Summary
Large bone defects are serious complications that are most commonly caused by extensive trauma, tumour, infection, or congenital musculoskeletal disorders. If nonunion occurs, implantation for repairing bone defects with biomaterials developed as a defect filler, which can promote bone regeneration, is essential. In order to evaluate biomaterials to be developed as bone substitutes for bone defect repair, it is essential to establish clinically relevant in vitro and in vivo testing models for investigating their biocompatibility, mechanical properties, degradation, and interactional with culture medium or host tissues. The results of the in vitro experiment contribute significantly to the evaluation of direct cell response to the substitute biomaterial, and the in vivo tests constitute a step midway between in vitro tests and human clinical trials. Therefore, it is essential to develop or adopt a suitable in vivo bone defect animal model for testing bone substitutes for defect repair. This review aimed at introducing and discussing the most available and commonly used bone defect animal models for testing specific substitute biomaterials. Additionally, we reviewed surgical protocols for establishing relevant preclinical bone defect models with various animal species and the evaluation methodologies of the bone regeneration process after the implantation of bone substitute biomaterials. This review provides an important reference for preclinical studies in translational orthopaedics.
Keywords: animal models, bone defect, bone regeneration, bone substitutes
Introduction
Bone defect healing is a process of reconstruction of the bone tissue, which generally undergoes a multidimensional procedure with an overlapping timeline [1]. The vast majority of bone defects can heal spontaneously under suitable physiological environmental conditions due to the regeneration ability of bone. However, the healing process of bone defect is time consuming, and new bone generation takes place slowly because of decreased blood supply to the fracture site and insufficiency of calcium and phosphorus to strengthen and harden new bone. In addition, large defects, also known as critical bone defects, may not heal spontaneously and lead to nonunion prognosis due to the size of defects or unstable biomechanical properties, unfavourable wound environment, suboptimal surgical technique, metabolic factors, hormones, nutrition, and applied stress [2], [3]. Bone grafts or substitute biomaterials are commonly used therapeutic strategies for clinical bone surgery to fill the bone defects for reconstructing large bone segments. Although autografts are the current gold standard treatment for bone defect regeneration [4], [5], it still has disadvantages such as limitation in donor supply [6], donor site pain, or haemorrhage [7]. Other disadvantages of allograft are the risk of immune-mediated rejection, the transmission of infectious diseases and the negative effect on the mechanical and biological properties of graft [8], [9], [10], [11]. In order to overcome the limitations associated with the current standard treatment of bone grafts, there has been an increasing interest in studying substitutes biomaterials, which are made of naturally derived and/or synthetic materials, during the past decades throughout the world [12], [13], [14], [15], [16]. The ideal bone graft substitutes should be biocompatible, bioresorbable, osteoconductive, osteoinductive, structurally similar to nature bone, and easy or ready to use.
Prior to testing in human beings, an ideal bone substitute should be tested both in vivo and in vitro, so as to make sure that it works effectively and safely. Therefore, to establish a suitable animal model is an indispensable step when evaluating the mechanical property and biocompatibility of bone substitute biomaterials. In this review, we discuss the speciality of different species for estimating bone defect substitute biomaterials in different bone defect sites, such as crania [17], [18], [19], femora [20], [21], [22], and ulna [23], [24], [25]. We evaluated the advantages and disadvantages of each species for estimating specific defects, analysed and compared the similarities between animal models and human clinical situations, and emphasised the factors we need to consider when choosing animals.
General selection criteria
A number of animal test models, such as rat/mouse [26], [27], [28], [29], [30], rabbit [31], [32], [33], [34], dog [35], [36], [37], [38], sheep [39], [40], [41], goat [42], [43], [44], and pig [45], [46], [47], [48], have been developed to simulate human in vivo environment and physical conditions to test the availability and comparability of bone substitute biomaterials. In order to mimic various orthopaedic situations, many defect sites have been explored, such as calvaria [17], [18], [19], femora [20], [21], [22], and ulna [23], [24], [25]. A prerequisite for such a model is that no spontaneous complete osseous regeneration of the created defects occurs during the lifetime of the animals [49]. The critical size defect is defined as the smallest osseous wound that does not heal spontaneously over a long period of time. For practical purposes, if there is no mineralised area of ≥30% after 52 weeks, there would never be complete bony regeneration. Although the minimum size that renders a defect “critical” is not well understood, it has been defined as a segmental bone deficiency of a length exceeding 2–2.5 times the diameter of the affected bone [11], [50].
Various factors have to be considered for selecting a specific animal species as a testing model. First and foremost, the chosen animal model should clearly demonstrate both significant physiological and pathophysiological analogies in comparison to humans. Second, it must be manageable to operate and observe a multiplicity of study objects postsurgery over a relatively short period of time [51]. Other selection criteria include costs for acquisition and care, animal availability, acceptability to society, tolerance to captivity, and ease of housing [52]. According to the international standard, we should also consider the size of the implant test specimens, number of implants per animal, intended duration of the test, and potential species' differences with regard to biological responses [53].
The following are the most frequently used animal models for creating bone defects to test conventional and innovative biological biomaterials to be used as bone substitutes.
Rabbits
Advantage and disadvantage of rabbit models
Rabbit is one of the most commonly used animal models, and it ranks first among all the animals used for musculoskeletal research [54]. However, regarding the assessment of multiple substitute biomaterials, the small size of rabbits is the major drawback for studying orthopaedic implants. However, it was reported that there were similarities in bone mineral density and the fracture toughness of mid-diaphyseal bone between rabbits and human [55]. Besides, in comparison with other species, such as primates or some rodents, rabbit has faster skeletal change and bone turnover [56]. Rabbits are easily available, and easy to house and handle. These characteristics make rabbits the first choice when researchers develop animal model for the in vivo test of a new bone substitute biomaterials.
Application of bone defect model for testing bone substitute biomaterials in rabbits
In recent years, several rabbit models have been used to test new bone substitute biomaterials. The most common implantation sites include bilateral tibiae and distal femur (Table 1). Walsh et al [57] investigated three commercially available and clinically used β-tricalcium phosphate (TCP) bone graft substitutes with the same chemistry (Vitoss, Osferion, Chronos), but with various macro- and microscopic characteristics, using a bilateral tibial metaphyseal defect model on New Zealand white rabbits. Bilateral defects (5 mm wide and 15 mm long) spanning the metaphyseal and diaphyseal regions were created 3 mm below the joint line in the anteromedial cortex of the proximal tibia. It turns out that all three β-TCP bone graft substitutes performed well in this rabbit model. Young et al [58] developed an easily accessible and reproducible, nonhealing, alveolar, 10 mm “full-thickness” cylindrical defect removing both cortical plates and the intervening trabecular bone and tooth roots bone defect in the rabbit mandible. Gauthier et al [59] used a cylindrical, 7–10 mm critical-size bone defect rabbit model to investigate the efficiency of an injectable calcium phosphate bone substitute for bone regeneration. The critical-size bone defect rabbit model has been used successfully to carry out a histomorphometric analysis of a new, highly porous, biphasic calcium phosphate bone substitute by Calvo-Guirado et al [60]. Delgado-Ruiz et al [61] also used a critical-size tibiae defect rabbit model to test the behaviour of porous titanium granules, with and without membranes being covered. The test result showed that the porous titanium particles must be covered by a membrane, when grafting larger defects. Chen et al [62] established a critical-size bone defect model to test poly(lactic-co-glycolic acid) (PLGA)/TCP/icaritin 3D printing scaffold on the ulnar site.
Table 1.
Rabbit bone defect models for testing bone substitute biomaterials.
| Defect site | Weight (kg) | Defect size | Substitute biomaterials |
|---|---|---|---|
| Tibiae | 3–3.5 | 5 mm wide & 15 mm long [57], 6 mm in diameter [60], [61]; 5 mm in length [77] | β-TCP bone graft substitutes [57]; hydroxyapatite 60%/B-tricalcium phosphate 40% [60]; porous titanium granules [61]; β-TCP, type I collagen, & rhFGF-2 [77] |
| Femur | 3–5 | 7 × 10 mm2 cylinder [59], 3 mm in diameter, 15 mm long [75]; 6 mm diameter × 5 mm cylinder [64], [68] | Injectable calcium phosphate bone substitute [59]; PLGA/TCP/icaritin [75]; magnesium alloy AZ91D [63]; micro/ma-MCP [64]; CMMS/rhBMP-2 [65]; magnesium calcium phosphate biocement [66]; magnesium scaffolds [68]; magnesium silicate (m-MS) [67]; poly(epsilon-caprolactone)–poly(ethyleneglycol)–poly(epsilon-caprolactone) composite scaffolds [67] |
| Calvaria | 2.0–3 | 10 mm diameter × 1.2 mm [78]; 9 mm diameter [79] | Apatite-coated zirconia [78]; low-molecular-weight silk fibroin [79] |
| Ulna | 3.5–4 | 12 mm segment of midshaft ular [80]; 15 mm segment of midshaft ular [13] | PLGA/tricalcium phosphate/icaritin/BMP-2 scaffolds [80]; BMP-2/PLGA-coated gelatin sponge [13] |
PLGA = poly(lactic-co-glycolic acid); TCP = tricalcium phosphate.
Protocol for developing steroid-associated osteonecrosis rabbit model for testing bone substitute biomaterials
Distal femur defect rabbit model has commonly been used to test substitute biomaterials by a variety of researchers [59], [63], [64], [65], [66], [67], [68]. Although distal femur is not the commonly observed location of osteonecrosis in clinic, distal femur defects are frequently observed after the removal of malignant bone tumours [69] and total knee replacement [70], [71], [72]. Distal femur defects may lead to the failure of a total knee arthroplasty if left untreated [73]. Therefore, the use of a distal femur defect model is meaningful and constructive in testing substitute biomaterials prior to conducting human clinical trials. As the distal femoral defect rabbit model is one of the most commonly used animal models to test substitute biomaterials, here we review the surgical protocols for establishing steroid-associated osteonecrosis (SAON) rabbit models with distal femoral defect for testing bone substitute materials. SAON, which would subsequently lead to subchondral joint collapse, is caused by the frequent prescription of pulsed steroids as a life-saving agent in a situation such as severe acute respiratory syndromes. Core decompression is the major treatment method for SAON in the early stage. However, the nonunion bone defect that remained after the surgery may lead to insufficient mechanical support of the femur and result in joint collapse, seriously affecting the prognosis. Therefore, the use of suitable bone substitute materials to fill in the defect and provide a mechanical support to the femur is essential for the process of SAON bone defect repair. In order to test the substitute materials' biocompatibility, mechanical properties, and availability, a model of SAON is necessary.
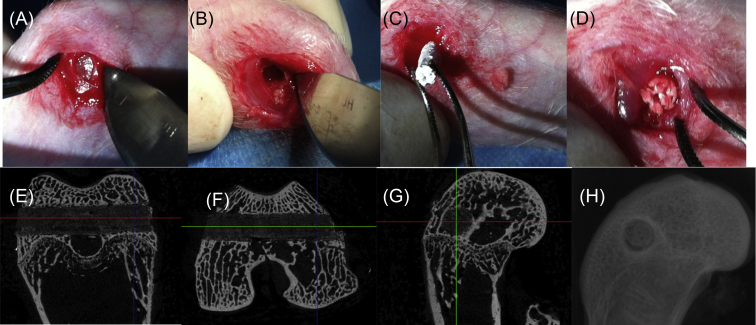
The protocol for establishing a SAON rabbit model is based on our previously published work [74], [75]. First of all, rabbits should be in healthy condition, weigh 3.0–3.5 kg, and reach an age of 28 weeks, which is similar to the adult age of human beings. Then, one injection of 10 mg/kg of lipopolysaccharide was given intravenously to the dopey rabbits. A day after that, three injections of 20 mg/kg of methylprednisolone were given intramuscularly at time intervals of 24 hours. Two weeks later, 93% of the rabbits developed osteonecrosis and none of them died during the procedure. This procedure is considered to be more effective or efficient, with a lower death rate compared with other published methods [76]. When the SAON rabbit model was established, we performed core decompression in rabbits by drilling a 3.0 mm tunnel transversely through the distal femora (Figure 1). Using this model, we have tested the in vivo bone defect repairing ability of a new bioactive PLGA/TCP composite scaffold incorporating the phytomolecule icaritin [75].
Figure 1.
Surgical protocol for the establishment of core-decompression at the distal femur in a SAON rabbit model for implantation of the PLGA/TCP/icaritin substitute biomaterial. (A) The surgical site is exposed by an operating scalpel. (B) A 3.0 mm tunnel is drilled transversely through the distal femora by a trephine. (C and D) The biomaterial is implanted into the bone tunnel. (E–G) Micro-CT three-dimensional image of the bone defect site. (H) X-ray image of the bone defect site. CT = computed tomography; PLGA = poly(lactic-co-glycolic acid); SAON = steroid-associated osteonecrosis; TCP = tricalcium phosphate.
Assessment of bone regeneration is a key step to estimate the osteoconductive and osteoinductive abilities of bone substitute biomaterials. It is also an essential step for evaluating the function of a rabbit model. The most commonly used methodologies are histological analysis, microcomputed tomography (micro-CT) analysis, mechanical test analysis, radiograph analysis, sequential fluorescence labelling analysis, and dynamic contrast-enhanced magnetic resonance imaging. Using dynamic contrast-enhanced magnetic resonance imaging, we evaluated the effectiveness of the SAON rabbit model [74]. It has also been shown that using a SAON rabbit the in vivo osteogeneticability of PLGA/TCP/icaritin substitute biomaterial could be tested successfully [75].
Rodents
Anatomical advantages and disadvantages of rodents
As rodents are small in size and easy to handle, these are one of the most commonly used animal models, considered useful in preclinical studies for testing biomaterials as bone substitutes, and regarded as one of the first-choice models for in vivo test for regeneration of the bone tissue [81]. However, limitations of rodent models are also obvious. Compared with other larger animals such as rabbits, canine, and pigs, rodents have small-sized long bones and thin and fragile cortices [82]. Besides, rodent models do not show Haversian-type remodelling in the cortex, while lager animals do.
Application of rodent bone defect model for testing substitute biomaterials
Surgical implantation of substitute materials, such as β-TCP, calcium phosphate, and collagen, has been commonly conducted in rodents (Table 2). Kondo et al [83] investigated the biocompatibility of highly purified β-TCP bone graft substitutes using a rat femur defect model. Their study suggested that purified β-TCP was biocompatible and resorbable. In a study of 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration, a critical murine femur defect model was used to demonstrate the in vivo properties of substitute biomaterials [84]. Ye et al [85] established a 4-mm-diameter calvaria critical-size defect model in 6–8-week-old nu/nu mice. Based on this model, the efficacy of iPSCs/silk scaffold in increasing bone formation was revealed. Those rodent bone defect models have all been used successfully to test the in vivo osteoconductive and osteoinductive abilities of bone substitute materials.
Table 2.
Rodent bone defect models for testing new substitute biomaterials.
| Defect site | Animal | Age/weight | Defect size | Substitute biomaterials |
|---|---|---|---|---|
| Distal femur | F344/Fisher [83]; male Wistar rat [87] | 8 wk [83]; 12–14 wk [87] | 2 mm diameter & depth [83]; 2 mm in diameter & 3 mm in length [87] | β-TCP bone graft substitutes [83]; polymer containing TGF-β1 [87] |
| Midfemur | Female BALB/cJ [84]; male Fisher [88]; nude rat [89] | 13–15 wk [84]; 253 g [88]; 325–400 g [89] | 2 mm in length [84]; 5 mm in length [88]; 5 mm in length [89] | Composite calcium phosphate & collagen [84]; marrow cells & porous ceramic [88]; BMP–silk composite matrices [89] |
| Calvaria | Nu/nu mice [85]; nude rat [90] | 6–8 wk [85]; 12 wk [90] | 4 mm diameter [85]; 8 mm diameter [90] | iPSCs/silk scaffold [85]; PLGA & adipose-derived stem cells [90] |
BMP = bone morphogenetic protein; PLGA = poly(lactic-co-glycolic acid)
Protocol for establishing calvaria critical-size defect nude mice model for testing bone substitute biomaterials
Critical-size defects are considered the smallest wounds established intraosseously, which cannot heal spontaneously during the lifetime of the animal [86]. In nude mice, a defect of 3 mm in size has been reported to be necessary to create a persisting femur bone defect [26]. Nude mice were anaesthetised according to the recommended routines for this species. A 4-mm-diameter calvaria critical-size defect was created on each side of the cranium using a dental bur attached to a slow-speed hand-piece with minimal invasion of the dura mater. Critical-size defects were created, which received implantation of substitute biomaterials later [85].
Assessment of bone regeneration was performed later by micro-CT scan and reconstruction, which showed that the majority of the calvaria defects were filled with a substantial amount of newly formed bone tissue in the defect site treated with the SATB2-transduced iPSC implants 5 weeks postoperation. Histological analysis of bone regeneration further demonstrates radiographic results, indicating that the SATB2-transduced group showed nearly complete osseous closure of the defect [85].
Pigs
Anatomical advantage and disadvantage of pigs
Pigs are considered to be close representative models of human bone regeneration processes with regard to bone anatomy, morphology, healing capacity, remodelling, mineral density, and concentration [91], [92]. Moreover, similarities have been found in the femur cross-sectional diameter and area between humans and pigs [93]. Besides, pigs also have a lamellar bone structure similar to that of humans [94]. However, pigs have a denser trabecular network, which considered intricate, difficult to handle, noisy, and aggressive; hence, pigs are often neglected in favour of more amenable species such as sheep and goats [95], [96], [97]. Furthermore, the length of the tibiae and femora in pigs is relatively small, which cannot meet the special implant needs of humans. Pig was the animal of choice for critical-size defect models to test bone substitute biomaterials because its bone regeneration rate (1.2–1.5 μm/d) is comparable to that of humans (1.0–1.5 μm/d) [49]. Commercial pigs are generally considered undesirable for orthopaedic research because of their large growth rates and very high body weight. However, the development of miniature pigs and micropigs has overcome this problem to some extent (Table 3).
Table 3.
Pig bone defect models used to test bone substitute biomaterials.
| Animal | Defect site | Defect size | Substitute biomaterials |
|---|---|---|---|
| Porcine | Craniofacial | 10 mm diameter & 10 mm depth | HA/TCP, PEG membrane, BMP-2 [45] |
| Göttinger minipigs | Tibial | 11 mm diameter & 25 mm depth | Granular calcium phosphate, bone marrow aspiration concentrate; platelet-rich plasma [98] |
| Minipig | Parietal | 2 × 4 cm2 | Particulate iliac bone graft, rhBMP-7 composite [48] |
| Pig | Orbital | 2 × 2 cm2 | Bone-marrow-coated polycaprolactone scaffolds [99] |
BMP = bone morphogenetic protein; PEG = poly(ethyleneglycol).
Application of pig bone defect models for testing substitute biomaterials
Wehrhan et al [45] created a gene delivery method to increase bone formation in a porcine craniofacial bone defect model. The results showed that the gene delivery method formed more new bone in the defect site. Riegger et al [98] created circumscribed cylindrical bone defects of 11 mm diameter and 25 mm depth without penetration of the lateral cortex in the medial plateau of the tibia of 16 minipigs. The defect model was created to test the in vivo effect of the granular calcium phosphate composites and bone marrow aspiration concentrate. They found that there was a significant correlation between the two detective methods, showing that multidetector CT could be a promising tool for monitoring bone healing. A minipig infant model with craniofacial bone defect was created to test the in vivo effect of autologous bone grafts and bone morphogenetic protein-7 (rhBMP-7) by Springer et al [48]. Rohner et al [99] used a pig orbital defect model to show the in vivo efficacy of bone marrow-coated polycaprolactone scaffolds. Their studies showed that this bone marrow-coated 3D polycaprolactone scaffold is a promising substitute biomaterial for enhancing bone regeneration.
Protocols for developing a porcine craniofacial bone defect model and testing substitute biomaterials
The porcine craniofacial bone defect model is used widely for testing bone substitute biomaterials. A commonly used protocol for developing a porcine craniofacial bone defect model and testing substitute materials was reported by Wehrhan et al [45]. Briefly, after anaesthetising domestic pigs and exposing the skull, nine defects of 10 mm diameter and 10 mm depth were created on it. Three testing groups, i.e., HA/TCP covered by poly(ethyleneglycol) (PEG) membrane, HA/TCP mixed with PEG matrix, and HA/TCP mixed with BMP-2 transfected hFOB cells and PEG matrix, were filled in three out of nine defects. The remaining six defects were filled with HA/TCP. After 2 weeks, 4 weeks, and 12 weeks, the animals were sacrificed and the os frontale was harvested for the following histological and immunohistochemical analyses.
Sheep/goats
Advantage and disadvantage of sheep or goats
It has been reported that adult sheep offer the advantage of possessing a body weight similar to adult humans, and having long bones of dimensions suitable for testing human implants and prostheses [96], which is not possible in small species such as rabbits and dogs. Sheep bones have similar macrostructure to human bones, but histologically, the bone structure of sheep is different from that of humans. In sheep, bone consists predominantly of the primary bone structure [100] in comparison with the largely secondary bone structure of humans [101]. Secondary bone remodelling in sheep does not take place until an average age of 7–9 years [96], while at 3–4 years of age they have a plexiform bone structure comprising a combination of woven and lamellar bones within which vascular plexuses are sandwiched [51]. Mature sheep have a significantly higher trabecular bone density and subsequently greater bone strength when compared to humans [51], [102]. However, differences may change with location. Some researchers argue that sheep are still valuable models for human bone turnover and remodelling activity, although differences in bone structure were defined [103], [104], [105]. Sheep are shown to have a larger amount of bone ingrowth than humans; this is probably due to the greater amount of cancellous bone in the distal femur of sheep compared with humans [106].
Application of sheep bone defect models for testing substitute biomaterials
Maissen et al [107] used an ovine segmental defect model to investigate the influence of rhTGFβ-3 on mechanical and radiological parameters of a healing bone defect. The osteogenesis and remodelling effects of a biphasic synthetic bone graft material (Genex Paste; Biocomposites, Staffordshire, England), composed of calcium sulphate and β-TCP, on the healing of a sheep vertebral defect model was described in a canine model by Yang et al [39]. Zhu et al [40] developed a sheep vertebral bone defect model to evaluate the new bioactive materials and assessed the feasibility of the model in vivo. Reichert et al [41] developed a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Lippens et al [42] used a 6-mm-size unicortical tibia defect goat model to evaluate the in vivo bone formation effect of an injectable polymerisable pluronic F127 hydrogel derivative combined with autologous mesenchymal stem cells. Kobayashi et al [108] used a 8-mm-diameter and 15-mm-deep sheep vertebral bone void model to investigate the histological properties of three formulations of calcium sodium phosphosilicate.
Protocols for developing a sheep tibia defect model for testing bone substitute biomaterials
The sheep tibia defect model has been used to test bone substitute by many researchers [41], [42], [107]. Here we review the protocol of an 18-mm-long mid-diaphysis tibia defect created in sheep for testing substitute materials and autologous bone graft. The defect was created in a 4–5-year-old sheep model and stabilized with a unilateral external fixator. The implant to the defects was divided into four groups. Assessment of in vivo stiffness was performed every week in a 4-week period by a custom-made device [107]. The radiology result revealed that only the bone graft group showed obvious recovery. Radiographic as well as computer tomographic evaluation was used to assess bone regeneration of the defect side.
Conclusion
Animal models play an indispensable role in testing bone substitute biomaterials for understanding their osteoconductivity, biocompatibility, mechanical properties, degradation, and interaction with host tissues. In this review, we summarised the most commonly and successfully used animal models, and the protocols that may be used as references to establish relevant preclinical experimental animal model(s) for testing both biosafety and treatment efficacy of bone substitutes (Table 4). After reviewing >100 publications about in vivo tests of biomaterials, we conclude that most authors fail to discuss the reason for choosing the animal model that they established and the clinical indication that they are stimulating. Although no animal model is perfect to simulate clinical conditions, we recommend that animal models should be established based on clinical indications. Finally, anaesthesia practice and specific surgical protocol should be included in the publications so as to make sure that animal welfare is well established.
Table 4.
Summary of advantages and disadvantages of different bone defect animal models.
| Animal species | Bone defect site | Advantages | Disadvantages |
|---|---|---|---|
| Pig | Craniofacial | Bone anatomy, morphology, healing capacity, & remodelling similar to humans; similar bone structure with respect to bone mineral density & concentration; a lamellar bone structure | Denser trabecular network, intricate & difficult to handle, noisy & aggressive, shorter tibiae & femur, large growth rates, & very high body weight |
| Sheep | Tibiae | Body weight similar to adult humans, easy to handle & house, relatively inexpensive, available in large numbers | Significantly higher trabecular bone density & subsequently greater bone strength, larger amount of bone ingrowth than humans |
| Rabbit | Tibiae femur | Easy to handle & small size, reaching skeletal maturity shortly after sexual maturity at ∼6 mo of age | Small size; differences in bone anatomy, such as size & shape of the bones & also in loading; faster skeletal change & bone turnover |
| Rodent | Femur calvaria | Easy to handle & small size, life span suitable for postsurgery observation | Small-sized long bones & thin & fragile cortices, no showing of Haversian-type remodelling in the cortex |
Conflicts and interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors are grateful for the financial support from NSFC-DG-RTD Joint Scheme (Project No. 51361130034), the European Union's 7th Framework Program under grant agreement No. NMP3-SL-2013-604517, NSFC grant (Project No. 51203178), and Shenzhen Fundamental Research Foundation (Project No. JCYJ20120617114912864).
Contributor Information
Xin-Luan Wang, Email: xl.wang@siat.ac.cn.
Yu-Xiao Lai, Email: yx.lai@siat.ac.cn.
References
- 1.Blumenfeld I., Srouji S., Lanir Y., Laufer D., Livne E. Enhancement of bone defect healing in old rats by TGF-β and IGF-1. Exp Gerontol. 2002;37:553–565. doi: 10.1016/s0531-5565(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 2.Perry C.R. Bone repair techniques, bone graft, and bone graft substitutes. Clin Orthop Relat Res. 1999;360:71–86. doi: 10.1097/00003086-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Clements J.R., Carpenter B.B., Pourciau J.K. Treating segmental bone defects: a new technique. J Foot Ankle Surg. 2008;47:350–356. doi: 10.1053/j.jfas.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Liu G., Zhao L., Zhang W., Cui L., Liu W., Cao Y. Repair of goat tibial defects with bone marrow stromal cells and beta-tricalcium phosphate. J Mater Sci Mater Med. 2008;19:2367–2376. doi: 10.1007/s10856-007-3348-3. [DOI] [PubMed] [Google Scholar]
- 5.Theos C.K.P., Kottakis S., Demertzis N. Reconstruction of tibia defects by ipsilateral vascularized fibula transposition. Arch Orthop Trauma Surg Neurol. 2008;128:179–184. doi: 10.1007/s00402-007-0301-3. [DOI] [PubMed] [Google Scholar]
- 6.Oest M.E.D.K., Kong H.J., Mooney D.J., Guldberg R.E. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J Orthop Res. 2007;25:941–950. doi: 10.1002/jor.20372. [DOI] [PubMed] [Google Scholar]
- 7.den Boer F.C., Wippermann B.W., Blokhuis T.J., Patka P., Bakker F.C., Haarman H.J. Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-1 or autologous bone marrow. J Orthop Res. 2003;21:521–528. doi: 10.1016/S0736-0266(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 8.Chapman M.W., Bucholz R., Cornell C. Treatment of acute fractures with a collagen-calcium phosphate graft material. A randomized clinical trial. J Bone Jt Surg Am. 1997;79:495–502. doi: 10.2106/00004623-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Muscolo D.L., Ayerza M.A., La A.-T. Massive allograft use in orthopedic oncology. Orthop Clin North Am. 2006;37:65–74. doi: 10.1016/j.ocl.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Gazdag A.R., Lane J.M., Glaser D., Forster R.A. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg. 1995;3:1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Lindsey R.W., Gugala Z., Milne E., Sun M., Gannon F.H., Latta L.L. The efficacy of cylindrical titanium mesh cage for the reconstruction of a critical-size canine segmental femoral diaphyseal defect. J Orthop Res. 2006;24:1438–1453. doi: 10.1002/jor.20154. [DOI] [PubMed] [Google Scholar]
- 12.Williams D.F. On the nature of biomaterials. Biomaterials. 2009;30:5897–5909. doi: 10.1016/j.biomaterials.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Kokubo S., Fujimoto R., Yokota S., Fukushima S., Nozaki K., Takahashi K. Bone regeneration by recombinant human bone morphogenetic protein-2 and a novel biodegradable carrier in a rabbit ulnar defect model. Biomaterials. 2003;24:1643–1651. doi: 10.1016/s0142-9612(02)00551-3. [DOI] [PubMed] [Google Scholar]
- 14.Han S.-O., Mahato R.I., Sung Y.K., Kim S.W. Development of biomaterials for gene therapy. Mol Ther. 2000;2:302–317. doi: 10.1006/mthe.2000.0142. [DOI] [PubMed] [Google Scholar]
- 15.Hubbell J.A. Biomaterials in tissue engineering. Nat Biotechnol. 1995;13:565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 16.Richards R.G. AO Research Institute Davos within the AO Foundation: a model for translation of science to the clinics. J Orthop Transl. 2013;1:11–18. [Google Scholar]
- 17.Rahman C.V., Ben-David D., Dhillon A., Kuhn G., Gould T.W., Muller R. Controlled release of BMP-2 from a sintered polymer scaffold enhances bone repair in a mouse calvarial defect model. J Tissue Eng Regen Med. 2014;8:59–66. doi: 10.1002/term.1497. [DOI] [PubMed] [Google Scholar]
- 18.Lim H.P., Mercado-Pagan A.E., Yun K.D., Kang S.S., Choi T.H., Bishop J. The effect of rhBMP-2 and PRP delivery by biodegradable beta-tricalcium phosphate scaffolds on new bone formation in a non-through rabbit cranial defect model. J Mater Sci Mater Med. 2013;24:1895–1903. doi: 10.1007/s10856-013-4939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das A., Tanner S., Barker D.A., Green D., Botchwey E.A. Delivery of S1P receptor-targeted drugs via biodegradable polymer scaffolds enhances bone regeneration in a critical size cranial defect. J Biomed Mater Res A. 2014;102:1210–1218. doi: 10.1002/jbm.a.34779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R., Nauth A., Li C., Qamirani E., Atesok K., Schemitsch E.H. Expression of VEGF gene isoforms in a rat segmental bone defect model treated with EPCs. J Orthop Trauma. 2012;26:689–692. doi: 10.1097/BOT.0b013e318266eb7e. [DOI] [PubMed] [Google Scholar]
- 21.Yoshii T., Hafeman A.E., Esparza J.M., Okawa A., Gutierrez G., Guelcher S.A. Local injection of lovastatin in biodegradable polyurethane scaffolds enhances bone regeneration in a critical-sized segmental defect in rat femora. J Tissue Eng Regen Med. 2014;8:589–595. doi: 10.1002/term.1547. [DOI] [PubMed] [Google Scholar]
- 22.Cheng C., Alt V., Dimitrakopoulou-Strauss A., Pan L., Thormann U., Schnettler R. Evaluation of new bone formation in normal and osteoporotic rats with a 3-mm femur defect: functional assessment with dynamic PET-CT (dPET-CT) using 2-deoxy-2-[(18)F]fluoro-D-glucose ((18)F-FDG) and (18)F-fluoride. Mol Imaging Biol. 2013;15:336–344. doi: 10.1007/s11307-012-0592-9. [DOI] [PubMed] [Google Scholar]
- 23.Mohan B.G., Shenoy S.J., Babu S.S., Varma H.K., John A. Strontium calcium phosphate for the repair of leporine (Oryctolagus cuniculus) ulna segmental defect. J Biomed Mater Res A. 2013;101:261–271. doi: 10.1002/jbm.a.34324. [DOI] [PubMed] [Google Scholar]
- 24.Smith M.R., Atkinson P., White D., Piersma T., Gutierrez G., Rossini G. Design and assessment of a wrapped cylindrical Ca-P AZ31 Mg alloy for critical-size ulna defect repair. J Biomed Mater Res B Appl Biomater. 2012;100:206–216. doi: 10.1002/jbm.b.31940. [DOI] [PubMed] [Google Scholar]
- 25.Kim A., Kim D.H., Song H.R., Kang W.H., Kim H.J., Lim H.C. Repair of rabbit ulna segmental bone defect using freshly isolated adipose-derived stromal vascular fraction. Cytotherapy. 2012;14:296–305. doi: 10.3109/14653249.2011.627915. [DOI] [PubMed] [Google Scholar]
- 26.Zwingenberger S., Niederlohmann E., Vater C., Rammelt S., Matthys R., Bernhardt R. Establishment of a femoral critical-size bone defect model in immunodeficient mice. J Surg Res. 2013;181:e7–14. doi: 10.1016/j.jss.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanchetta P., Lagarde N., Uguen A., Marcorelles P. Mixture of hyaluronic acid, chondroitin 6 sulphate and dermatan sulphate used to completely regenerate bone in rat critical size defect model. J Craniomaxillofac Surg. 2012;40:783–787. doi: 10.1016/j.jcms.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Skaliczki G., Weszl M., Schandl K., Major T., Kovacs M., Skaliczki J. Compromised bone healing following spacer removal in a rat femoral defect model. Acta Physiol Hung. 2012;99:223–232. doi: 10.1556/APhysiol.99.2012.2.16. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S., Ponnazhagan S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone. 2012;50:1012–1018. doi: 10.1016/j.bone.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bateman J.P., Safadi F.F., Susin C., Wikesjo U.M. Exploratory study on the effect of osteoactivin on bone formation in the rat critical-size calvarial defect model. J Periodontal Res. 2012;47:243–247. doi: 10.1111/j.1600-0765.2011.01428.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X., Cai Q., Liu H., Heng B.C., Peng H., Song Y. Osteoconductive effectiveness of bone graft derived from antler cancellous bone: an experimental study in the rabbit mandible defect model. Int J Oral Maxillofac Surg. 2012;41:1330–1337. doi: 10.1016/j.ijom.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W., Wang W., Chen Q.Y., Lin Z.Q., Cheng S.W., Kou D.Q. Effect of calcium citrate on bone integration in a rabbit femur defect model. Asian Pac J Trop Med. 2012;5:310–314. doi: 10.1016/S1995-7645(12)60045-5. [DOI] [PubMed] [Google Scholar]
- 33.Hussain I., Moharamzadeh K., Brook I.M. Jose de Oliveira Neto P, Salata LA. Evaluation of osteoconductive and osteogenic potential of a dentin-based bone substitute using a calvarial defect model. Int J Dent. 2012;2012:396316. doi: 10.1155/2012/396316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider G., Blechschmidt K., Linde D., Litschko P., Korbs T., Beleites E. Bone regeneration with glass ceramic implants and calcium phosphate cements in a rabbit cranial defect model. J Mater Sci Mater Med. 2010;21:2853–2859. doi: 10.1007/s10856-010-4143-0. [DOI] [PubMed] [Google Scholar]
- 35.Yano K., Namikawa T., Uemura T., Hoshino M., Wakitani S., Takaoka K. Regenerative repair of bone defects with osteoinductive hydroxyapatite fabricated to match the defect and implanted with combined use of computer-aided design, computer-aided manufacturing, and computer-assisted surgery systems: a feasibility study in a canine model. J Orthop Sci. 2012;17:484–489. doi: 10.1007/s00776-012-0235-7. [DOI] [PubMed] [Google Scholar]
- 36.Lee J., Tran Q., Seeba G., Wikesjo U.M., Susin C. The critical-size supraalveolar peri-implant defect model: reproducibility in histometric data acquisition of alveolar bone formation and osseointegration. J Clin Periodontol. 2009;36:1067–1074. doi: 10.1111/j.1600-051X.2009.01487.x. [DOI] [PubMed] [Google Scholar]
- 37.Jang B.J., Byeon Y.E., Lim J.H., Ryu H.H., Kim W.H., Koyama Y. Implantation of canine umbilical cord blood-derived mesenchymal stem cells mixed with beta-tricalcium phosphate enhances osteogenesis in bone defect model dogs. J Vet Sci. 2008;9:387–393. doi: 10.4142/jvs.2008.9.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takigami H., Kumagai K., Latson L., Togawa D., Bauer T., Powell K. Bone formation following OP-1 implantation is improved by addition of autogenous bone marrow cells in a canine femur defect model. J Orthop Res. 2007;25:1333–1342. doi: 10.1002/jor.20411. [DOI] [PubMed] [Google Scholar]
- 39.Yang H.L., Zhu X.S., Chen L., Chen C.M., Mangham D.C., Coulton L.A. Bone healing response to a synthetic calcium sulfate/beta-tricalcium phosphate graft material in a sheep vertebral body defect model. J Biomed Mater Res B Appl Biomater. 2012;100:1911–1921. doi: 10.1002/jbm.b.32758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu X.S., Zhang Z.M., Mao H.Q., Geng D.C., Zou J., Wang G.L. A novel sheep vertebral bone defect model for injectable bioactive vertebral augmentation materials. J Mater Sci Mater Med. 2011;22:159–164. doi: 10.1007/s10856-010-4191-5. [DOI] [PubMed] [Google Scholar]
- 41.Reichert J.C., Epari D.R., Wullschleger M.E., Saifzadeh S., Steck R., Lienau J. Establishment of a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Tissue Eng Part B Rev. 2010;16:93–104. doi: 10.1089/ten.TEB.2009.0455. [DOI] [PubMed] [Google Scholar]
- 42.Lippens E., Vertenten G., Girones J., Declercq H., Saunders J., Luyten J. Evaluation of bone regeneration with an injectable, in situ polymerizable Pluronic F127 hydrogel derivative combined with autologous mesenchymal stem cells in a goat tibia defect model. Tissue Eng Part A. 2010;16:617–627. doi: 10.1089/ten.TEA.2009.0418. [DOI] [PubMed] [Google Scholar]
- 43.Koeter S., Tigchelaar S.J., Farla P., Driessen L., van Kampen A., Buma P. Coralline hydroxyapatite is a suitable bone graft substitute in an intra-articular goat defect model. J Biomed Mater Res B Appl Biomater. 2009;90:116–122. doi: 10.1002/jbm.b.31260. [DOI] [PubMed] [Google Scholar]
- 44.Yu D., Li Q., Mu X., Chang T., Xiong Z. Bone regeneration of critical calvarial defect in goat model by PLGA/TCP/rhBMP-2 scaffolds prepared by low-temperature rapid-prototyping technology. Int J Oral Maxillofac Surg. 2008;37:929–934. doi: 10.1016/j.ijom.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Wehrhan F., Amann K., Molenberg A., Lutz R., Neukam F.W., Schlegel K.A. PEG matrix enables cell-mediated local BMP-2 gene delivery and increased bone formation in a porcine critical size defect model of craniofacial bone regeneration. Clin Oral Implants Res. 2012;23:805–813. doi: 10.1111/j.1600-0501.2011.02223.x. [DOI] [PubMed] [Google Scholar]
- 46.Kropil P., Hakimi A.R., Jungbluth P., Riegger C., Rubbert C., Miese F. Cone beam CT in assessment of tibial bone defect healing: an animal study. Acad Radiol. 2012;19:320–325. doi: 10.1016/j.acra.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 47.Carstens M.H., Chin M., Li X.J. In situ osteogenesis: regeneration of 10-cm mandibular defect in porcine model using recombinant human bone morphogenetic protein-2 (rhBMP-2) and Helistat absorbable collagen sponge. J Craniofac Surg. 2005;16:1033–1042. doi: 10.1097/01.scs.0000186307.09171.20. [DOI] [PubMed] [Google Scholar]
- 48.Springer I.N., Acil Y., Kuchenbecker S., Bolte H., Warnke P.H., Abboud M. Bone graft versus BMP-7 in a critical size defect—cranioplasty in a growing infant model. Bone. 2005;37:563–569. doi: 10.1016/j.bone.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Schlegel K.A., Lang F.J., Donath K., Kulow J.T., Wiltfang J. The monocortical critical size bone defect as an alternative experimental model in testing bone substitute materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:7–13. doi: 10.1016/j.tripleo.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Gugala Z., Lindsey R.W., Gogolewski s New Approaches in the treatment of critical-size segmental defects in long bones. Macromol Symp. 2007;253:147–161. [Google Scholar]
- 51.Liebschner M.A. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials. 2004;25:1697–1714. doi: 10.1016/s0142-9612(03)00515-5. [DOI] [PubMed] [Google Scholar]
- 52.Pearce A.I., Richards R.G., Milz S., Schneider E., Pearce S.G. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007;13:1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- 53.Upman P.J. ISO 10993-6: test for local effects after implantation. BONEZone. 2006;5:50–52. [Google Scholar]
- 54.Neyt J., Buckwalter J.A., Carroll N. Use of animal models in musculoskeletal research. Iowa Orthop J. 1998;18:118–123. [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Mabrey J.D., Agrawal C.M. An interspecies comparison of bone fracture properties. Bio-med Mater Eng. 1998;8:1–9. [PubMed] [Google Scholar]
- 56.Castaneda S., Largo R., Calvo E., Rodriguez-Salvanes F., Marcos M.E., Diaz-Curiel M. Bone mineral measurements of subchondral and trabecular bone in healthy and osteoporotic rabbits. Skelet Radiol. 2006;35:34–41. doi: 10.1007/s00256-005-0022-z. [DOI] [PubMed] [Google Scholar]
- 57.Walsh W.R., Vizesi F., Michael D., Auld J., Langdown A., Oliver R. Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials. 2008;29:266–271. doi: 10.1016/j.biomaterials.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 58.Young S., Bashoura A.G., Borden T., Baggett L.S., Jansen J.A., Wong M. Development and characterization of a rabbit alveolar bone nonhealing defect model. J Biomed Mater Res A. 2008;86:182–194. doi: 10.1002/jbm.a.31639. [DOI] [PubMed] [Google Scholar]
- 59.Gauthier O., Müller R., von Stechow D., Lamy B., Weiss P., Bouler J.-M. In vivo bone regeneration with injectable calcium phosphate biomaterial: a three-dimensional micro-computed tomographic, biomechanical and SEM study. Biomaterials. 2005;26:5444–5453. doi: 10.1016/j.biomaterials.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 60.Calvo-Guirado J., Delgado-Ruíz R., Ramírez-Fernández M., Maté-Sánchez J., Ortiz-Ruiz A., Marcus A. Histomorphometric and mineral degradation study of Ossceram®: a novel biphasic B-tricalcium phosphate, in critical size defects in rabbits. Clin Oral Implants Res. 2012;23:667–675. doi: 10.1111/j.1600-0501.2011.02193.x. [DOI] [PubMed] [Google Scholar]
- 61.Delgado-Ruiz R.A., Calvo-Guirado J.L., Abboud M., Ramirez-Fernández M.P., Maté-Sánchez J.E., Negri B. Porous titanium granules in critical size defects of rabbit tibia with or without membranes. Int J Oral Sci. 2014;6:105–110. doi: 10.1038/ijos.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S.-H., Lei M., Xie X.-H., Zheng L.-Z., Yao D., Wang X.-L. PLGA/TCP composite scaffold incorporating bioactive phytomolecule icaritin for enhancement of bone defect repair in rabbits. Acta Biomater. 2013;9:6711–6722. doi: 10.1016/j.actbio.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 63.Witte F., Ulrich H., Palm C., Willbold E. Biodegradable magnesium scaffolds: part II: peri-implant bone remodeling. J Biomed Mater Res A. 2007;81A:757–765. doi: 10.1002/jbm.a.31293. [DOI] [PubMed] [Google Scholar]
- 64.Wei J., Jia J., Wu F., Wei S., Zhou H., Zhang H. Hierarchically microporous/macroporous scaffold of magnesium-calcium phosphate for bone tissue regeneration. Biomaterials. 2010;31:1260–1269. doi: 10.1016/j.biomaterials.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Dai C., Guo H., Lu J., Shi J., Wei J., Liu C. Osteogenic evaluation of calcium/magnesium-doped mesoporous silica scaffold with incorporation of rhBMP-2 by synchrotron radiation-based mu CT. Biomaterials. 2011;32:8506–8517. doi: 10.1016/j.biomaterials.2011.07.090. [DOI] [PubMed] [Google Scholar]
- 66.Li X., Niu Y., Guo H., Chen H., Li F., Zhang J. Preparation and osteogenic properties of magnesium calcium phosphate biocement scaffolds for bone regeneration. J Instrum. 2013;8:C07010. [Google Scholar]
- 67.He D., Dong W., Tang S., Wei J., Liu Z., Gu X. Tissue engineering scaffolds of mesoporous magnesium silicate and poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) composite. J Mater Sci Mater Med. 2014;25:1415–1424. doi: 10.1007/s10856-014-5183-7. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y.J., Yang Z.Y., Tan L.L., Li H., Zhang Y.Z. An animal experimental study of porous magnesium scaffold degradation and osteogenesis. Braz J Med Biol Res. 2014;47:715–720. doi: 10.1590/1414-431X20144009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campanacci M., Costa P. Total resection of distal femur or proximal tibia for bone tumours. Autogenous bone grafts and arthrodesis in twenty-six cases. J Bone Jt Surg Br. 1979;61:455–463. doi: 10.1302/0301-620X.61B4.227909. [DOI] [PubMed] [Google Scholar]
- 70.Engh G.A., Herzwurm P.J., Parks N.L. Treatment of major defects of bone with bulk allografts and stemmed components during total knee arthroplasty. J Bone Jt Surg. 1997;79:1030–1039. doi: 10.2106/00004623-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Kraay M.J., Goldberg V.M., Figgie M.P., Figgie H.E. Distal femoral replacement with allograft/prosthetic reconstruction for treatment of supracondylar fractures in patients with total knee arthroplasty. J Arthroplasty. 1992;7:7–16. doi: 10.1016/0883-5403(92)90025-l. [DOI] [PubMed] [Google Scholar]
- 72.Stockley I., McAuley J.P., Gross A.E. Allograft reconstruction in total knee arthroplasty. J Bone Jt Surg Br. 1992;74:393–397. doi: 10.1302/0301-620X.74B3.1587885. [DOI] [PubMed] [Google Scholar]
- 73.Ghazavi M.T., Stockley I., Yee G., Davis A., Gross A.E. Reconstruction of massive bone defects with allograft in revision total knee arthroplasty. J Bone Jt Surg. 1997;79:17–25. [PubMed] [Google Scholar]
- 74.Qin L., Zhang G., Sheng H., Yeung K.W., Yeung H.Y., Chan C.W. Multiple bioimaging modalities in evaluation of an experimental osteonecrosis induced by a combination of lipopolysaccharide and methylprednisolone. Bone. 2006;39:863–871. doi: 10.1016/j.bone.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X.L., Xie X.H., Zhang G., Chen S.H., Yao D., He K. Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair. J Orthop Res. 2013;31:164–172. doi: 10.1002/jor.22188. [DOI] [PubMed] [Google Scholar]
- 76.Zhang G., Qin L., Sheng H. Establishment of steroid-associated osteonecrosis rabbit model. Pract Man Musculoskelet Res. 2008;30:495–510. [Google Scholar]
- 77.Komaki H., Tanaka T., Chazono M., Kikuchi T. Repair of segmental bone defects in rabbit tibiae using a complex of β-tricalcium phosphate, type I collagen, and fibroblast growth factor-2. Biomaterials. 2006;27:5118–5126. doi: 10.1016/j.biomaterials.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 78.Kim H.-W., Shin S.-Y., Kim H.-E., Lee Y.-M., Chung C.-P., Lee H.-H. Bone formation on the apatite-coated zirconia porous scaffolds within a rabbit calvarial defect. J Biomater Appl. 2008;22:485–504. doi: 10.1177/0885328207078075. [DOI] [PubMed] [Google Scholar]
- 79.Lee E.-H., Kim J.-Y., Kweon H.Y., Jo Y.-Y., Min S.-K., Park Y.-W. A combination graft of low-molecular-weight silk fibroin with Choukroun platelet-rich fibrin for rabbit calvarial defect. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2010;109:e33–e38. doi: 10.1016/j.tripleo.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 80.Chen S.-H., Zheng L.-Z., Xie X.-H., Wang X.-L., Lai Y.-X., Chen S.-K. Comparative study of poly (lactic-co-glycolic acid)/tricalcium phosphate scaffolds incorporated or coated with osteogenic growth factors for enhancement of bone regeneration. J Orthop Transl. 2014;2:91–104. [Google Scholar]
- 81.Gomes P., Fernandes M. Rodent models in bone-related research: the relevance of calvarial defects in the assessment of bone regeneration strategies. Lab Anim. 2011;45:14–24. doi: 10.1258/la.2010.010085. [DOI] [PubMed] [Google Scholar]
- 82.An Y.H., Freidman R.J. CRC Press; Boca Raton, FL: 1998. Animal models in orthopaedic research. [Google Scholar]
- 83.Kondo N., Ogose A., Tokunaga K., Ito T., Arai K., Kudo N. Bone formation and resorption of highly purified β-tricalcium phosphate in the rat femoral condyle. Biomaterials. 2005;26:5600–5608. doi: 10.1016/j.biomaterials.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 84.Inzana J.A., Olvera D., Fuller S.M., Kelly J.P., Graeve O.A., Schwarz E.M. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ye J.-H., Xu Y.-J., Gao J., Yan S.-G., Zhao J., Tu Q. Critical-size calvarial bone defects healing in a mouse model with silk scaffolds and SATB2-modified iPSCs. Biomaterials. 2011;32:5065–5076. doi: 10.1016/j.biomaterials.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmitz J.P., Hollinger J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308. [PubMed] [Google Scholar]
- 87.Tielinen L., Manninen M., Puolakkainen P., Kellomäki M., Törmälä P., Rich J. Inability of transforming growth factor-β1, combined with a bioabsorbable polymer paste, to promote healing of bone defects in the rat distal femur. Arch Orthop Trauma Surg. 2001;121:191–196. doi: 10.1007/s004020000206. [DOI] [PubMed] [Google Scholar]
- 88.Ohgushi H., Goldberg V.M., Caplan A.I. Repair of bone defects with marrow cells and porous ceramic: experiments in rats. Acta Orthop. 1989;60:334–339. doi: 10.3109/17453678909149289. [DOI] [PubMed] [Google Scholar]
- 89.Kirker-Head C., Karageorgiou V., Hofmann S., Fajardo R., Betz O., Merkle H.P. BMP-silk composite matrices heal critically sized femoral defects. Bone. 2007;41:247–255. doi: 10.1016/j.bone.2007.04.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoon E., Dhar S., Chun D.E., Gharibjanian N.A., Evans G.R.D. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619–627. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- 91.Thorwarth M., Schultze-Mosgau S., Kessler P., Wiltfang J., Schlegel K.A. Bone regeneration in osseous defects using a resorbable nanoparticular hydroxyapatite. J Oral Maxillofac Surg. 2005;63:1626–1633. doi: 10.1016/j.joms.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Aerssens J., Boonen S., Lowet G., Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139:663–670. doi: 10.1210/endo.139.2.5751. [DOI] [PubMed] [Google Scholar]
- 93.Raab D.M., Crenshaw T.D., Kimmel D.B., Smith E.L. A histomorphometric study of cortical bone activity during increased weight-bearing exercise. J Bone Min Res. 1991;6:741–749. doi: 10.1002/jbmr.5650060712. [DOI] [PubMed] [Google Scholar]
- 94.Mosekilde L., Kragstrup J., Richards A. Compressive strength, ash weight, and volume of vertebral trabecular bone in experimental fluorosis in pigs. Calcif Tissue Int. 1987;40:318–322. doi: 10.1007/BF02556693. [DOI] [PubMed] [Google Scholar]
- 95.Mosekilde L., Weisbrode S.E., Safron J.A., Stills H.F., Jankowsky M.L., Ebert D.C. Calcium-restricted ovariectomized Sinclair S-1 minipigs: an animal model of osteopenia and trabecular plate perforation. Bone. 1993;14:379–382. doi: 10.1016/8756-3282(93)90167-9. [DOI] [PubMed] [Google Scholar]
- 96.Newman E., Turner A.S., Wark J.D. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone. 1995;16:277S–284S. doi: 10.1016/8756-3282(95)00026-a. [DOI] [PubMed] [Google Scholar]
- 97.Swindle M.M., Smith A.C., Hepburn B.J. Swine as models in experimental surgery. J Invest Surg. 1988;1:65–79. doi: 10.3109/08941938809141077. [DOI] [PubMed] [Google Scholar]
- 98.Riegger C., Kropil P., Jungbluth P., Lanzman R.S., Miese F.R., Hakimi A.R. Quantitative assessment of bone defect healing by multidetector CT in a pig model. Skelet Radiol. 2012;41:531–537. doi: 10.1007/s00256-011-1203-6. [DOI] [PubMed] [Google Scholar]
- 99.Rohner D., Hutmacher D.W., Cheng T.K., Oberholzer M., Hammer B. In vivo efficacy of bone-marrow-coated polycaprolactone scaffolds for the reconstruction of orbital defects in the pig. J Biomed Mater Res B Appl Biomater. 2003;66:574–580. doi: 10.1002/jbm.b.10037. [DOI] [PubMed] [Google Scholar]
- 100.V DK . Development of bone. In: Sumner-Smith G., editor. Bone in clinical orthopedics. W.B. Saunders Co.; Philadelphia: 2006. pp. 1–80. [Google Scholar]
- 101.Eitel F., Klapp F., Jacobson W., Schweiberer L. Bone regeneration in animals and in man. A contribution to understanding the relative value of animal experiments to human pathophysiology. Arch Orthop Trauma Surg. 1981;99:59–64. doi: 10.1007/BF00400911. [DOI] [PubMed] [Google Scholar]
- 102.Nafei A., Danielsen C.C., Linde F., Hvid I. Properties of growing trabecular ovine bone. Part I: mechanical and physical properties. J Bone Jt Surg Br. 2000;82:910–920. doi: 10.1302/0301-620x.82b6.9836. [DOI] [PubMed] [Google Scholar]
- 103.Chavassieux P., Pastoureau P., Boivin G., Charhon S., Chapuy M., Delmas P. Effects of sodium fluoride on bone remodeling in ewes. J Bone Min Res. 1987;2(Suppl. 1) 359 [abstract] [Google Scholar]
- 104.Pastoureau P., Arlot M., Caulin F., Barlet J., Meunier P., Delmas P.D. Effects of oophorectomy on biochemical and histological indices of bone turnover in ewes. J Bone Min Res. 1989;4 S237; abstract 477. [Google Scholar]
- 105.den Boer F.C., Patka P., Bakker F.C., Wippermann B.W., van Lingen A., Vink G.Q. New segmental long bone defect model in sheep: quantitative analysis of healing with dual energy x-ray absorptiometry. J Orthop Res. 1999;17:654–660. doi: 10.1002/jor.1100170506. [DOI] [PubMed] [Google Scholar]
- 106.Willie B.M., Bloebaum R.D., Bireley W.R., Bachus K.N., Hofmann A.A. Determining relevance of a weightbearing ovine model for bone ingrowth assessment. J Biomed Mater Res A. 2004;69:567–576. doi: 10.1002/jbm.a.30038. [DOI] [PubMed] [Google Scholar]
- 107.Maissen O., Eckhardt C., Gogolewski S., Glatt M., Arvinte T., Steiner A. Mechanical and radiological assessment of the influence of rhTGFbeta-3 on bone regeneration in a segmental defect in the ovine tibia: pilot study. J Orthop Res. 2006;24:1670–1678. doi: 10.1002/jor.20231. [DOI] [PubMed] [Google Scholar]
- 108.Kobayashi H., Turner A.S., Seim H.B., 3rd, Kawamoto T., Bauer T.W. Evaluation of a silica-containing bone graft substitute in a vertebral defect model. J Biomed Mater Res A. 2010;92:596–603. doi: 10.1002/jbm.a.32397. [DOI] [PubMed] [Google Scholar]