Summary
Mesenchymal stem cells (MSCs) from bone marrow are main cell source for tissue repair and engineering, and vehicles of cell-based gene therapy. Unlike other species, mouse bone marrow derived MSCs (BM-MSCs) are difficult to harvest and grow due to the low MSCs yield. We report here a standardised, reliable, and easy-to-perform protocol for isolation and culture of mouse BM-MSCs. There are five main features of this protocol. (1) After flushing bone marrow out of the marrow cavity, we cultured the cells with fat mass without filtering and washing them. Our method is simply keeping the MSCs in their initial niche with minimal disturbance. (2) Our culture medium is not supplemented with any additional growth factor. (3) Our method does not need to separate cells using flow cytometry or immunomagnetic sorting techniques. (4) Our method has been carefully tested in several mouse strains and the results are reproducible. (5) We have optimised this protocol, and list detailed potential problems and trouble-shooting tricks. Using our protocol, the isolated mouse BM-MSCs were strongly positive for CD44 and CD90, negative CD45 and CD31, and exhibited tri-lineage differentiation potentials. Compared with the commonly used protocol, our protocol had higher success rate of establishing the mouse BM-MSCs in culture. Our protocol may be a simple, reliable, and alternative method for culturing MSCs from mouse bone marrow tissues.
Keywords: bone marrow, isolation, mesenchymal stem cells, mouse, protocol
Introduction
Mesenchymal stem cells (MSCs) are multipotent stem cells that have the potential to self-renew and differentiate into a variety of specialised cell types such as osteoblasts, chondrocytes, adipocytes, and neurons [1], [2]. MSCs are easily accessible, expandable, immunosuppressive and they do not elicit immediate immune responses [3], [4]. Therefore, MSCs are an attractive cell source for tissue engineering and vehicles of cell therapy.
MSCs can be isolated from various sources such as adipose tissue, tendon, peripheral blood, and cord blood [5], [6], [7]. Bone marrow (BM) is the most common source of MSCs. MSCs have been successfully isolated and characterised from many species including mouse, rat, rabbit, dog, sheep, pig, and human [8], [9], [10], [11], [12]. Mice are one of the most commonly used experimental animals in biology and medicine primarily because they are mammals, small, inexpensive, easily maintained, can reproduce quickly, and share a high degree of homology with humans [13]. However, the isolation and purification of MSCs from mouse bone marrow is more difficult than other species due to their heterogeneity and low percentage in the bone marrow [1], [14], [15].
Two main stem cell populations and their progenies, haematopoietic stem cells and BM-MSCs, are the main residents of bone marrow [1], [15]. BM-MSCs are usually isolated and purified through their physical adherence to the plastic cell culture plate [16]. Several techniques have been used to purify or enrich MSCs including antibody-based cell sorting [17], low and high-density culture techniques [18], [19], positive and negative selection method [20], frequent medium changes [21], and enzymatic digestion approach [22]. However, they all had some short falls: the standard MSCs culture method based on plastic adherence has been confirmed to have lower successful rate [23]; whereas the cell sorting approach reduced the osteogenic potentials of MSCs [17]. Negative selection method leads to granulocyte–monocyte lineage cells reappearing after 1 week of culture [24]. Cells obtained using a positive selection method show higher proliferation ability compared with the negative selection method, but the method was only repeated in the C57B1/6 mice and failed to repeat in other strains of mice [25]. Frequent medium change method is inconvenient because it is required to change the culture medium every 8 hours during the first 72 hours of the initial culture [21]. Therefore, an easy and effective protocol for isolation of mouse BM-MSCs is needed.
Materials and methods
Reagents
Reagents used included: 0.25% trypsin–EDTA (1×) with phenol red; penicillin–streptomycin– neomycin (PSN; Life Technologies, Carlsbad, CA, USA) antibiotic mixture; foetal bovine serum, qualified, heat-inactivated (Life Technologies); minimal essential medium (MEM) α, nucleosides, powder (Life Technologies); and NaHCO3 (Sigma–Aldrich, St Louis, MO, USA).
Reagent setup
Stock of α-MEM was made up with 1 bag of α-MEM powder (1 L) and 2.2 g NaHCO3 in 1000 mL of Milli-Q water, adjusted to pH 7.2, filtered to sterilise, and stored for 1–2 weeks at 4°C. Complete α-MEM medium was α-MEM medium stock supplemented with 15% foetal bovine serum and 1% PSN, stored at 4°C. Phosphate-buffered saline (PBS) included: NaCl 8.0 g, KCl 0.2 g, KH2PO4 0.24 g, and Na2HPO4 1.44 g in 1 L Milli-Q water (pH 7.4, sterilised and stored at 4°C).
Animals
In this study, two mouse strains (ICR and C57) with different ages (4 weeks and 8 weeks, males and females) were tested using our protocol. All mice were purchased from and housed in a designated and government approved animal facility at The Chinese University Hong Kong, Hong Kong SAR, China, in according to The Chinese University Hong Kong's animal experimental regulations. All efforts were made to minimise animal suffering.
BM-MSCS culture protocol
Isolation and culture of mouse BM-MSCs
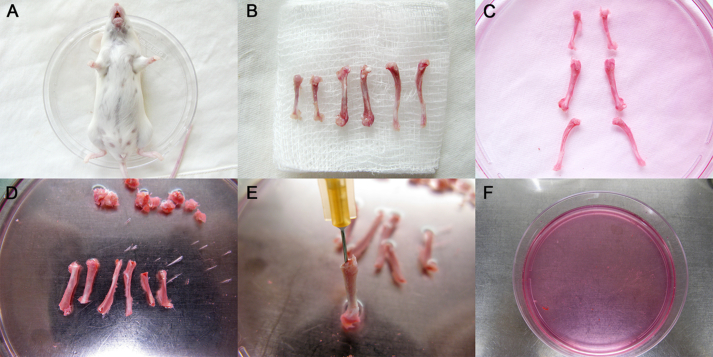
Mice aged 4 weeks or 8 weeks are terminated by cervical dislocation and placed in a 100-mm cell culture dish (Becton Dickinson, Franklin Lakes, NJ, USA), where the whole body is soaked in 70% (v/v) ethanol for 2 minutes, and then the mouse is transferred to a new dish (Fig. 1A). Four claws are dissected at the ankle and carpal joints, and incisions made around the connection between hindlimbs and trunk, forelimbs, and trunk. The whole skin is then removed from the hind limbs and forelimbs by pulling toward the cutting site of the claw. Muscles, ligaments, and tendons are carefully disassociated from tibias, femurs, and humeri using microdissecting scissors and surgical scalpel. Tibias, femurs, and humeri are dissected by cutting at the joints, and the bones are transferred onto sterile gauze. Bones are carefully scrubbed to remove the residual soft tissues (Fig. 1B), and transferred to a 100-mm sterile culture dish with 10 mL complete α-MEM medium on ice (Fig. 1C). All samples are processed within 30 minutes following animal death to ensure high cell viability. The soft tissues are completely dissociated from the bones to avoid contamination.
Figure 1.
Illustrations of mouse bone marrow cell collection procedures. (A) The mouse was terminated by cervical dislocation, placed in a 100-mm culture dish, and washed with 70% (vol/vol) ethanol for 2 minutes. (B) Tibias, femurs, and humeri were dissected; muscles, ligaments, and tendons were removed and the bones transferred onto sterile gauzes. (C) Bones were transferred to a 100-mm sterile culture dish with 10 mL complete α-minimal essential medium on ice. (D) The dish was transferred into the biosafety cabinet and washed twice to flush away impurities; the two ends just below the end of the marrow cavity were excised with microdissecting scissors. (E) A 23-gauge needle was inserted into the bone cavity and used to slowly flush the marrow out. The bone cavities were washed twice again until the bones became pale. (F) All the bone pieces were removed from the dish and the fat mass was left in the medium. Then the dish was incubated at 37°C in a 5% CO2 incubator.
In a biosafety cabinet, the bones are washed twice with PBS containing 1% PSN to flush away the blood cells and the residual soft tissues, then bones are transferred into a new 100-mm sterile culture dish with 10 mL complete α-MEM medium. The bone is held with forceps and the two ends excised just below the end of the marrow cavity using microdissecting scissors (Fig. 1D). A 23-gauge needle attached to a 5 mL syringe is used to draw 5 mL complete α-MEM medium from the dish; then the needle is inserted into the bone cavity. The marrow out is slowly flushed and the bone cavities washed twice again until the bones become pale (Fig. 1E). All the bone pieces are removed from the dish using forceps, leaving the solid mass in the medium, and the dish is incubated at 37°C in a 5% CO2 incubator for 5 days (Fig. 1F). In order to obtain enough marrow cells, the bone cavities are flushed repeatedly until the bones appear to be pale.
The initial spindle-shaped cells appear on Day 3 in phase-contrast microscopy, and then culture becomes more confluent and reaches 70–90% confluence within only 2 days. Cells are washed with PBS twice, and digested with 2.5 mL of 0.25% trypsin for 2 minutes at 37°C, then the trypsin neutralised with 7.5 mL complete α-MEM medium. The bottom of the plate is flushed using pipet-aid and the cells transferred to a 15 mL Falcon tube (Becton Dickinson), which is centrifuged at 800g for 5 minutes, and the cells resuspended in a 75 cm2 cell culture flask (Corning Inc, Corning, NY, USA) at a split ratio of 1:3. Note: Washing the cells with PBS prior to digestion is important, as it removes the residual medium and cell secretion and loosens the adhesive force of MSCs to the dish. The digestion should be limited to 2 minutes, as longer digestion is harmful for MSCs, and could lift non-MSCs from the dish.
Passaging should be performed every 4–6 days at a split ratio of 1:3. Normally, the cells at Passage 3 contain fewer macrophages and blood cells, and less fat than those at Passages 1 and 2, and can be readily used for experiments. Challenges and possible solutions in mouse BM-MSCs culture are summarised in Table 1.
Table 1.
Challenges and possible solutions in mouse BM-MSCs culture.
| Problem | Possible cause | Solution |
|---|---|---|
| Few harvested cells from bone marrow | Incomplete bone marrow cavity flushing | Repeatedly flush bone cavities until the bones appear to be pale |
| The bone was broken and cells leaked out | Carefully dissect bones and dissociate soft tissue from bones | |
| Cells were dead during harvesting | Prepare the bone marrow within 30 min following animal death, and keep bones in complete α-MEM medium on ice | |
| Microbial contamination | Contaminated during bone sample harvesting | Wash the mouse body with 70% ethanol for at least 2 min Avoid bones touching the mouse skin during dissection Keep bones in complete α-MEM medium with 1% PSN |
| Contaminated during cell culture period | Wipe the dish with 70% ethanol prior to transferring it into the cabinet Wash bones twice using pipet-aid to flush away impurities, blood cells and residual soft tissue that slightly connect to the bone with complete α-MEM medium containing 1% PSN |
|
| Cells are not digested off by trypsin | Cells not washed with PBS prior to digestion | Wash the cells twice with PBS prior to digestion to remove any residual serum |
| Cells grow slowly after passaging | Trypsin cells for >2 min | Digest cells with trypsin for <2 min |
| The initial total MSC numbers are low | Do not disturb the cells for the first 3 days and do not passage the cells until they reach at least 70% confluence |
The commonly used protocol for establishing (mouse) BM-MSC culture
The commonly accepted isolation strategy for BM-MSCs was initially reported by Nadri et al. [16]. Briefly, a mouse is terminated; tibias, femurs, and humeri are dissected. Both ends of the bones are removed with sharp scissors. Bone marrow is flushed out from the bone cavity using plain culture medium, then filtered through a 70-mm filter mesh, washed, and resuspended. The dish is then incubated at 37°C in a 5% CO2 incubator. Nonadherent cells are removed 24–72 hours later by changing the medium. When culture reaches 70–90% confluence, cells are subcultured at a split ratio of 1:3.
Phenotypic characterisation and cell growth rate of the mouse BM-MSCs
BM-MSCs at Passage 3 were used for characterisation. Mesenchymal stem cell markers CD44, CD90, endothelial cell marker CD31 and haematopoietic marker CD45 were examined by flow cytometry according to a previously published paper [26]. The trilineage differentiation abilities were tested according to previous published protocols [26], [27]. We have used Alizarin red, Oil Red O, and toluidine blue staining as indicators for osteogenic, adipogenic, and chondrogenic differentiation according to our previously published methods [27].
In order to compare the differences between our protocol and the commonly used protocol for mouse BM-MSCs culture, we used 16 mice of two different strains (ICR and C57) with two different ages (4 weeks and 8 weeks, males and females) and assigned them into four groups as shown in Table 2. Left/right side of the femurs were chosen for isolation of BM-MSCs using our protocol and the contralateral femur was then used for isolation of BM-MSCs using the commonly used method. To calculate the cell growth rate, when the cultured cells reached about 90% confluence, they were trypsinised, cell numbers were counted using a handheld automatic cell counter (Scepter 2.0 Cell Counter, Merck Millipore, Darmstadt, Germany) and recorded. The passaged cells were then subcultured into new flasks at a split ratio of 1:3.
Table 2.
Animal group details.
| Groups (Our protocol) |
Strain | Numbers | Age (wk) | Femur useda | Control groups (Standard protocol) |
Femur useda |
|---|---|---|---|---|---|---|
| I4 | ICR | 4 | 4 | Random | I4-C | Contralateral |
| I8 | ICR | 4 | 8 | Random | I8-C | Contralateral |
| C4 | C57 | 4 | 4 | Random | C4-C | Contralateral |
| C8 | C57 | 4 | 8 | Random | C8-C | Contralateral |
Left or right side of the femur was randomly used for cell culture using our protocol, and the contralateral femur was then used for cell culture using the standard protocol.
Statistical analysis
All quantitative data were transferred to statistical spreadsheets and analysed by a commercially available statistical program SPSS version 16.0 (IBM SPSS Inc., Chicago, IL, USA), one-way analysis of variance were used for comparison of mean values with p < 0.05 considered statistically significant.
Results
Morphological features of cultured mouse BM-MSCs
Morphological features of the cultured mouse BM-MSCs using our protocol were similar to those of the BM-MSCs cultures using the commonly used method. On Day 1, most of the cells were still mononuclear cells and fat droplets were frequently seen (Fig. 2A). On Day 2, some spindle-shaped cells (arrows) appeared among the mononuclear cells and fat droplets (Fig. 2B). On Day 3, the number of spindle-shaped cells continued increasing (Fig. 2C). On Day 4, the spindle-shaped cells reached about 60–80% confluence (Fig. 2D), the cell growth was slower in the flasks using the commonly used method compared to the new method. On Day 5, the spindle-shaped cells already formed cell layers (Fig. 2E) using the new protocol, but this is not seen in the flasks under the commonly used protocol. Using our protocol, on Day 5, fibroblast-like cell grew out from a dense cell nodule (Fig. 2F), and the cells were passaged on this day; on Day 7, cells reached 100% confluence when left without passaging, multiple cell layers and dense cell nodules were formed in some areas of the culture dish (Fig. 2G and H). By contrast, the cells cultured using the commonly used method only reach 60–70% confluence at Day 7. After the cells reached 90% confluence, they were passed and split; after Passage 3, the cells had uniform fibroblast-like morphology (Fig. 2I) regardless of the initial cell culture protocol. Using our protocol, we could obtain approximately 5 × 107–1.5 × 108 BM-MSCs from one mouse bone marrow preparation in about 2 weeks, whereas it would take at least 3 weeks for the BM-MSCs to reach a similar cell number using the commonly used protocol.
Figure 2.
Morphological features of the cultured mouse bone marrow mesenchymal stem cells using our protocol. (A) On Day 1, most of the cells were still mononuclear and fat droplets (arrows) were frequently seen. (B) On Day 2, some spindle-shaped cells (arrows) appeared among the mononuclear cells and fat droplets. (C) On Day 3, the numbers of spindle-shaped cells (arrows) continued increasing. (D) On Day 4, the spindle-shaped cells reached about 60–80% confluence. (E) On Day 5, the spindle-shaped cells formed cell layers (the circle). (F) On Day 5, fibroblast-like cells (arrows) grew out from a dense cell nodule, and the cells were passaged on this day. (G, H) On Day 7, cells reached 100% confluence when left without passaging; multiple cell layers and dense cell nodules were formed in some areas of the culture dish (the circle). (I) At Passage 3, the cells have uniform fibroblast-like morphology. Scale bar = 200 μm.
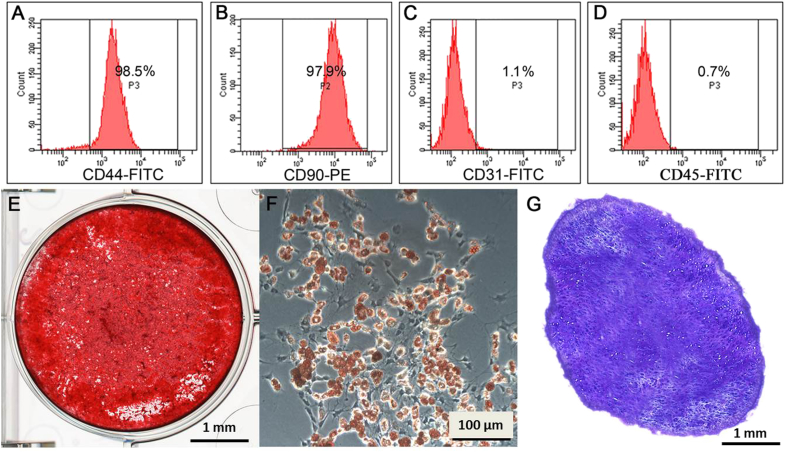
Confirmation of phenotype and differentiation capacities of mouse BM-MSCs
Once established in the culture dishes and passaged three times, the MSCs retain similar phenotypes or differentiation potentials regardless of the protocol initially used. Results showed that the established cells were strongly positive for MSC markers CD44, CD90 (Fig. 3A and B), and negative for endothelial cell marker CD31 (Fig. 3C), and the haematopoietic cell marker CD45 (Fig. 3D). The isotype control was negative.
Figure 3.
Confirmation of mesenchymal stem cell (MSC) surface markers and differentiation capacities of mouse bone marrow (BM)-MSCs. (A, B) Flow cytometry analysis results showed that these cells were positive for MSC markers CD44 (A) and CD90 (B). (C) Cells were negative for endothelial cell marker CD31. (D) Cells were negative for haematopoietic cell marker CD45. (E) Alizarin red staining demonstrated that mineralised nodules formed in the BM-MSCs after 4 weeks under the osteogenic induction. (F) Intracellular Oil-red-O staining showed lipid-rich vacuole formation of the mouse BM-MSCs after 2 weeks, adipogenic induction. (G) After 3 weeks' chondrogenic induction, the cell pellet was sectioned and stained with toluidine blue; the positive acidic proteoglycan indicated the chondrocyte-like cell formation. Scale bar = 1 mm (F and H) and 100 μm (G).
The differentiation capacities of BM-MSCs obtained using either protocol remain similar. Alizarin red staining demonstrated that mineralised nodules formed in the BM-MSCs after 4 weeks under the osteogenic induction (Fig. 3E). Intracellular Oil-red-O staining showed lipid-rich vacuoles formation of the mouse BM-MSCs after 2 weeks adipogenic induction (Fig. 3F). After 3 weeks chondrogenic induction, the cell pellet was sectioned and stained with toluidine blue; the positive acidic proteoglycan indicated the chondrocyte-like cells formation (Fig. 3G).
Comparison of the cell growth rate using the new protocol versus the commonly used protocol
As shown in Table 3, the successful isolation rate of MSCs was not strain- or sex-dependent. Cells are able to reach 70–90% confluence from Passage 0–1 using both protocols; however, cells in Groups I4, I8, C4, and C8 grew significantly faster than that in control groups, it only takes 5 days when cells reached 70–90% confluence from P0 to P1 and from P1 to P2 compared with 9 days, and the cell numbers are also significantly higher than those using the standard culture protocol (p = 0.037). After the first passage, cells in about one in four of the cell culture dishes in the control groups (using the commonly used cell culture method) grew very slowly or stopped growing, and they were not able to reach 70–90% confluence even after culture for 1 month. These cells accumulated internal fatty vacuoles, and displayed the typical large and flat senescent morphology.
Table 3.
Comparison of cell growth rate using the two protocols among different mice.
| Groups | Passage 0–1 |
Passage 1–2 |
||||
|---|---|---|---|---|---|---|
| Cells (× 106) | Durationa | Success rate (%)b | Cells (× 106) | Duration (d)a | Success (%)b | |
| I4-C | 2.27 ± 0.06 | 9 | 100 | 3.43 ± 0.08 | 9 | 75 |
| I4 | 2.82 ± 0.11 | 5 | 100 | 4.20 ± 0.15 | 5 | 100 |
| I8-C | 2.26 ± 0.10 | 9 | 100 | 3.31 ± 0.10 | 9 | 75 |
| I8 | 2.77 ± 0.09 | 5 | 100 | 4.27 ± 0.09 | 5 | 100 |
| C4-C | 2.27 ± 0.03 | 9 | 100 | 3.32 ± 0.09 | 9 | 50 |
| C4 | 2.85 ± 0.09 | 5 | 100 | 4.21 ± 0.09 | 5 | 100 |
| C8-C | 2.23 ± 0.10 | 9 | 100 | 3.38 ± 0.06 | 9 | 75 |
| C8 | 2.83 ± 0.16 | 5 | 100 | 4.32 ± 0.09 | 5 | 100 |
Duration: defined as the time needed for cells to reach 70–90% confluence after passage.
Success rate of cell culture: defined as cells reaching 70–90% confluence after passage.
Discussion
Isolation of MSCs from bone marrow is far more challenging in mouse than other species. We compared several reported isolation strategies and developed a new protocol for standardised, reliable and easy-to-perform isolation of mouse MSCs from bone marrow. There are five main features of our method: First, after flushing bone marrow out of the marrow cavity, we cultured the cells with fat mass without filtering them. Our experience showed that the initial phase of culture is crucial for later yield of cells. The number of MSCs in mouse bone marrow is much lower than that of rat or human MSCs [1], if we filtered the bone marrow, some MSCs attached to the fat masses will be entrapped onto the filter, thus further reducing the MSCs yields. Furthermore, BM-MSCs are maintained in their niche composing of stromal cells, extracellular matrix elements, and secreting factors to nurture and regulate MSCs self-renewal and differentiation [28]. Our method is simple, but keeping the MSCs in their initial niche with minimal disturbance to allow the initial adjusting time for the MSCs in culture. Second, the culture medium is not supplemented with any additional growth factor. It is reported that supplement of growth factors in culture may modify MSC protein synthesis and intracellular trafficking, and affect MSC proliferation and differentiation potentials [21]. Third, our method does not need to separate the haematopoietic stem cells, which are preferentially localised in the endosteal regions of the bone [29] using flow cytometry or immunomagnetic sorting techniques. Our method simply use the culture medium to select the plastic adherent MSCs. Fourth, this method has been tested in several mouse strains (ICR, FVB/N, CMV-Luc, C57) as well as in mice with different ages (2–8 weeks, males and females) and the results are reproducible in all tested mice. The successful isolation rate of MSCs was not strain- or sex-dependent. Fifth, we have optimised our culture protocol, and listed detailed problems that may arise in cell culture and trouble-shooting tricks (Table 1). The cells established using our culture method are mesenchymal stem cells, which are not contaminated with haematopoietic cell lineages and endothelial cells, and are able to differentiate into osteoblasts, adipocytes and chondrocytes when respectively cultured with osteogenic, adipogenic, and chondrogenic medium.
The current study focuses on the development of a simple cell culture protocol for mouse BM-MSCs. We used minimal manipulations during the initial cell harvest (no filtering, no enzyme digestion). Once the MSCs established in either using our protocol or the commonly used protocol, the MSCs remain similar phenotypes or differentiation potentials.
In conclusion, compared with the commonly used mouse BM-MSCs culture protocol, our protocol results in a higher success rate of MSCs isolation and establishment in culture; the cells displayed higher growth rate and maintained the multipotent differentiation potentials. Our protocol may be a simple, reliable, and alternative method for culturing MSCs from mouse bone marrow tissues.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgements
This work was supported by a grant from the Hong Kong Government Research Grant Council, General Research Fund (CUHK470813), and a grant from the China Shenzhen City Science and Technology Bureau under the Shenzhen City Knowledge Innovation Plan, Basic Research Project (JCYJ20130401171935811) to G.L. This study was also supported in part by the SMART program, Lui Che Woo Institute of Innovative Medicine, Faculty of Medicine, The Chinese University of Hong Kong. This research project was made possible by resources donated by Lui Che Woo Foundation Limited.
References
- 1.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Ramos J.R. Neural cells derived from adult bone marrow and umbilical cord blood. J Neurosci Res. 2002;69:880–893. doi: 10.1002/jnr.10337. [DOI] [PubMed] [Google Scholar]
- 3.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 4.Kassem M., Abdallah B.M. Human bone-marrow-derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res. 2008;331:157–163. doi: 10.1007/s00441-007-0509-0. [DOI] [PubMed] [Google Scholar]
- 5.Erices A., Conget P., Minguell J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 6.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campagnoli C., Roberts I.A., Kumar S., Bennett P.R., Bellantuono I., Fisk N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 8.Meirelles Lda S., Nardi N.B. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702–711. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- 9.Kadiyala S., Young R.G., Thiede M.A., Bruder S.P. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997;6:125–134. doi: 10.1177/096368979700600206. [DOI] [PubMed] [Google Scholar]
- 10.Lennon D.P., Caplan A.I. Isolation of rat marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1606–1607. doi: 10.1016/j.exphem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Ringe J., Kaps C., Schmitt B., Buscher K., Bartel J., Smolian H. Porcine mesenchymal stem cells. Induction of distinct mesenchymal cell lineages. Cell Tissue Res. 2002;307:321–327. doi: 10.1007/s00441-002-0525-z. [DOI] [PubMed] [Google Scholar]
- 12.Caterson E.J., Nesti L.J., Danielson K.G., Tuan R.S. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245–256. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- 13.Crow J.F.C. C. Little, cancer and inbred mice. Genetics. 2002;161:1357–1361. doi: 10.1093/genetics/161.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvorakova J., Hruba A., Velebny V., Kubala L. Isolation and characterization of mesenchymal stem cell population entrapped in bone marrow collection sets. Cell Biol Int. 2008;32:1116–1125. doi: 10.1016/j.cellbi.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H., Guo Z.K., Jiang X.X., Li H., Wang X.Y., Yao H.Y. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 16.Nadri S., Soleimani M., Hosseni R.H., Massumi M., Atashi A., Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007;51:723–729. doi: 10.1387/ijdb.072352ns. [DOI] [PubMed] [Google Scholar]
- 17.Van Vlasselaer P., Falla N., Snoeck H., Mathieu E. Characterization and purification of osteogenic cells from murine bone marrow by two-color cell sorting using anti-Sca-1 monoclonal antibody and wheat germ agglutinin. Blood. 1994;84:753–763. [PubMed] [Google Scholar]
- 18.Eslaminejad M.B., Nadri S. Murine mesenchymal stem cell isolated and expanded in low and high density culture system: surface antigen expression and osteogenic culture mineralization. In Vitro Cell Dev Biol Anim. 2009;45:451–459. doi: 10.1007/s11626-009-9198-1. [DOI] [PubMed] [Google Scholar]
- 19.Eslaminejad M.B., Nikmahzar A., Taghiyar L., Nadri S., Massumi M. Murine mesenchymal stem cells isolated by low density primary culture system. Dev Growth Differ. 2006;48:361–370. doi: 10.1111/j.1440-169X.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 20.Baddoo M., Hill K., Wilkinson R., Gaupp D., Hughes C., Kopen G.C. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- 21.Soleimani M., Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 22.Siclari V.A., Zhu J., Akiyama K., Liu F., Zhang X., Chandra A. Mesenchymal progenitors residing close to the bone surface are functionally distinct from those in the central bone marrow. Bone. 2013;53:575–586. doi: 10.1016/j.bone.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun S., Guo Z., Xiao X., Liu B., Liu X., Tang P.H. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21:527–535. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- 24.Tropel P., Noël D., Platet N., Legrand P., Benabid A.L., Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Nadri S., Soleimani M. Isolation murine mesenchymal stem cells by positive selection. In Vitro Cell Dev Biol Anim. 2007;43:276–282. doi: 10.1007/s11626-007-9041-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T., Lee Y.W., Rui Y.F., Cheng T.Y., Jiang X.H., Li G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 2013;4:70. doi: 10.1186/scrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X., McClurg A., Zhou G.Q., McCaigue M., Armstrong M.A., Li G. Chondrogenic differentiation alters the immunosuppressive property of bone marrow-derived mesenchymal stem cells, and the effect is partially due to the upregulated expression of B7 molecules. Stem Cells. 2007;25:364–370. doi: 10.1634/stemcells.2006-0268. [DOI] [PubMed] [Google Scholar]
- 28.Kolf C.M., Cho E., Tuan R.S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nombela-Arrieta C., Pivarnik G., Winkel B., Canty K.J., Harley B., Mahoney J.E. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]