matheny L.M., Briggs K.K., Rodkey W.G., Carreira D.S. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25:255–260. doi: 10.1055/s-0032-1313742. [DOI] [PubMed] [Google Scholar]
- 34.Almarza A.J., Yang G., Woo S.L.Y., Nguyen T., Abramowitch S.D. Positive changes in bone marrow-derived cells in response to culture on an aligned bioscaffold. Tissue Eng Part A. 2008;14:1489–1495. doi: 10.1089/ten.tea.2007.0422. [DOI] [PubMed] [Google Scholar]
- 35.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(–Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Londono R., Badylak S.F. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577–592. doi: 10.1007/s10439-014-1103-8. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert T.W., Stewart-Akers A.M., Sydeski J., Nguyen T.D., Badylak S.F., Woo S.L.Y. Gene expression by fibroblasts seeded on small intestinal submucosa and subjected to cyclic stretching. Tissue Eng. 2007;13:1313–1323. doi: 10.1089/ten.2006.0318. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Palencia D.M., D'Amore A., Gonzalez-Mancera A., Wagner W.R., Briceno J.C. Effects of fabrication on the mechanics, microstructure and micromechanical environment of small intestinal submucosa scaffolds for vascular tissue engineering. J Biomech. 2014;47:2766–2773. doi: 10.1016/j.jbiomech.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 39.Hodde J., Record R., Tullius R., Badylak S. Fibronectin peptides mediate HMEC adhesion to porcine-derived extracellular matrix. Biomaterials. 2002;23:1841–1848. doi: 10.1016/s0142-9612(01)00310-6. [DOI] [PubMed] [Google Scholar]
- 40.Agrawal V., Tottey S., Johnson S.A., Freund J.M., Siu B.F., Badylak S.F. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A. 2011;17:2435–2443. doi: 10.1089/ten.tea.2011.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bray R.C., Leonard C.A., Salo P.T. Vascular physiology and long-term healing of partial ligament tears. J Orthop Res. 2002;20:984–989. doi: 10.1016/S0736-0266(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 42.Hsu S.L., Liang R., Woo S.L.Y. Functional tissue engineering of ligament healing. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:12. doi: 10.1186/1758-2555-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi S.M., Mastrangelo A.N., Magarian E.M., Fleming B.C., Murray M.M. Collagen–platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37:2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen D.T., Geel J., Schulze M., Raschke M.J., Woo S.L.Y., van Dijk C.N. Healing of the goat anterior cruciate ligament after a new suture repair technique and bioscaffold treatment. Tissue Eng Part A. 2013;19:2292–2299. doi: 10.1089/ten.tea.2012.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konishi A., Konishi A., Tazawa C., Miki Y., Darnel A.D., Suzuki T. The possible roles of mineralocorticoid receptor and 11beta-hydroxysteroid dehydrogenase type 2 in cardiac fibrosis in the spontaneously hypertensive rat. J Steroid Biochem Mol Biol. 2003;85:439–442. doi: 10.1016/s0960-0760(03)00198-5. [DOI] [PubMed] [Google Scholar]
- 46.Lo C.S., Chen C.H., Hsieh T.J., Lin K.D., Hsiao P.J., Shin S.J. Local action of endogenous renal tubular atrial natriuretic peptide. J Cell Physiol. 2009;219:776–786. doi: 10.1002/jcp.21728. [DOI] [PubMed] [Google Scholar]